Manifestation of Novel Social Challenges of the European













- Slides: 54

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Zoltan Balajthy Molecular Therapies- Lecture 4 IN VIVO AND EX VIVO GENE THERAPY

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Learning objectives of chapter 4. We are going to learn several methods by which we can introduce genetic material into a cell to treat disease, and in this manner, the aim of gene therapy is to introduce therapeutic material into the target cells, for this to become active inside the patient and exert the intended therapeutic effect. Topics in chapter 4 4. 1. Gene therapy Main criterias of the effective gene therapy In vivo gene therapy Ex vivo gene therapy 4. 2. Types of gene transfer, vectors for gene therapy Liposomes, naked DNA Retrovirus vector Adeno-associated viral vector (AAV) 4. 3. General gene therapy strategies Targeted killing of specific cells Targeted inhibition of gene expression Targeted mutation correction 4. 4. 3. 4. Human gene therapy Severe combined immunodeficiency (SCID) Genetic defects in the LDL receptor Cystic fibrosis (CF)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 4. 1. Gene Therapy Gene therapy may be used for treating, or even curing, genetic and acquired diseases by using normal genes to supplement or replace defective genes or to bolster a normal function. • Somatic : gene is introduced into specific somatic cells; not heritable • Germline : gene is introduced into emryonic cells or fertilized egg; heritable (ethical, legal and religious questions in human use)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Main criterias of the effective gene therapy Well-designed and manufactured gene The gene introduction into the right cells Safe integration of the gene into the chromosome without disturbing the surrounding genes The control of the gene, so the protein is produced only when it is needed

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Somatic gene therapy targets Cancer: main target of the current clinical trials Muscle: easily accessible, has a good blood supply and abundant tissue Endothelium: can directly secrete therapeutic protein into the bloodstream Skin: skin grafts also can secrete the necessary proteins Liver: has many functions and great regeneration Lung: easily accessible with aerosol sprays Nervous tissues: many illnesses and injuries can affect it, not easy to modify the neurons

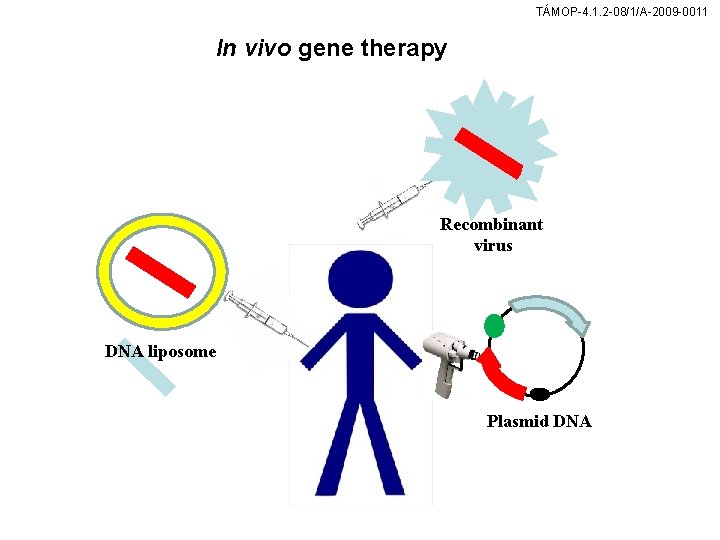
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 In vivo gene therapy Recombinant virus DNA liposome Plasmid DNA

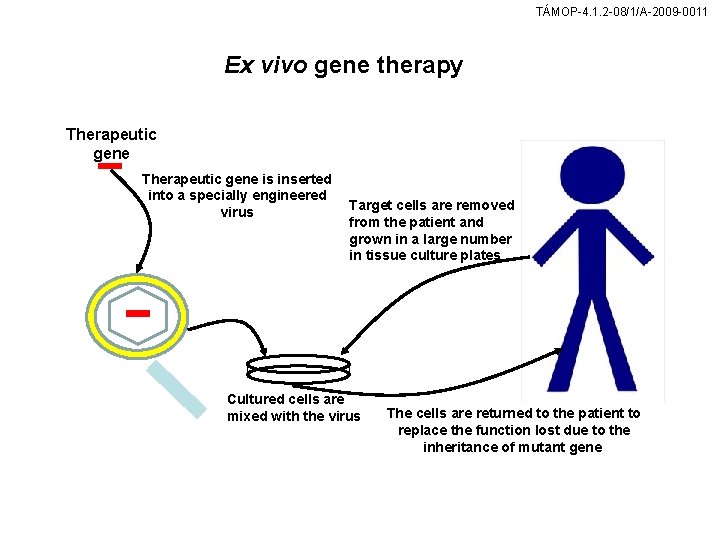
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Ex vivo gene therapy Therapeutic gene is inserted into a specially engineered virus Target cells are removed from the patient and grown in a large number in tissue culture plates Cultured cells are mixed with the virus The cells are returned to the patient to replace the function lost due to the inheritance of mutant gene

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Properties of the ideal gene therapy vector Safe (no side effects) Immunologically inert Can be targeted to a specific cell type or tissue Can be used to deliver any gene whatever its size Easy large scale production Cost effective The ideal vector does not exist (yet)!

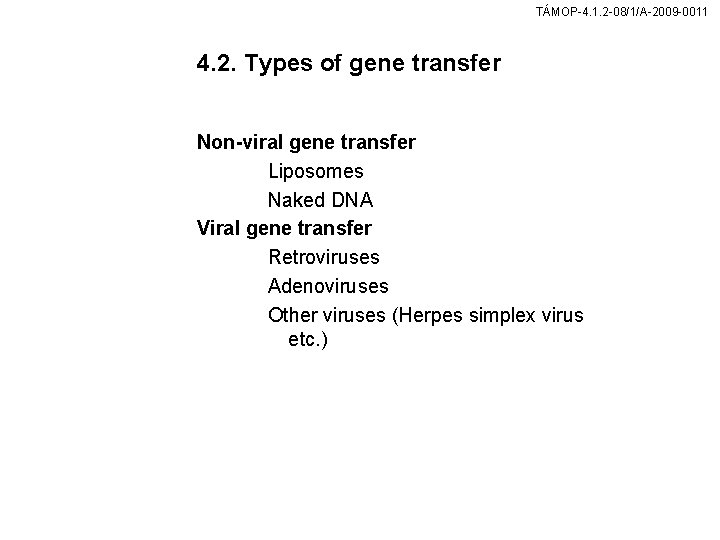
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 4. 2. Types of gene transfer Non-viral gene transfer Liposomes Naked DNA Viral gene transfer Retroviruses Adenoviruses Other viruses (Herpes simplex virus etc. )

