New Contract Quality Criteria January Session Fiona Lowe
























































- Slides: 63

New Contract Quality Criteria January Session Fiona Lowe Chief Officer Coventry, Warwickshire, Herefordshire & Worcestershire LPCs This meeting has been supported by the following Companies: Pfizer, Teva and Chiesi through the purchase of the Exhibition stand space. These Companies have had no involvement with the Speaker selection or content of this meeting

Registration & Stands (09: 00 – 09: 30) Breakout Room – Basics & Gateway Practical Support 09: 00 – 10: 00

Introduction (09: 30) Fiona Lowe

Welcome & Introduction • Welcome & introducing the team • Domestics ▫ Fire Alarms ▫ Toilets ▫ Phones ▫ Sponsors • Pre-work and Handouts • How the day will work ▫ Main Room & Breakout Room

Agenda • 09: 00 Registration, refreshments and view stands (Basics, Gateway and other QC practical support in breakout room until 10 am) • • • 09: 30 Introduction followed by Dementia Friends session with Geraldine Flavell 10: 30 Asthma – Clinical Effectiveness with Fiona Lowe 11: 00 Coffee Break 11: 15 Patient Safety session with Surinderpal Virdee 12: 45 Healthy Living Pharmacy Introduction with Fiona Lowe (Basics, Gateway and other QC practical support sessions in breakout room) • 12: 45 Q&A • 13: 00 Lunch and stands (Basics, Gateway and other QC practical support sessions in breakout room) • 13: 30 Dementia Friends session if required in breakout room • 14: 00 – 16: 30 Safeguarding Level 2 (CPPE) with Hayley Berry (refreshments available from 15: 30) • 16: 30 Wrap Up and any remaining Q&A

Quality Payments in 17/18 • Total of 100 points • £ 64 per point • = £ 6, 400 per contract • Could increase as additional allocation if less than 100% uptake • To a max of £ 128 per point Gateway ▫ EPS is active and used ▫ NHS Choices is up to date and maintained (7 th Feb onwards) ▫ Active NHS Mail address and active (apply during January for generic email) ▫ Delivery of at least ONE specified Advanced service

Quality Payments Total points Points at any Number of review points over the two one review at which it can be claimed review points Domain Criteria Patient Safety Written safety report at premises level available for inspection at review point, covering analysis of incidents and incident patterns (taken from an One ongoing log), evidence of sharing learning locally and nationally, and actions taken in response to national patient safety alerts. 20 20 Patient Safety On the day of the review 80% of registered pharmacy professionals working at the pharmacy have achieved level 2 safeguarding status for children and Two vulnerable adults in the last two years. 5 10 5 5 20 20 On the day of the review, the results of the Community Pharmacy Patient Experience Questionnaire from the last 12 months is publicly available on the pharmacy’s NHS Choices page. One Public Health On the day of the review, the pharmacy is a Healthy Living Pharmacy level 1 One (self-assessment).

Quality Payments Total points Points at any Number of review points over the two one review at which it can be claimed review points Domain Criteria Digital On the day of the first review, the pharmacy can demonstrate a total increase in access to Summary Care Records between 1 December 2016 and 28 April 2017 in comparison to the previous 5 months; and on the day of the Two second review, the pharmacy can demonstrate a total increase to access to Summary Care Records between 1 May 2017 and 24 November in comparison to the previous 7 months. 5 10 Digital On the day of the review, the pharmacy’s NHS 111 Directory of Services entry is up to date. Two 2. 5 5 Clinical Effectiveness On the day of the review, the pharmacy can show evidence of asthma patients, for whom more than 6 short acting bronchodilator inhalers were dispensed without any corticosteroid inhaler within a 6 month period, are referred to an appropriate health care professional for an asthma review. Two 10 20 Workforce On the day of the review, 80% of all pharmacy staff working in patient facing roles are trained ‘Dementia Friends’. Two 5 10 Total number of points: 100

LPC Communications



A Dementia Friend learns a little bit more about what it's like to live with dementia and then turns that understanding into action - anyone of any age can be a Dementia Friends (9: 45) Geraldine Flavell https: //www. dementiafriends. org. uk/

