Chemical Nomenclature and Formulas for Ionic Compounds Ionic









- Slides: 20

Chemical Nomenclature and Formulas for Ionic Compounds

Ionic Compounds n Consist of cations (positive ions) and anions (negative ions) n Usually composed of metals (cations) and nonmetals (anions) n Oxidation numbers (charges of ions) are important
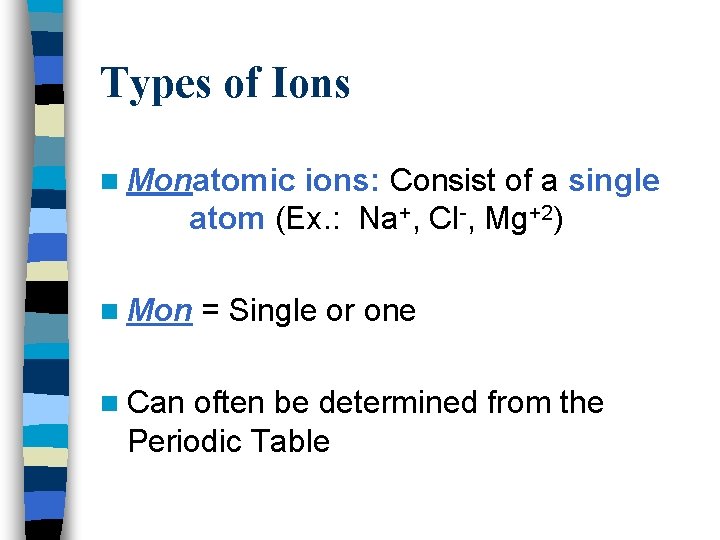
Types of Ions n Monatomic ions: Consist of a single atom (Ex. : Na+, Cl-, Mg+2) n Mon n Can = Single or one often be determined from the Periodic Table

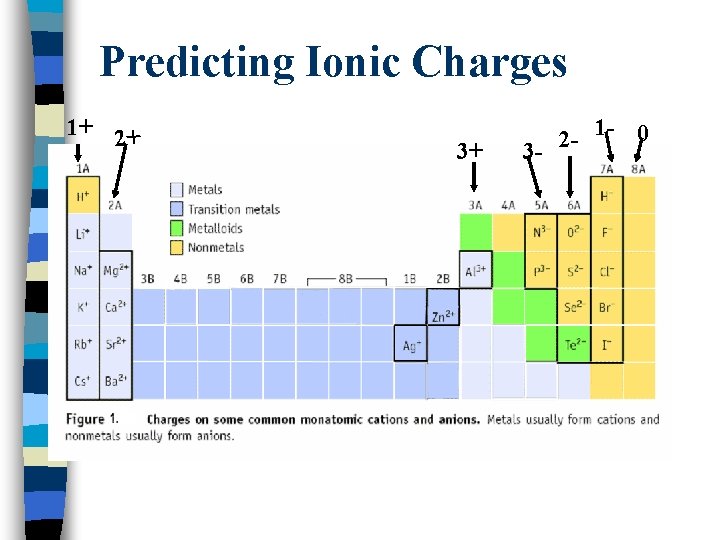
Predicting Ionic Charges 1+ 2+ 3+ 3 - 2 - 1 - 0

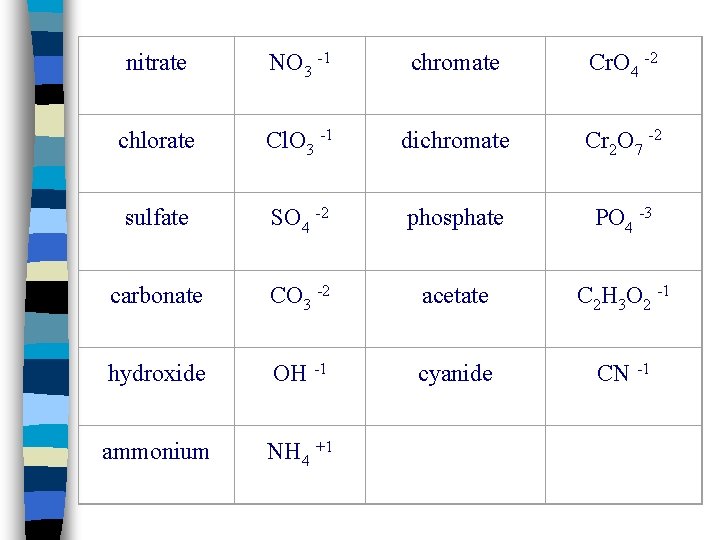
Types of Ions n Polyatomic ions: Groups of atoms that behave as a unit and carry a charge (Ex. : NO 3 -, OH-, SO 4 -2) n Poly n You = Many or several will need a list of polyatomic ions to determine the name and formula