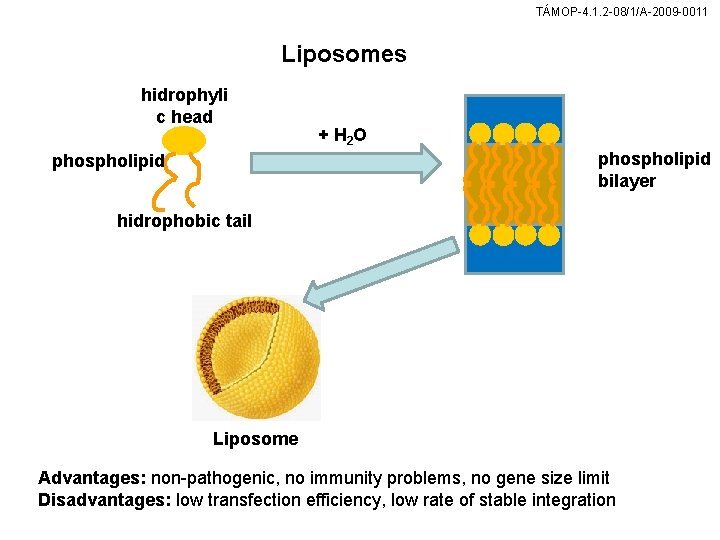
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Liposomes hidrophyli c head + H 2 O phospholipid bilayer phospholipid hidrophobic tail Liposome Advantages: non-pathogenic, no immunity problems, no gene size limit Disadvantages: low transfection efficiency, low rate of stable integration

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Naked DNA Plasmids, PCR products Advantages: simplest Disadvantages: very low transfection effectivity

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Gene particle bombardment (bioballistic delivery system) Advantages: same as liposome-mediated transfer, promising as a vaccination method Disadvantages: limited to dermal tissue, low rate of stable integration, difficult to QC

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Main criteria of the viral vectors Well-designed and manufactured gene The gene introduction into the right cells Safe integration of the gene into the chromosome without disturbing the surrounding genes The control of the gene, so the protein is produced only when it is needed

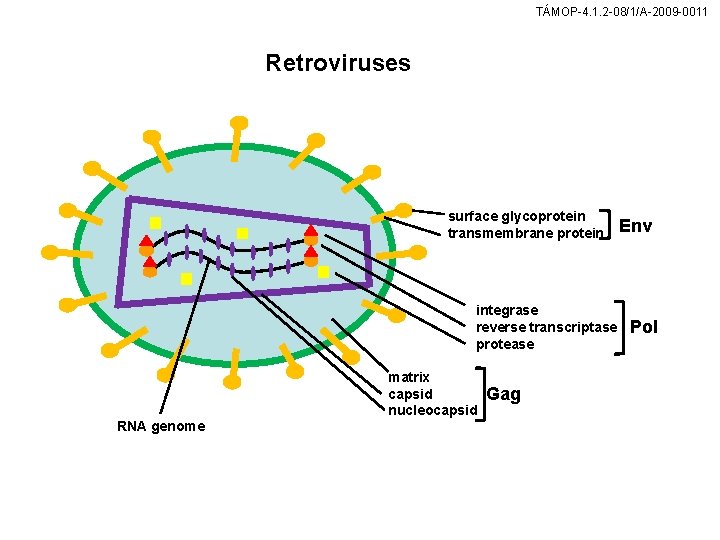
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Retroviruses surface glycoprotein transmembrane protein integrase reverse transcriptase protease RNA genome matrix capsid nucleocapsid Gag Env Pol

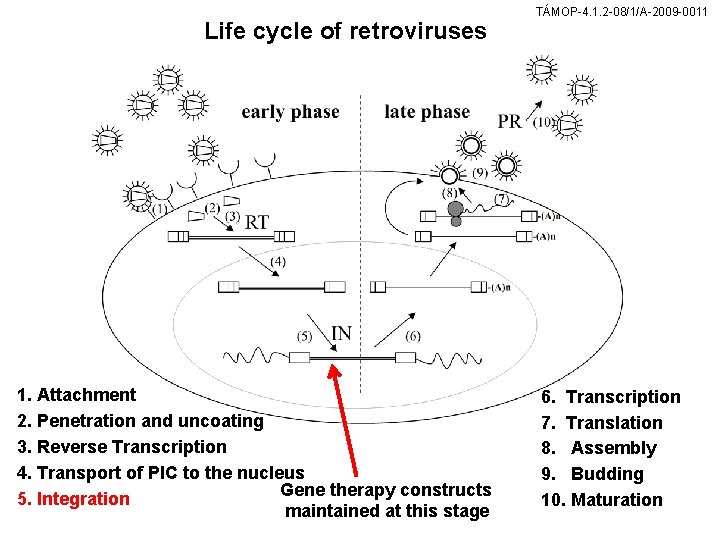
Life cycle of retroviruses 1. Attachment 2. Penetration and uncoating 3. Reverse Transcription 4. Transport of PIC to the nucleus Gene therapy constructs 5. Integration maintained at this stage TÁMOP-4. 1. 2 -08/1/A-2009 -0011 6. Transcription 7. Translation 8. Assembly 9. Budding 10. Maturation

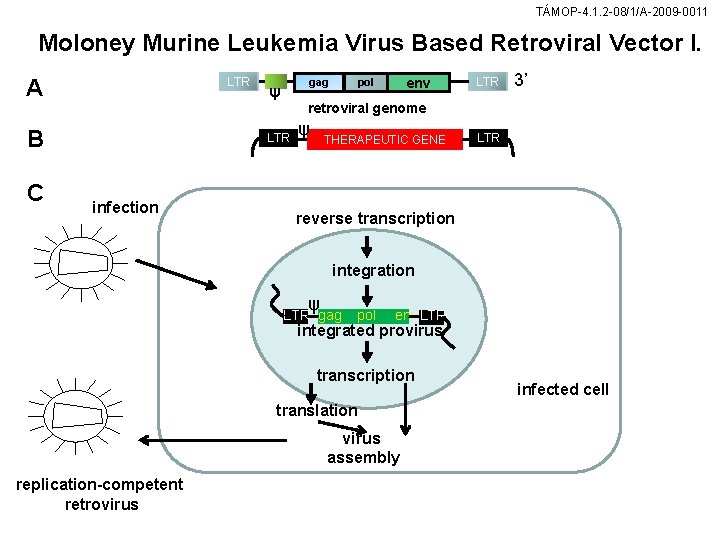
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Moloney Murine Leukemia Virus Based Retroviral Vector I. A LTR B C LTR 3’ retroviral genome LTR infection env pol gag ψ ψ THERAPEUTIC GENE LTR reverse transcription integration ψ LTR gag pol env LTR integrated provirus transcription translation virus assembly replication-competent retrovirus infected cell

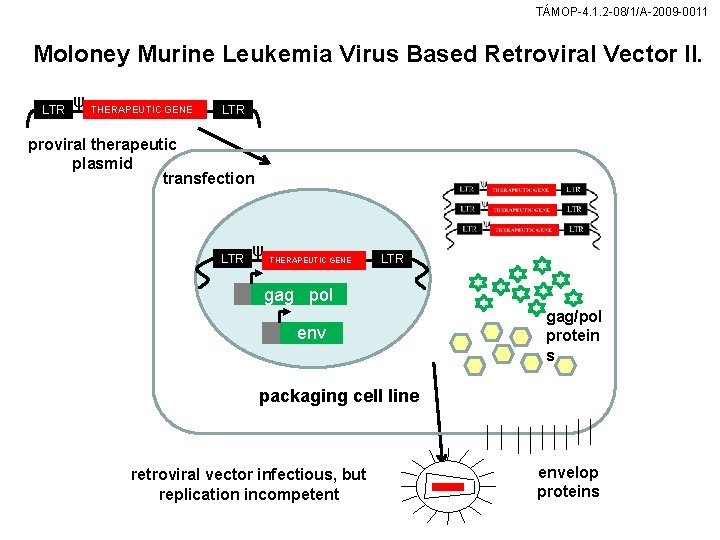
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Moloney Murine Leukemia Virus Based Retroviral Vector II. LTR ψ THERAPEUTIC GENE LTR proviral therapeutic plasmid transfection LTR ψ THERAPEUTIC GENE LTR gag pol env gag/pol protein s packaging cell line retroviral vector infectious, but replication incompetent envelop proteins

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mo. MLV-based retroviral vector (A) The retroviral genome contains the genes gag (structural proteins), pol (reverse polymerase), and env (envelope proteins). Ψ the packaging signal distinguishing cellular RNA from viral packaging proteins. The viral genome 5 flanked by long terminal repeats (LTR). (B) Gag, pol, and env in the vector genome have been replaced by a therapeutic gene. (C) Gag, pol, env are expressed by separate genes that are transfected into the packaging cell. lf the viral vector construct is cotransfected with the transgene into the packaging cell, the protein products of the vector genome recombine with gag/pol to form infectious viruses that cannot replicate.