Asthma – Clinical Effectiveness (10: 30) Fiona Lowe

Asthma – clinical effectiveness quality payment • Community pharmacy contractors passing the gateway criteria will receive a Quality Payment if they meet one or more of the Quality Payment criteria. One of these is: • ‘On the day of the review, the pharmacy can show evidence of asthma patients, for whom more than 6 short acting bronchodilator inhalers were dispensed without any corticosteroid inhaler within a 6 month period, are referred to an appropriate health care professional for an asthma review. ’ • Two opportunities to collect 10 points (each worth £ 64)

Asthma review • Requires pharmacists to refer asthma patients to an appropriate healthcare professional if they have been dispensed more than six shortacting bronchodilators without any corticosteroid inhaler within a six month period • Evidence required and if a minimum number of patients to be referred not yet clear • In the meantime, consider what changes may need to be made to, for example, standard operating procedures (SOPs), and Patient Medication Record (PMR) systems • Ensure pharmacy team can identify short-acting bronchodilator inhalers and corticosteroid inhalers

Asthma review • Eligibility 1. They have been diagnosed with asthma 2. They have been prescribed an inhaler containing one of the following short-acting bronchodilators: Short-acting beta-2 agonist Ipratropium bromide 3. They have been prescribed more than six inhalers containing a short acting bronchodilator in the last six months 4. Inhaled corticosteroid therapy has not been prescribed during this six month period

Asthma review Meeting the criterion • Identify potential patients that meet this criterion ▫ E. g. Checking patient medication record (PMR) to see if the patient is eligible when presenting with a prescription • Speak with the patient to understand their inhaler use ▫ Medicines Use Review (MUR) or their inhaler technique check may be undertaken • If an issue is identified: ▫ Refer patient to their GP using the pre-determined referral system • All referrals should be documented on the patients PMR ▫ It may also be appropriate to use a data collection form as evidence for the criterion being met

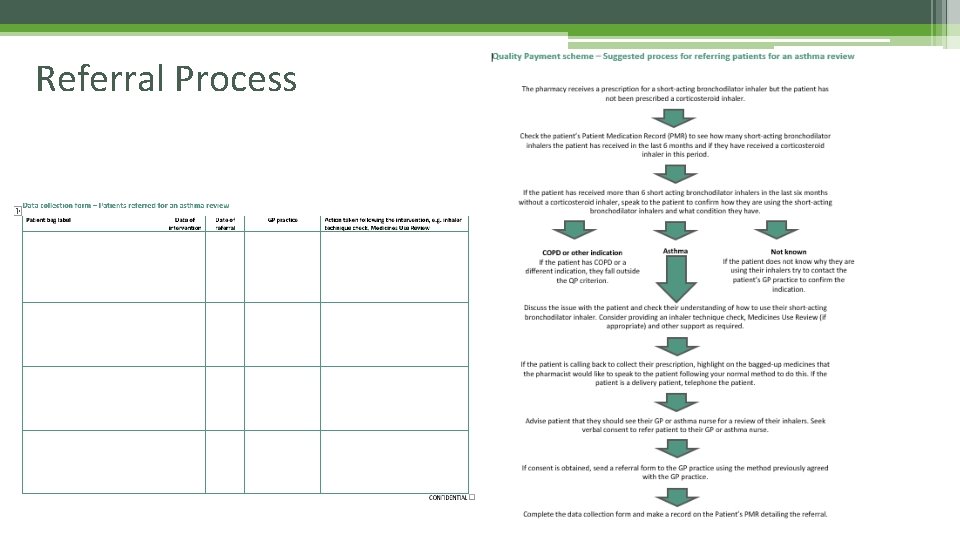
How to achieve the quality payment • Further information on this Quality Payment criterion is available in PSNC Briefing 068/16: Quality Payments – referrals for asthma reviews (November 2016) • Process • A suggested process for pharmacy teams to follow to incorporate this Quality Payment criterion into their daily practice can be found below. • Asthma referrals – Suggested process for referring patients for an asthma review (Word) • Asthma referrals – Suggested process for referring patients for an asthma review (PDF) • This process looks to identify patients who have been diagnosed with asthma and have been prescribed more than 6 short-acting bronchodilators in the last 6 months as these patients are likely to require their asthma to be assessed urgently.