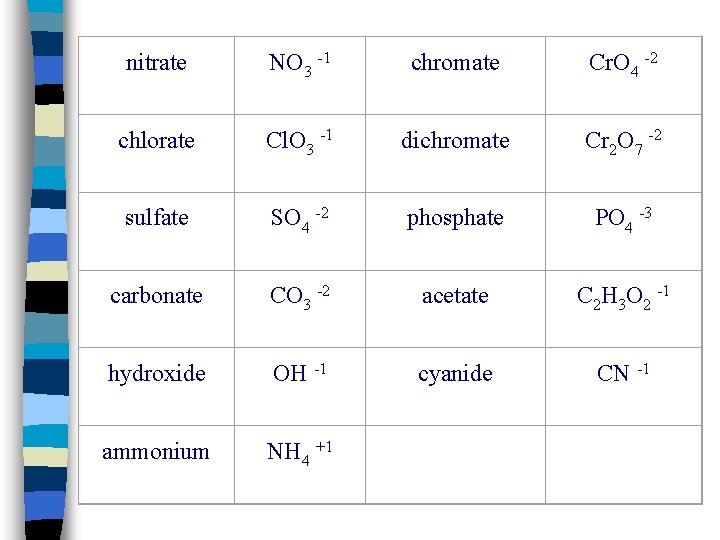
nitrate NO 3 -1 chromate Cr. O 4 -2 chlorate Cl. O 3 -1 dichromate Cr 2 O 7 -2 sulfate SO 4 -2 phosphate PO 4 -3 carbonate CO 3 -2 acetate C 2 H 3 O 2 -1 hydroxide OH -1 cyanide CN -1 ammonium NH 4 +1

Binary Ionic compounds Writing Formulas n Made up of two monatomic ions (metal and nonmetal) n Ex. : Potassium and Chlorine n Ex. : Calcium and Bromine

Binary ionic compounds Writing Formulas n Transition Metals (Groups 3 through 12) and some metals in Groups 3 A and 4 A (except aluminum, cadmium, silver, and zinc) can have several oxidation numbers n Ex. : Iron (III) and Oxygen n Ex. : Copper (II) and Oxygen

Ionic Compounds with Polyatomic ions Writing Formulas n Ions made up of more than one atom n Act as individual ions n Rules used for binary compounds still apply n Use parentheses when more than one polyatomic ion is needed and use the appropriate subscripts outside of the parentheses

nitrate NO 3 -1 chromate Cr. O 4 -2 chlorate Cl. O 3 -1 dichromate Cr 2 O 7 -2 sulfate SO 4 -2 phosphate PO 4 -3 carbonate CO 3 -2 acetate C 2 H 3 O 2 -1 hydroxide OH -1 cyanide CN -1 ammonium NH 4 +1

Ionic Compounds with Polyatomic ions Writing Formulas n Example: ion Ammonium ion and chloride *IWB Calcium ion and phosphate *IWB

Practice Problems 1. sodium chloride 2. calcium oxide 3. potassium hydroxide 4. magnesium sulfide 5. copper(II) carbonate 6. aluminum oxide 7. iron(III) oxide 8. sodium carbonate 9. aluminum hydroxide 10. ammonium nitrate 11. zinc nitrate 12. magnesium carbonate

Naming ionic compounds: Rules for naming ionic compounds 1. Name cation first and then the anion Cs. Br Cs cation + - Br anion