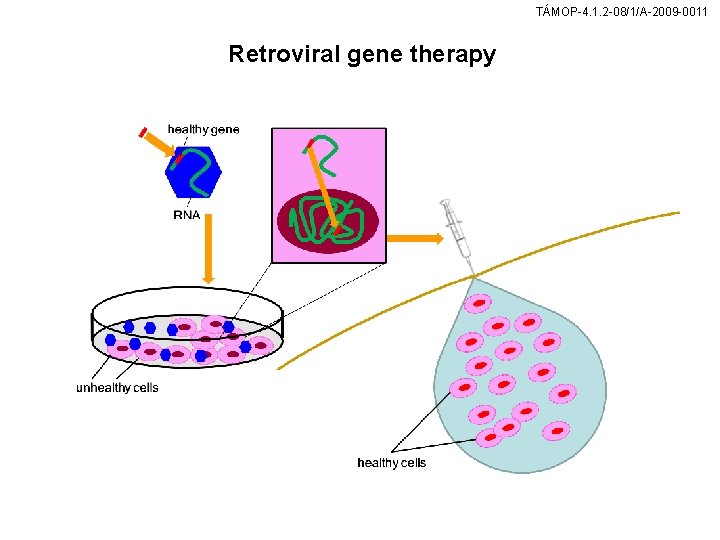
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Retroviral gene therapy

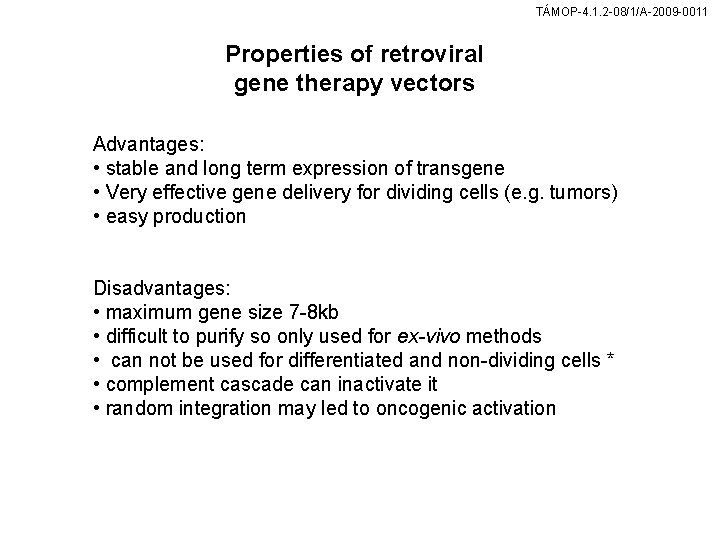
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Properties of retroviral gene therapy vectors Advantages: • stable and long term expression of transgene • Very effective gene delivery for dividing cells (e. g. tumors) • easy production Disadvantages: • maximum gene size 7 -8 kb • difficult to purify so only used for ex-vivo methods • can not be used for differentiated and non-dividing cells * • complement cascade can inactivate it • random integration may led to oncogenic activation

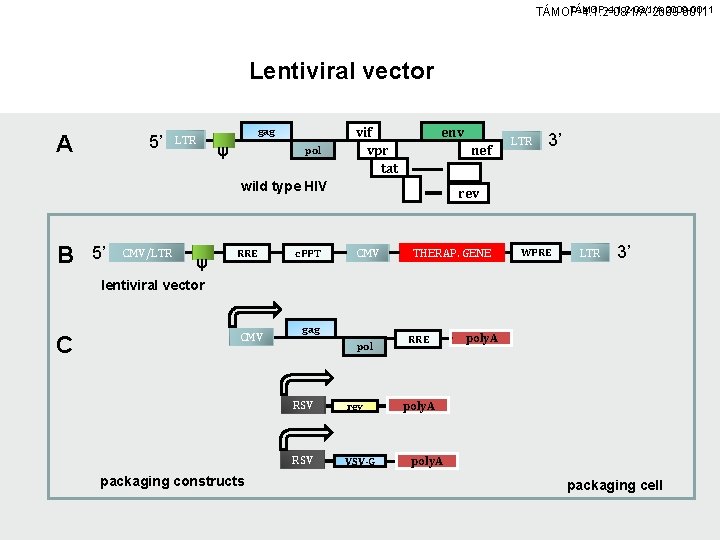
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Lentiviral vector A 5’ LTR gag ψ pol vif vpr tat env nef wild type HIV B 5’ CMV/LTR ψ RRE c. PPT LTR 3’ rev CMV THERAP. GENE WPRE LTR 3’ lentiviral vector C CMV packaging constructs gag pol RSV rev RSV VSV-G RRE poly. A packaging cell

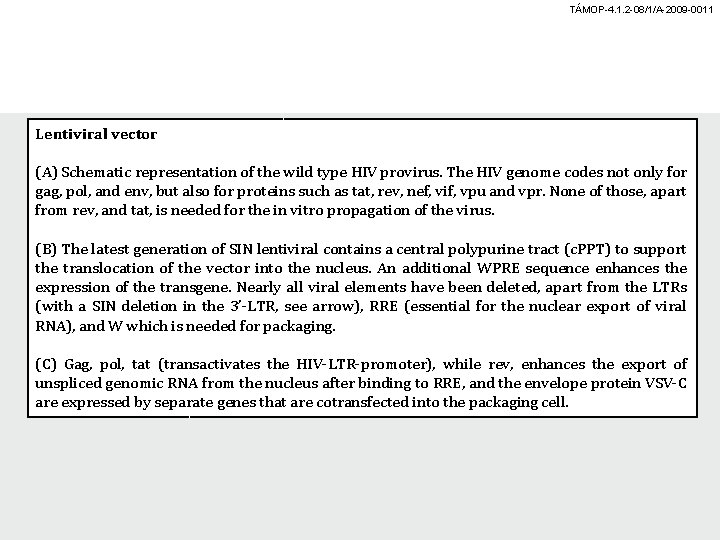
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Lentiviral vector (A) Schematic representation of the wild type HIV provirus. The HIV genome codes not only for gag, pol, and env, but also for proteins such as tat, rev, nef, vif, vpu and vpr. None of those, apart from rev, and tat, is needed for the in vitro propagation of the virus. (B) The latest generation of SIN lentiviral contains a central polypurine tract (c. PPT) to support the translocation of the vector into the nucleus. An additional WPRE sequence enhances the expression of the transgene. Nearly all viral elements have been deleted, apart from the LTRs (with a SIN deletion in the 3’-LTR, see arrow), RRE (essential for the nuclear export of viral RNA), and W which is needed for packaging. (C) Gag, pol, tat (transactivates the HIV-LTR-promoter), while rev, enhances the export of unspliced genomic RNA from the nucleus after binding to RRE, and the envelope protein VSV-C are expressed by separate genes that are cotransfected into the packaging cell.