Referral Process

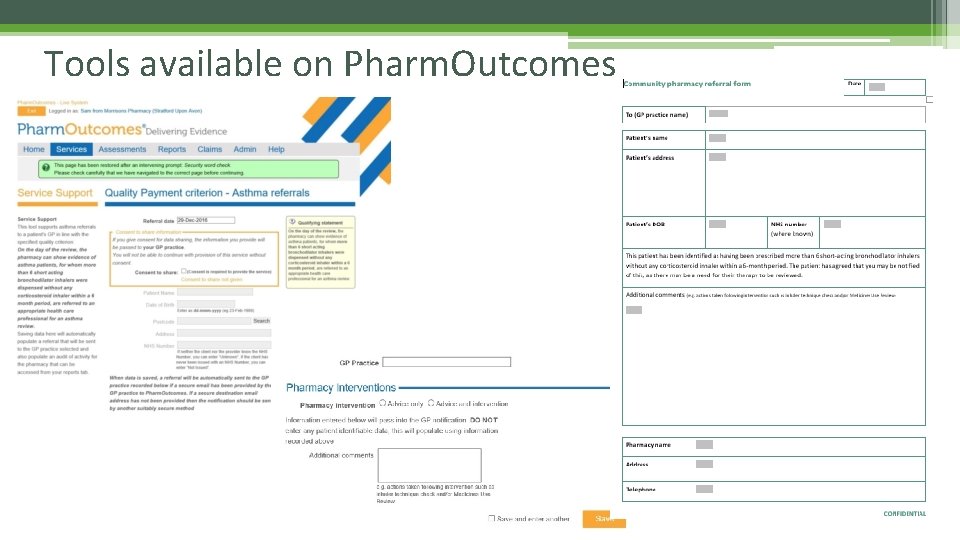
Tools available on Pharm. Outcomes



What added value is possible? • • • MUR NMS Inhaler technique checks Differential diagnosis from COPD Clinical audit Evidence of supporting patients with LTC Link to other services Build relationships with GP Practice team Future sessions – NMS/ MUR support and inhaler technique training

Refreshments (11 am)

Patient Safety & CPPQ (11: 15) Surinderpal Virdee MRPharm. S MFRPSII

Objectives To meet the following criteria that fall within the Quality Payments Scheme • Community Pharmacy Patient Questionnaire (CPPQ) results • Patient Safety Report

Details CPPQ • Results of the CPPQ from the last 12 months is publicly available on the pharmacy’s NHS Choices website Patient Safety Report A written safety report at premises level available for inspection at review point covering: - • Analysis of incidents and incident patterns • Evidence of sharing learning locally and nationally • Action taken in response to National Patient Safety Alerts

Community Pharmacy Patient Questionnaire (CPPQ) REPORT

• The CPPQ survey must be done annually • Each site has a minimum number of responses required based on their average monthly items Average monthly script volume (Items) Minimum number of returned surveys 0 -2000 50 2001 -4000 75 4001 -6000 100 6001 -8000 125 8001 -upwards 150 • The questionnaire is available on the PSNC website and available in 3 formats (PDF, Word document and a Large print Word document)

• The PSNC have also provided a worksheet tool to enable you to analyze the results Section 1 - Data collation Section 2 - Ranks each question Section 3 - Action plan. This is the part that will need to be published on your NHS Choices profile (for each site) • The survey needs to be completed by End March • Need to get started as soon as possible, you do not want to be chasing surveys close to the completion date • Most actions will actually be very simple and only require simple changes

Some examples of questions and potential solutions include: - Waiting area not of sufficient size and was left standing for prescription Solution - Review seating arrangement and added 2 more chairs Was not offered any lifestyle advice Solution - Pharmacy leaflet now directs patients to pharmacist when seeking such advice Not enough stock to fulfil my prescription Solution - Now have in place recommended average stock levels on electronic stock file