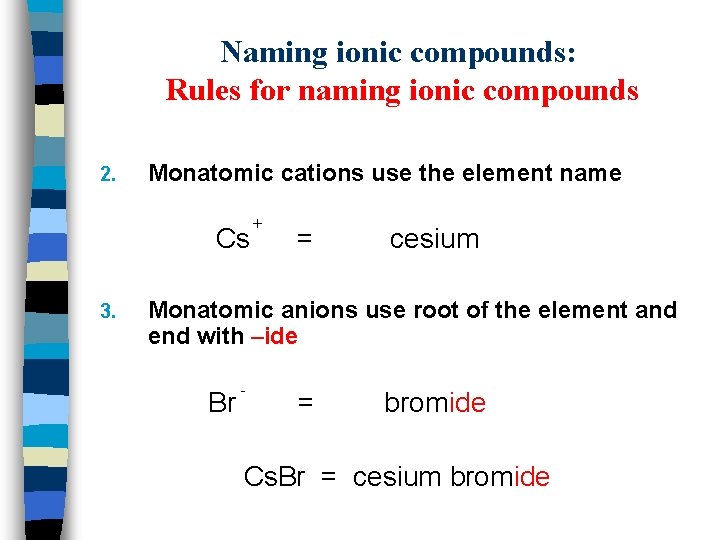
Naming ionic compounds: Rules for naming ionic compounds 2. Monatomic cations use the element name Cs 3. + = cesium Monatomic anions use root of the element and end with –ide Br - = bromide Cs. Br = cesium bromide


Naming ionic compounds: Rules for naming ionic compounds 4. This applies to transition metals and some metals in groups 3 A and 4 A (more than one oxidation number): Distinguish between different oxidation numbers of the cation in the name of the chemical formula Use a Roman numeral in parentheses after name of cation


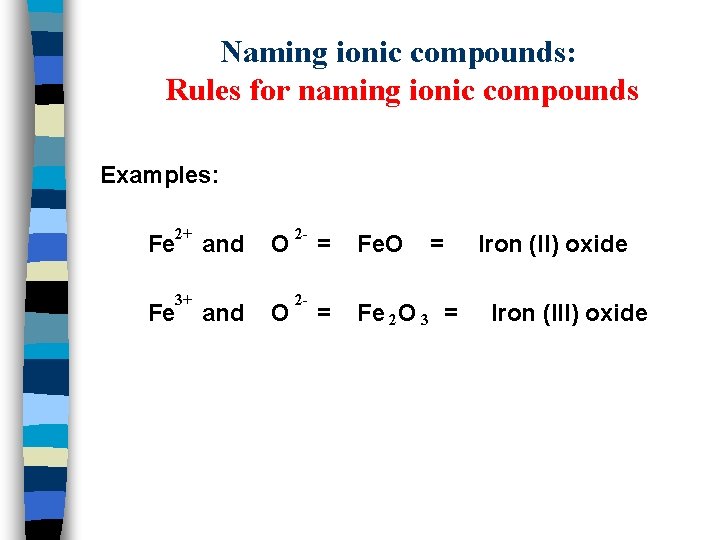
Naming ionic compounds: Rules for naming ionic compounds Examples: 2+ Fe 3+ Fe and O O 2 - 2 - = Fe. O = = Fe 2 O 3 = Iron (II) oxide Iron (III) oxide

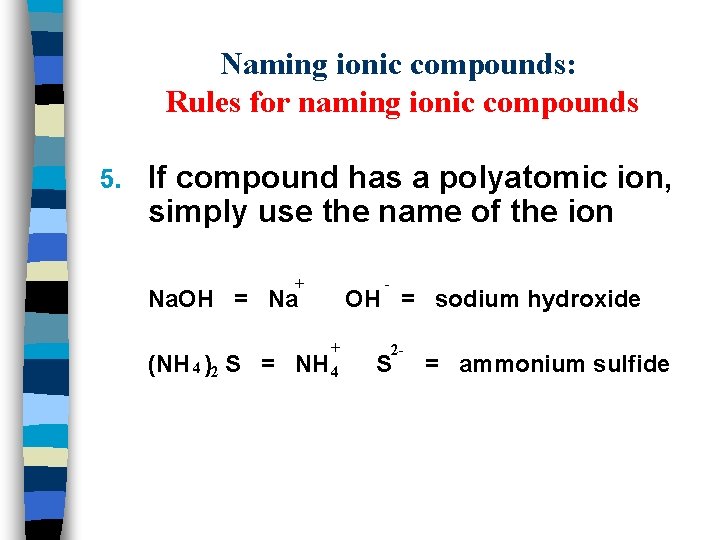
Naming ionic compounds: Rules for naming ionic compounds 5. If compound has a polyatomic ion, simply use the name of the ion + Na. OH = Na (NH 4 )2 S = + NH 4 - OH = sodium hydroxide 2 - S = ammonium sulfide

Practice Problems 1. Mg. Cl 2 2. Li. OH 3. Zn. CO 3 4. K 2 S 5. Fe. PO 4 6. Ag 3 N 7. Mn(CN)2 8. Ag. C 2 H 3 O 2 9. Ba. I 2 10. Pb. S 2