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adenoviruses

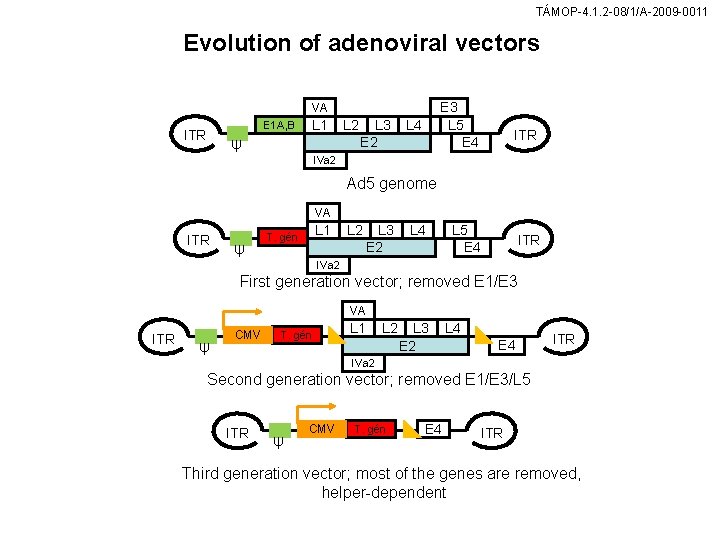
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Evolution of adenoviral vectors VA E 1 A, B ITR L 1 ψ L 2 L 3 E 2 L 4 E 3 L 5 E 4 ITR IVa 2 Ad 5 genome VA ITR ψ L 1 T. gén L 2 L 3 E 2 L 4 IVa 2 First generation vector; removed E 1/E 3 VA ITR ψ CMV T. gén L 1 L 2 L 3 E 2 L 4 E 4 ITR IVa 2 Second generation vector; removed E 1/E 3/L 5 ITR ψ CMV T. gén E 4 ITR Third generation vector; most of the genes are removed, helper-dependent

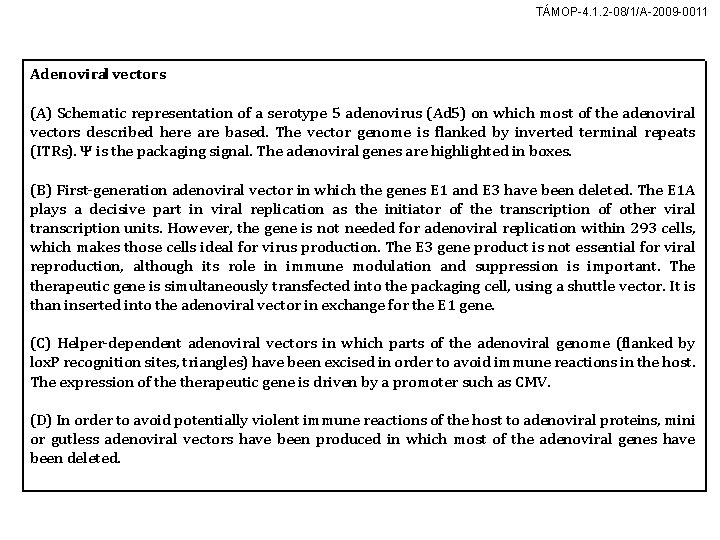
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adenoviral vectors (A) Schematic representation of a serotype 5 adenovirus (Ad 5) on which most of the adenoviral vectors described here are based. The vector genome is flanked by inverted terminal repeats (ITRs). Ψ is the packaging signal. The adenoviral genes are highlighted in boxes. (B) First-generation adenoviral vector in which the genes E 1 and E 3 have been deleted. The E 1 A plays a decisive part in viral replication as the initiator of the transcription of other viral transcription units. However, the gene is not needed for adenoviral replication within 293 cells, which makes those cells ideal for virus production. The E 3 gene product is not essential for viral reproduction, although its role in immune modulation and suppression is important. The therapeutic gene is simultaneously transfected into the packaging cell, using a shuttle vector. It is than inserted into the adenoviral vector in exchange for the E 1 gene. (C) Helper-dependent adenoviral vectors in which parts of the adenoviral genome (flanked by lox. P recognition sites, triangles) have been excised in order to avoid immune reactions in the host. The expression of therapeutic gene is driven by a promoter such as CMV. (D) In order to avoid potentially violent immune reactions of the host to adenoviral proteins, mini or gutless adenoviral vectors have been produced in which most of the adenoviral genes have been deleted.

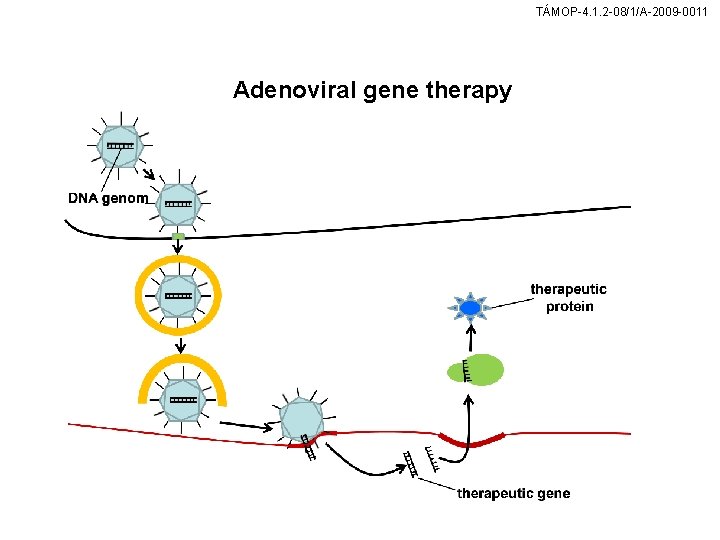
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adenoviral gene therapy

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Properties of adenoviral gene therapy vectors Advanteges: • no risk of oncogenic activation (no DNA integration) • can carry large genes (30 kb) • infect dividing and non-dividing cells • high level gene expression • easy production Dissadvantages: • short term gene expression • induce inflammatory and immune response • Cell-specific targeting difficult to achieve

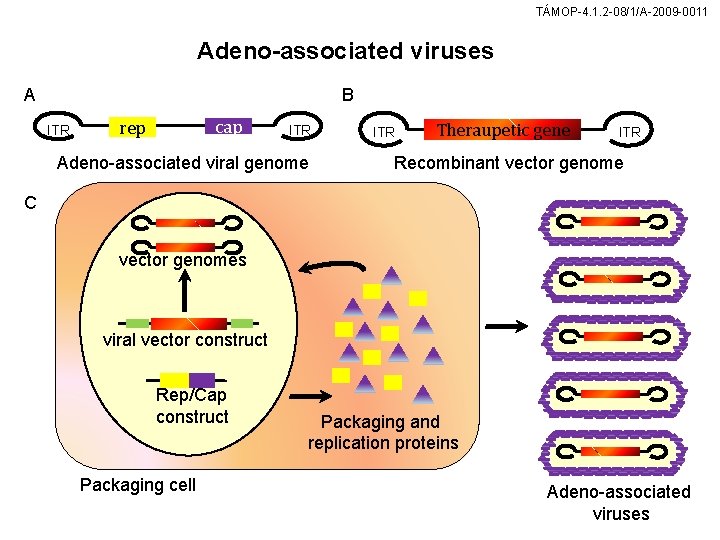
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adeno-associated viruses A B ITR cap rep ITR Adeno-associated viral genome ITR Theraupetic gene ITR Recombinant vector genome C vector genomes viral vector construct Rep/Cap construct Packaging cell Packaging and replication proteins Adeno-associated viruses