Patient Safety Report

Before a patient safety report can be written, a number of other forms require completion. These forms will enable you to be more thorough in your analysis and review. However…. . before we look at those forms…. let’s understand some key reasons why patient safety is important: • Firstly and most importantly, none of us want to harm a patient and therefore want to constantly improve our standards to avoid patient harm • GPh. C Standards for registered premises: Principle 1 ‘The governance arrangements safeguard the health, safety and wellbeing of patients and the public’ In particular, it focuses on identifying and managing risk i. e. ‘what happened, why did it happen and what do we now need to do to make sure it doesn't happen again • Finally, the RPS released a new document in November 2016 titled ‘Professional Standards for the reporting, learning sharing, taking action and review of incidents’ (Please note: further details of the GPh. C Principles and Standards can be found on the GPh. C website)


Forms* that will help you complete a patient safety report include: - • Near miss log • Near Miss Error Improvement Tool (RPS) • Community pharmacy medication safety incident (Pharmacy error) report form • Controlled Drug incident report form * There may be other forms that also require completion. The above list is an example

Near miss log • Templates available e. g. RPS website • Log must be readily available to all staff • Need to have an open culture of reporting • Have a no blame culture • Before we look at a good example of a completed near miss log…I have some questions for you……….


Near miss log Improvement Tool • Templates available from RPS website • To be used when reviewing the near miss log • Tool allows you to work out your near miss error percentage (calculated using your total items dispensed) • Review lets you understand ▫ ▫ What type of near misses occurred Why did they happen What needs to happen to minimise the risk of it happening again Review the actions within an agreed timescale to ensure action has been met • Complete as a team…not the pharmacist stuck in an office!!! • Does not have to be a drawn out process. If done weekly/monthly should not take > 30 - 60 mins • The more reviews you do…the easier they become…. and if the actions you put in place are correct, the error percentage will start to reduce

Before we carry on discussing the remaining forms………………. …. . using your own near miss logs you have bought with you today……spend 10 minutes to start to complete the improvement tool (at this stage only need to compete boxes 1, 2 and 3) We will walk around and field any queries or questions

Community Pharmacy Medication Safety Incident report form • Templates available from various sites e. g. PSNC (or if you have company specific version…use them) • NPA’s Chief Pharmacist Leyla Hannbeck is acting as the Medication Safety Officer (MSO) for all independent pharmacies in England with fewer than 50 branches • All incidents should be reported to the NPA via their website (you do not have to be a member of the NPA to do this) • Completed forms can be printed off and kept in the pharmacy • When reporting using the NPA website, you will not need to also report to the National Reporting and Learning System (NRLS) as the NPA will do this for you (Please note: these incidents will be anonymised prior to sending) • Where you have a Superintendent Pharmacist, please ensure they are notified of the incident and that you follow the approved SOP process for incident management for your business

Community Pharmacy Medication Safety Incident report form Key points to note on the incident form ▫ Date of incident - where possible include date of dispensing and date notified of incident ▫ Time ▫ Degree of harm - don’t forget to update if the patients condition worsens ▫ Patient details - Try and capture as much information as possible. It will make contacting the patient much easier especially to help resolve any issues ▫ Contributing factors - just be honest !!! ▫ Planned actions to prevent reoccurrence - The most important box !!!! ▫ Contacting the patient - where a representative wishes to be the point of contact…. don’t forget that patient consent must be received before you can discuss with anybody else

Controlled Drug (CD) incident report form • All healthcare professionals have a statutory duty to report all complaints, concerns or untoward incidents involving controlled drugs to the Controlled Drug Accountable Officer (CDAO) • All CD incidents need to be reported to the CDAO using the secure email account provided • Upon discovering the incident, report as soon as possible and definitely by close of the play of the following day. At this stage you may only have partial information, however, further details can be submitted as they come to light • CD incident report should include: ▫ ▫ Name, Role and Professional registration number (if applicable) Date and location and telephone number where incident occurred Drug name, form, strength and quantity Details of actual incident, action taken, action planned and measures to prevent recurrence (information below taken from Arden and GEM CD bulletin Issue 1 Dec 2016) tails of any internal investigation and learnings identified