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adeno-associated viral vector (A) The AAV genome contains sequences which are essential for the transduction process, such as inverted terminal repetitions (ITRs) and the genes rep and cap. (B) In the vector genome, rep and cap have been replaced by a therapeutic gene. lf therapeutic gene is larger than 4. 5 kb, it is distributed over two concatemeric vector constructs. (C) The REP and CAP proteins are expressed by the packaging cells and are needed for the production of single-stranded DNA genomes in a capsule consisting of proteins. A non-enveloped AAV virus collects in the nucleus. Helper proteins from adenoviruses, which are needed for replication, are also expressed in the packaging cell (not shown here). The AAV are released from the packaging cell through the lytic adenoviral replication process.

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Properties of adeno-associated viral vectors Advantages: • not associated to human diseases • effectively infects dividing and non-dividing cells • able to integrate into the specific site (chromosome 19) • small genom and easy to manipulate • can obtain high titered virus stock (109 -1010/ml) Dissadvantages: • limited gene size ~ 4. 5 kb

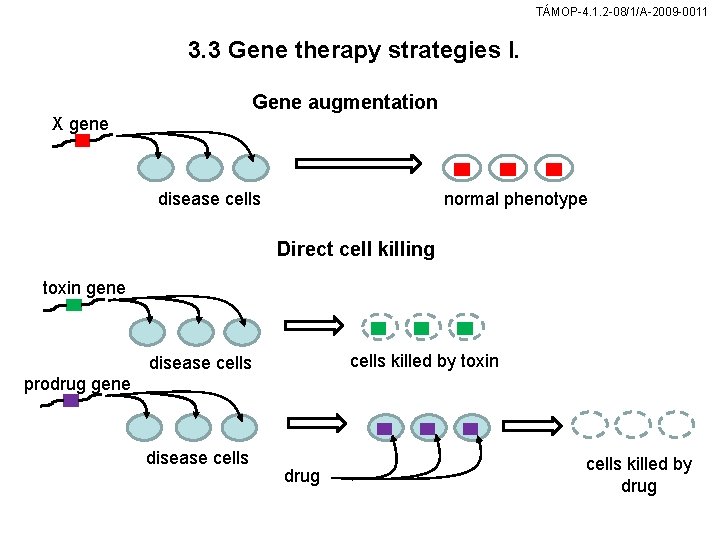
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 3. 3 Gene therapy strategies I. Gene augmentation X gene normal phenotype disease cells Direct cell killing toxin gene cells killed by toxin disease cells prodrug gene disease cells drug cells killed by drug

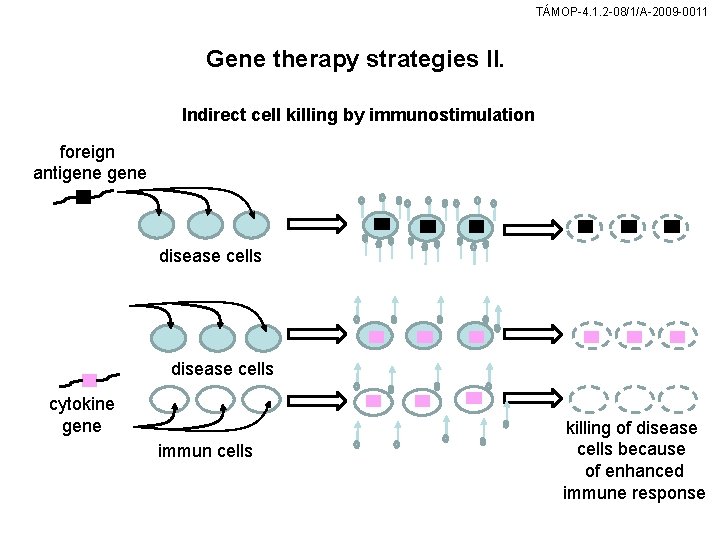
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Gene therapy strategies II. Indirect cell killing by immunostimulation foreign antigene disease cells cytokine gene immun cells killing of disease cells because of enhanced immune response

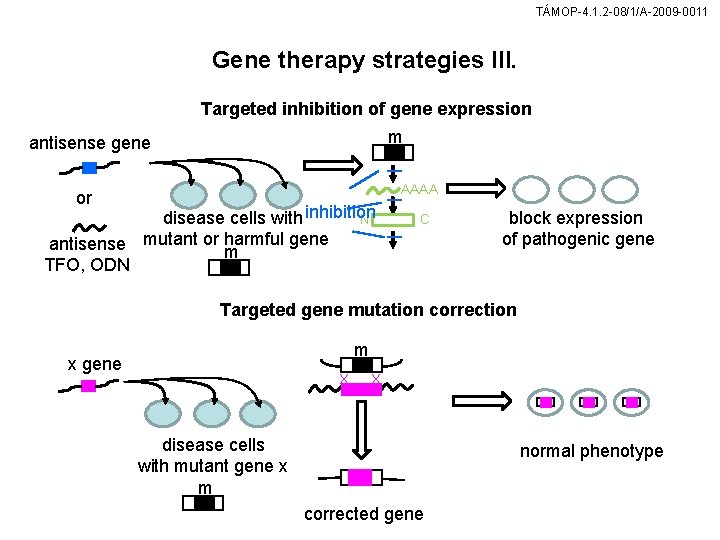
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Gene therapy strategies III. Targeted inhibition of gene expression m antisense gene AAAA or disease cells with inhibition N gene antisense mutant or harmful m TFO, ODN C block expression of pathogenic gene Targeted gene mutation correction m x gene X X disease cells with mutant gene x m normal phenotype corrected gene

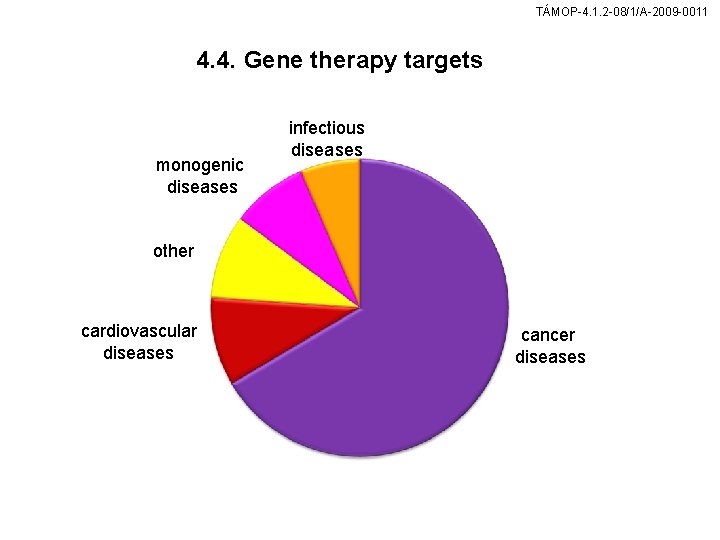
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 4. 4. Gene therapy targets monogenic diseases infectious diseases other cardiovascular diseases cancer diseases