Controlled Drug (CD) incident report form Examples of what to report: to CDAO • Stolen/missing/lost CD prescriptions • Fraud involving CD’s Supplying CD’s without a valid prescription • Incorrect item/quantity dispensed • Dispensing out of date CD items • Spillages and missing CD’s • Prescribing errors involving CD’s • Incorrect labelling of CD’s • Incidents involving CD instalment prescriptions (FP 10 MDA) • CD balance discrepancies • Issues with CD requisitions • Incidents involving CD deliveries • CD’s handed out to incorrect person

Patient Safety Alerts • NRLS (NRLS) leads and contributes to improved, safe patient care by informing, supporting and influencing the health sector • Types of alerts from the NRLS include: ▫ Rapid Response Reports ▫ Patient Safety Alerts ▫ Safer Practice Notices • Other places you can receive alerts from include: ▫ MHRA ▫ GOV. UK ▫ RPS (if registered with them, they can send you drug and device recalls, patient safety notices etc) ▫ NHS England ▫ Local teams

Patient Safety Alerts Things to think about: - • How do you get your alerts? • How do you know you are receiving all of them? • What do you do when you get the alerts? • What do you do with the alerts that are not applicable to you?

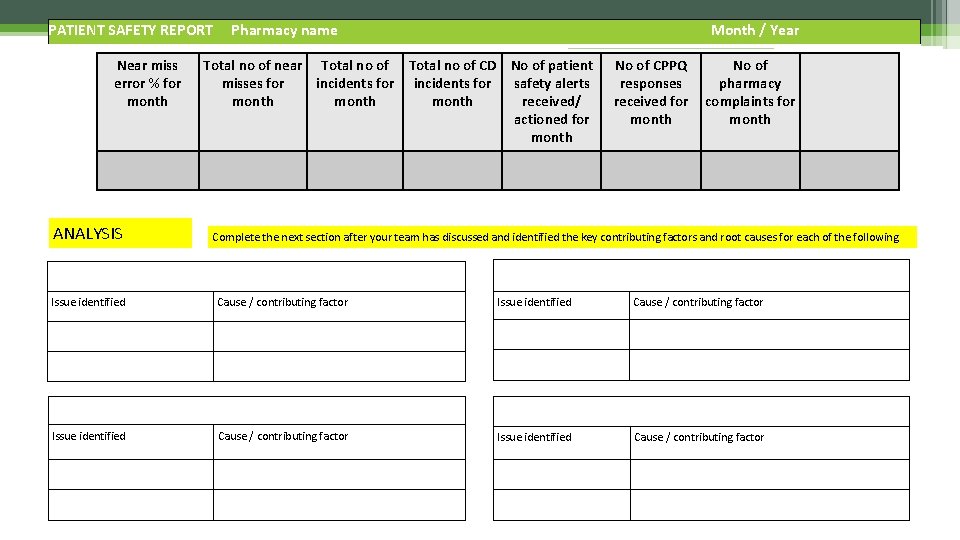
Case study The month has ended and you are required to complete a Patient Safety Report Using the information below…. start to complete the report • Total items dispensed for the month 9, 642 • Total number of near misses for the month 86 • Total incidents reported to NPA 2 • Incident 1 - Azithromycin dispensed instead of Azathioprine. No incorrect medication taken by patient. Contributing factor - messy shelves • Incident 2 - Patient given Insulin Humalog Mix 25 instead of Mix 50. Patient has used three doses and suffered mild side effects however patient is now OK. Contributing factors - disorganised fridge and dispenser who lacks knowledge of insulins • 1 CD discrepancy - 1 Concerta XL 18 mg tablet missing. Reported to CDAO • Zero patient safety alerts received • 15 CPPQ responses • Zero pharmacy complaints

PATIENT SAFETY REPORT Near miss error % for month Pharmacy name Month / Year Total no of near Total no of CD No of patient misses for incidents for safety alerts month received/ actioned for month No of CPPQ responses received for month No of pharmacy complaints for month ANALYSIS Complete the next section after your team has discussed and identified the key contributing factors and root causes for each of the following Near Misses Dispensing Incidents Issue identified Cause / contributing factor Pharmacy Complaints Issue identified Cause / contributing factor Other Issues e. g. CPPQ Cause / contributing factor Issue identified Cause / contributing factor

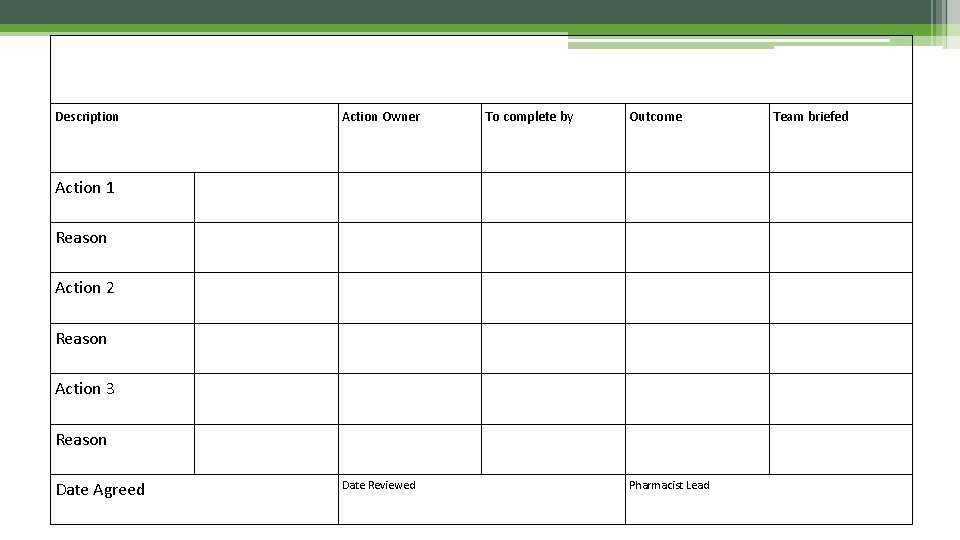
KEY ACTIONS: PHARMACY Description Action Owner To complete by Outcome Action 1 Reason Action 2 Reason Action 3 Reason Date Agreed Date Reviewed Pharmacist Lead Team briefed

Healthy Living Pharmacy Introduction (12: 45) Fiona Lowe Or in Breakout Room – Basics & Gateway Practical Support

Healthy Living Pharmacy – getting started

Healthy Living Pharmacy (HLP) • Can be claimed for at one of the two review points and is worth 20 points • Requires pharmacy to achieve HLP level 1 status – this is self-assessed • HLP level 1 status includes having at least one member of the pharmacy staff being a Health Champion, and at least one member of the team undergoing leadership training

Key Criteria • Meet all essential Contract Criteria including governance and health promotion requirements • Meet Gateway Criteria • Consultation Room • Providing MURs and NMS • Provide or signpost to provider of NHS Flu and NUMSAS • Health Promotion Zone in place and updated regularly • Proactively engage local commissioners, charities and signpost to other local services • One Leader pharmacy attended recent Leadership training from appropriate provider and has certificate • One FTE Health Champion per pharmacy completed RSPH Level 2 Understanding Health Improvement and passed exam with certificate • All pharmacy staff can provide brief health interventions (MECC) • Record evidence of activity including signposting and photos of health promotion events

Support available • Health Champion and Leadership training • Face to face options near to you • On line options • Expert advice and support from Michelle Dyoss – part of the national group • Prospectus with all the information that you need to meet National Criteria • Reaccreditation for HLPs > 2 years old, where criteria still met.