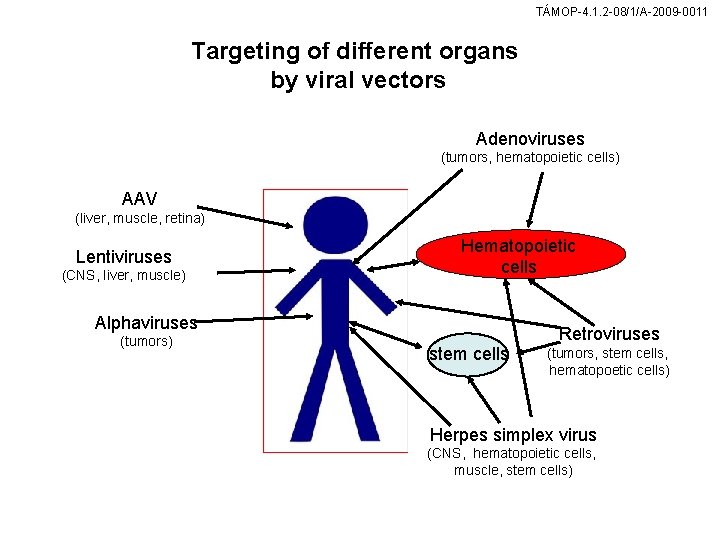
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Targeting of different organs by viral vectors Adenoviruses (tumors, hematopoietic cells) AAV (liver, muscle, retina) Lentiviruses (CNS, liver, muscle) Hematopoietic cells Alphaviruses (tumors) Retroviruses stem cells (tumors, stem cells, hematopoetic cells) Herpes simplex virus (CNS, hematopoietic cells, muscle, stem cells)

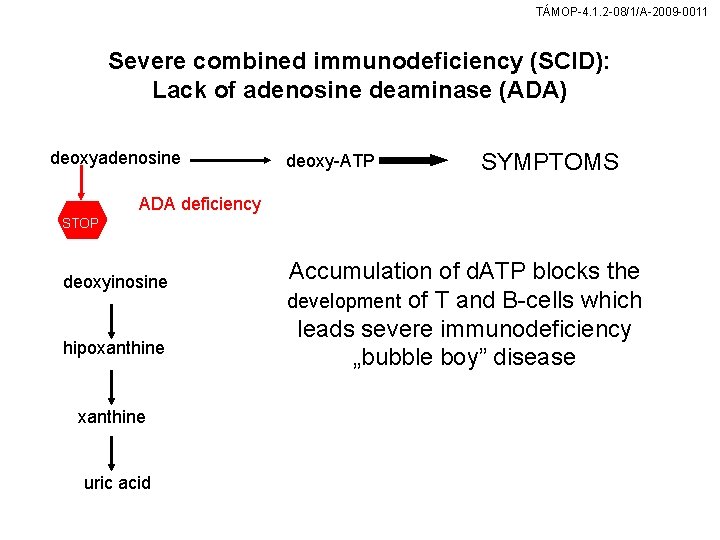
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Severe combined immunodeficiency (SCID): Lack of adenosine deaminase (ADA) deoxyadenosine deoxy-ATP SYMPTOMS ADA deficiency STOP deoxyinosine hipoxanthine uric acid Accumulation of d. ATP blocks the development of T and B-cells which leads severe immunodeficiency „bubble boy” disease

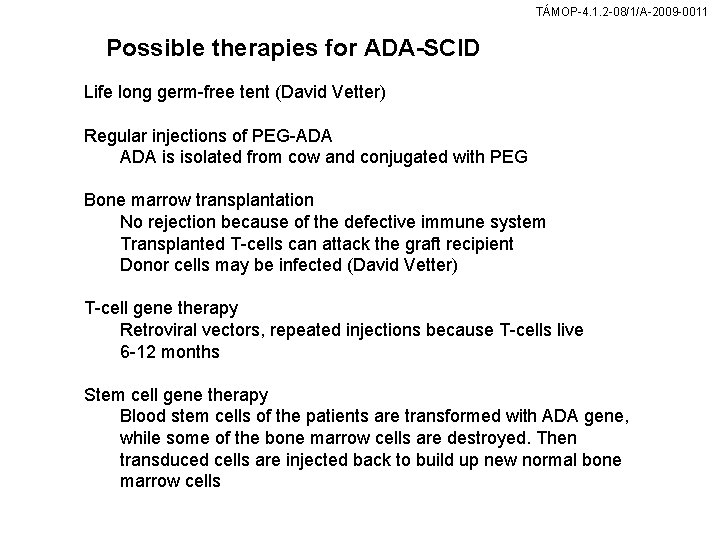
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Possible therapies for ADA-SCID Life long germ-free tent (David Vetter) Regular injections of PEG-ADA is isolated from cow and conjugated with PEG Bone marrow transplantation No rejection because of the defective immune system Transplanted T-cells can attack the graft recipient Donor cells may be infected (David Vetter) T-cell gene therapy Retroviral vectors, repeated injections because T-cells live 6 -12 months Stem cell gene therapy Blood stem cells of the patients are transformed with ADA gene, while some of the bone marrow cells are destroyed. Then transduced cells are injected back to build up new normal bone marrow cells

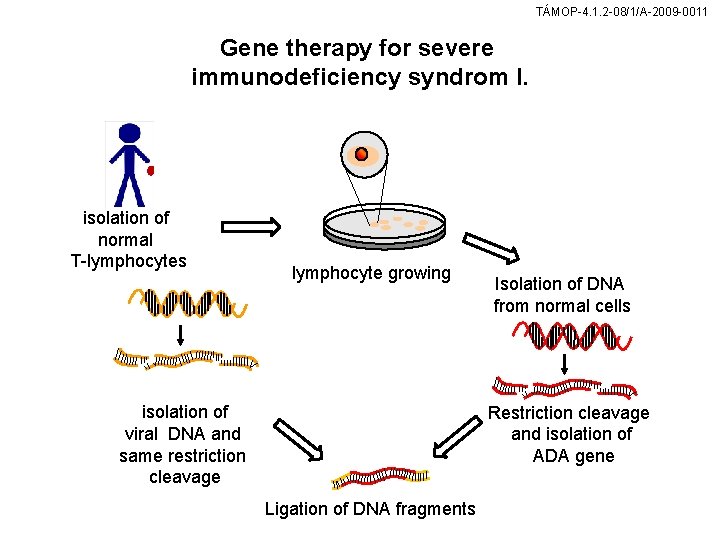
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Gene therapy for severe immunodeficiency syndrom I. isolation of normal T-lymphocytes lymphocyte growing isolation of viral DNA and same restriction cleavage Isolation of DNA from normal cells Restriction cleavage and isolation of ADA gene Ligation of DNA fragments