Examples of evidence collection • Certificates – e. g. HLC, leadership training (required when claiming completion of training; certificates for assessment and/or training) • Minutes of pharmacy meeting notes showing HLC sharing learning with the pharmacy team • HLP action plan • Written feedback from pharmacy team members to their team leader • Photos of the pharmacy team engaging with the public (consent), consultation room, pharmacy, HLP logo location, recycling bins, paper disposal system, outreach work

Examples of evidence collection • Case studies of local outreach programmes conducted by pharmacy • Health and wellbeing notice board – local public health services / initiatives information • Health and wellbeing information in a range of formats (e. g. DVDs, plasma screen, leaflets etc) • Signposting information folder • Lists of local public health commissioners and their contact details along with examples of correspondence with them (e. g. emails, letters)

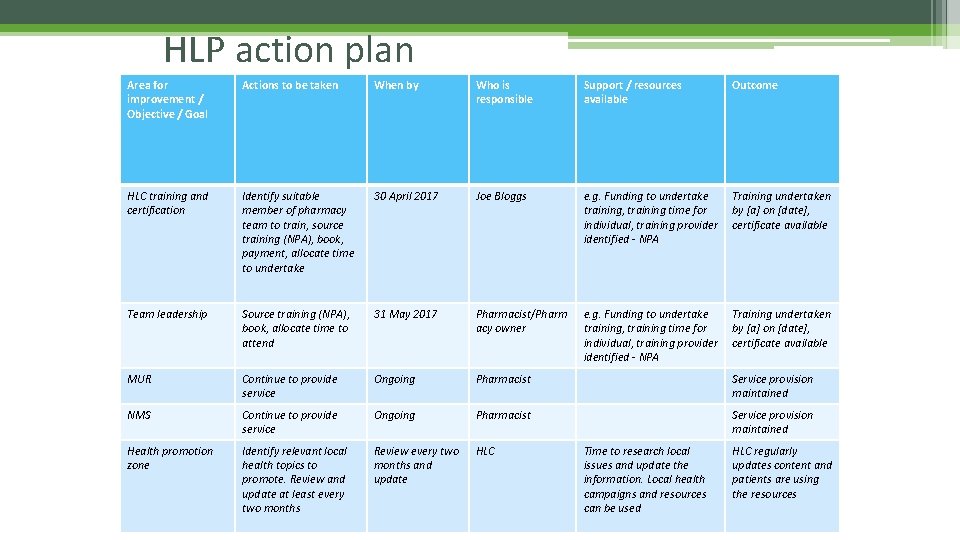
HLP action plan Area for improvement / Objective / Goal Actions to be taken When by Who is responsible Support / resources available Outcome HLC training and certification Identify suitable member of pharmacy team to train, source training (NPA), book, payment, allocate time to undertake 30 April 2017 Joe Bloggs e. g. Funding to undertake training, training time for individual, training provider identified - NPA Training undertaken by [a] on [date], certificate available Team leadership Source training (NPA), book, allocate time to attend 31 May 2017 Pharmacist/Pharm acy owner e. g. Funding to undertake training, training time for individual, training provider identified - NPA Training undertaken by [a] on [date], certificate available MUR Continue to provide service Ongoing Pharmacist Service provision maintained NMS Continue to provide service Ongoing Pharmacist Service provision maintained Health promotion zone Identify relevant local health topics to promote. Review and update at least every two months Review every two months and update HLC Time to research local issues and update the information. Local health campaigns and resources can be used HLC regularly updates content and patients are using the resources

Community pharmacies in England are now able to register as a Level 1 Healthy Living Pharmacy (HLP) under the new self-assessment process. The Royal Society for Public Health (RSPH) is the quality assurance provider for the HLP self-assessment scheme, and contractors can register their pharmacy as a Level 1 HLP by completing the registration process on the RSPH website. Achieving HLP Level 1 status is one of the quality criteria under the new quality payments scheme for community pharmacies in England. This criterion is worth 20 quality points, which amounts to a minimum payment of £ 1, 280. The self-assessment process requires pharmacy teams to work through the HLP Level 1 quality criteria, provide appropriate evidence and complete an online assessment of compliance form. Once this is completed satisfactorily and submitted to the RSPH, pharmacies should receive confirmation of registration and an HLP certificate within ten working days. Currently, registration is free, as this pilot scheme is being funded by PHE. HLPs will need to go through the self-assessment process and registration every two years. Pharmacies already accredited as HLPs by their Local Authority will not be able to complete the registration process with the RSPH, as this pilot scheme is for community pharmacies that are yet to achieve level 1 HLP status. Information on the NPA training available to meet these criteria is on the NPA website.