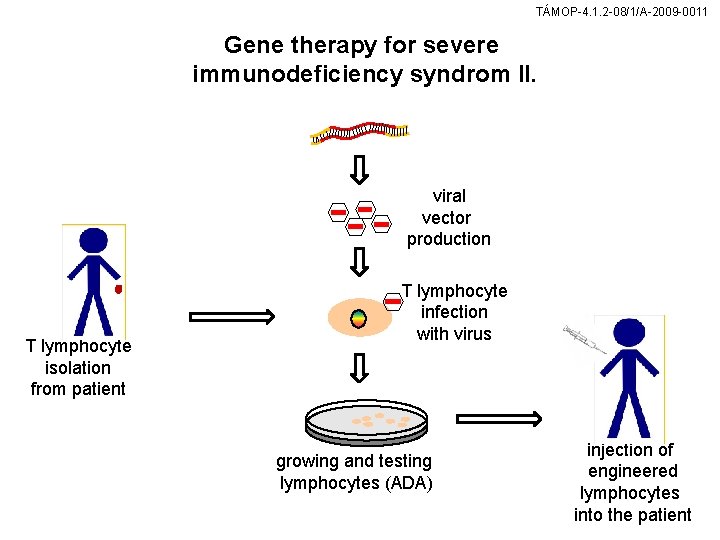
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Gene therapy for severe immunodeficiency syndrom II. viral vector production T lymphocyte isolation from patient T lymphocyte infection with virus growing and testing lymphocytes (ADA) injection of engineered lymphocytes into the patient

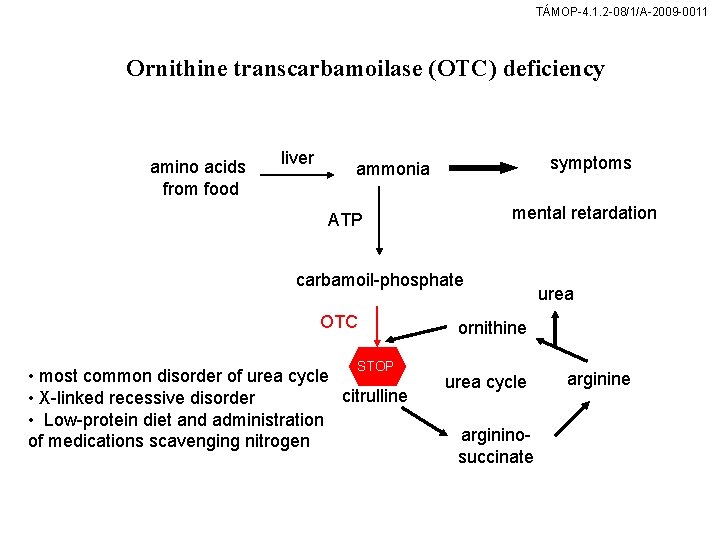
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Ornithine transcarbamoilase (OTC) deficiency amino acids from food liver symptoms ammonia mental retardation ATP carbamoil-phosphate OTC STOP • most common disorder of urea cycle citrulline • X-linked recessive disorder • Low-protein diet and administration of medications scavenging nitrogen urea ornithine urea cycle argininosuccinate arginine

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Setbacks in gene therapy Jesse Gelsinger (1999) • had a mild OTC deficiency, which was controlled by diet and regular therapy • volunteered for the OTC gene therapy where normal OTC gene was in vivo transferred into his liver using by adenovirus • felt in coma after few hours of treatment then died 3 days afterwards • he had extreme high virus level which caused strong immune response led to his death French X-SCID (2002) • one of the eleven „bubble boys” did not respond to the treatment • eight children cured • leukemia was developed in two children

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Limitating factors of gene therapy • short-lived nature of therapy - multiple periodic treatments required • immune response - hard for repeated treatments • problems with viral vectors - could regain virulence - could cause toxicity, immun and imflammation response - could control and activate genes • multi-gene disorders - challenge for gene therapy (high blood pressure, Alzheimer)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Familial hypercholesterolemia • Genetic disorder - mostly resulted by the mutation of the LDL receptor gene - homozygous frequency 1: 500 - heterozygous frequency 1: 1 000 • High cholesterol and LDL levels in blood - homozygous have 6 -7 X higher - heterozygous have 2. 5 X higher compared to normal values • Early cardiovascular diseases (heart attack / stroke) - for homozygous in childhood at the age of 5 and 10 - for heterozygous at the age of 35 and 40

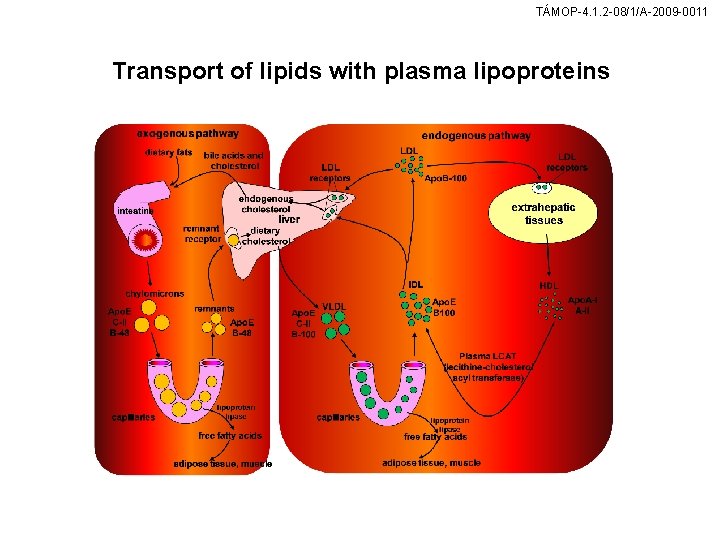
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Transport of lipids with plasma lipoproteins

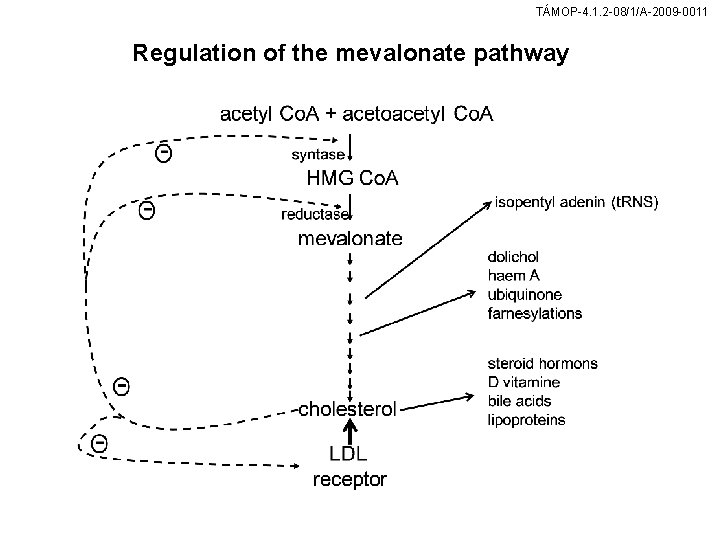
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Regulation of the mevalonate pathway

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Main facts of the cholesterol question • most of the cells permanently synthetize cholesterol • daily cholesterol intake is significant even from a normal diet • cholesterol does not degrade, removed with biles • inhibition of the cholesterol synthesis could block the formation of other important compounds which may lead severe side effects