Q&A (13: 00)

Lunch & stands (13: 15 – 14: 00) Breakout Room – Basics & Gateway Practical Support 13: 00 – 13: 30 https: //www. youtube. com/watch? v=Fak. BT 7 o. Ys. KE

A Dementia Friend learns a little bit more about what it's like to live with dementia and then turns that understanding into action - anyone of any age can be a Dementia Friends (13: 30) In Breakout Room https: //www. dementiafriends. org. uk/

Safeguarding (14: 00 – 16: 30) Hayley Berry

Refreshments ( available at 15: 30)

Wrap Up (16: 30) Thank You Feedback Forms & Packs Q&A Next Sessions Safe Journey Home Or in Breakout Room – Basics & Gateway Practical Support http: //psnc. org. uk/cp-west-midlands/
 Old quality vs new quality
Old quality vs new quality Contract assets and contract liabilities
Contract assets and contract liabilities Contingent contract and wagering agreement
Contingent contract and wagering agreement William lloyd garrison jr 1934
William lloyd garrison jr 1934 Analyze the response of franklin
Analyze the response of franklin Ali eats
Ali eats Distinctive image features from scale-invariant keypoints
Distinctive image features from scale-invariant keypoints Feature transform
Feature transform Tom duerig
Tom duerig Distinctive image features from scale invariant keypoints
Distinctive image features from scale invariant keypoints Löwe lateinischer name
Löwe lateinischer name Bernie lowe and associates
Bernie lowe and associates Dr robert lowe
Dr robert lowe Procare citrus floor cleaner lowe's
Procare citrus floor cleaner lowe's Susan suchting lowe
Susan suchting lowe David lowe sift
David lowe sift Jason lowe-power
Jason lowe-power Prof sandra lowe
Prof sandra lowe Who founded lowes
Who founded lowes Gilly salmon 5 stage model
Gilly salmon 5 stage model Contract review in software quality assurance
Contract review in software quality assurance Alternative quality contract
Alternative quality contract Welcome to new session 2020-21
Welcome to new session 2020-21 Quality criteria
Quality criteria Criteria of food quality
Criteria of food quality Jetblue alpa contract
Jetblue alpa contract Naac criteria 7 best practices
Naac criteria 7 best practices Naac criteria 6
Naac criteria 6 Modified new york criteria
Modified new york criteria Quality control and quality assurance
Quality control and quality assurance Quality control vs quality assurance pmp
Quality control vs quality assurance pmp Pmbok quality management
Pmbok quality management Quality assurance cycle in nursing
Quality assurance cycle in nursing Compliance vs quality
Compliance vs quality Basic concept of quality management
Basic concept of quality management Quality management gurus
Quality management gurus Quality is free: the art of making quality certain
Quality is free: the art of making quality certain Fiona pilkington
Fiona pilkington Fiona list
Fiona list Fiona couper
Fiona couper Dr fiona campbell leeds
Dr fiona campbell leeds Fyo uga
Fyo uga Fiona forde bl
Fiona forde bl Fiona blyth
Fiona blyth Fiona fights robin hood
Fiona fights robin hood Dr fiona shaw
Dr fiona shaw Fiona langdon review
Fiona langdon review Margaret panting 2004
Margaret panting 2004 Fiona pilkington
Fiona pilkington Fiona pocock
Fiona pocock Dr fiona beck
Dr fiona beck Fiona moss
Fiona moss Fiona grant
Fiona grant Fiona gil
Fiona gil York carers centre
York carers centre Fiona beal
Fiona beal Fiona goddard
Fiona goddard Fiona told the truth to julian change into passive voice
Fiona told the truth to julian change into passive voice Fiona harden
Fiona harden Dr fiona wong
Dr fiona wong Fiona rennie dubai
Fiona rennie dubai Fiona list
Fiona list Fiona meechan
Fiona meechan Roethlisberger y dickson
Roethlisberger y dickson