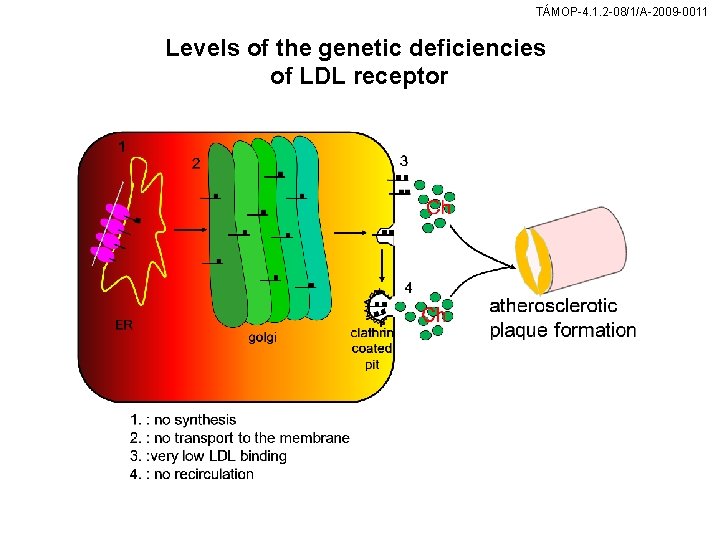
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Levels of the genetic deficiencies of LDL receptor

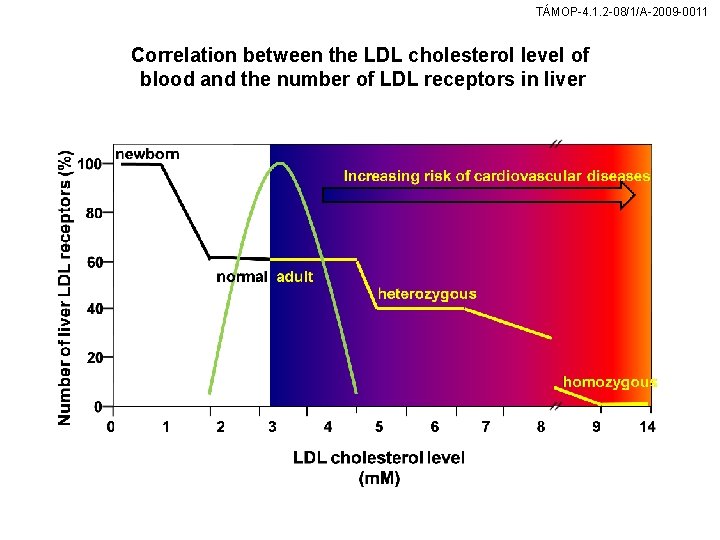
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Correlation between the LDL cholesterol level of blood and the number of LDL receptors in liver

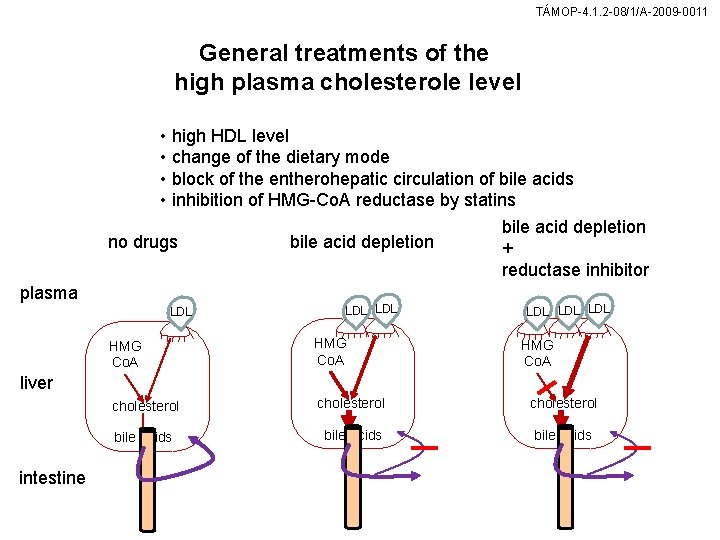
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 General treatments of the high plasma cholesterole level • high HDL level • change of the dietary mode • block of the entherohepatic circulation of bile acids • inhibition of HMG-Co. A reductase by statins bile acid depletion no drugs bile acid depletion + reductase inhibitor plasma LDL LDL LDL HMG Co. A cholesterol bile acids HMG Co. A liver intestine

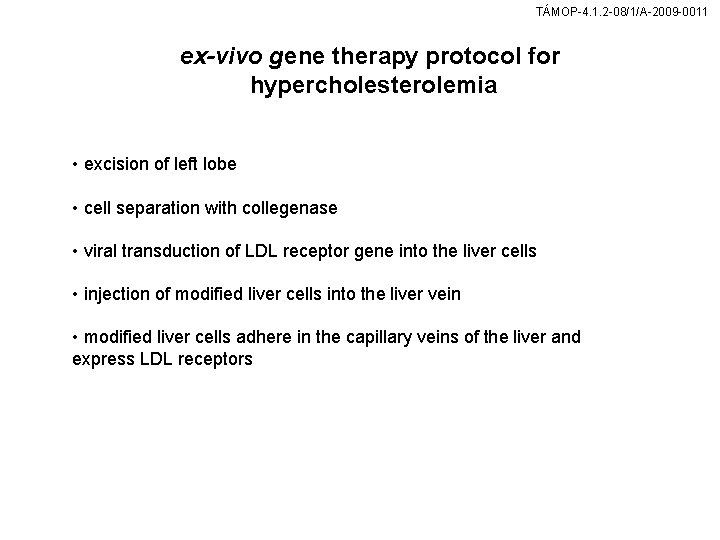
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 ex-vivo gene therapy protocol for hypercholesterolemia • excision of left lobe • cell separation with collegenase • viral transduction of LDL receptor gene into the liver cells • injection of modified liver cells into the liver vein • modified liver cells adhere in the capillary veins of the liver and express LDL receptors

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cystic fibrosis • autosomal recessive disease - frequency 1: 3000 (in Caucasians) • mutations in the CFTR gene - cystic fibrosis transmembrane conductance regulator protein (CFTR) responsible for the viscosity of mucus in glands (lung, liver, pancreas, digestive and reproductive tract, skin); - lack of CFTR results consistent mucus (infection risk) • symptoms: - digestive and absorption problems - poor height and weight gain - cronic pneumonia - infertility in the 95 % of the male - diseases of vitamin deficiencies (ADEK)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Treatments for cystic fibrosis • high energy diet with Na. Cl and vitamines(ADEK) supplements • pancreatic enzyme supplements • mechanical devices and medications to clear the mucus • antibiotic treatment for bacterial infections • lung transplantation • gene therapy - introducing normal CFTR gene into the affected cells - liposomic and adenoviral vectors in aerosol spray (not enough efficient) - 5 - 10 % infection would be needed to recover the normal function - Gentamicin treatment to support synthesis of full-length CFTR protein (promising results in experimental phase)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Some ethical concerns about gene therapy Is it possible to distinguish „good” and „bad” gene therapy? Who decides which traits are normal and which constitute a disability or disorder? Is it available only for the rich? Could the comprehensive use of gene therapy make people less accepting of different individuals? Should people be allowed to use gene therapy to change their basic human traits (height, intelligence etc. ) ?
 Challenges of novel drug delivery system
Challenges of novel drug delivery system Régis gaudemer émotions
Régis gaudemer émotions Manifestation commerciale exemple
Manifestation commerciale exemple Manifestation determination meeting agenda
Manifestation determination meeting agenda Manifestation determination flowchart
Manifestation determination flowchart Manifestation determination flowchart
Manifestation determination flowchart Rayon rubis doré
Rayon rubis doré The manifestation of the holy spirit
The manifestation of the holy spirit Concept de virginia henderson
Concept de virginia henderson Manifestation determination definition
Manifestation determination definition A failure is a manifestation of an
A failure is a manifestation of an Manifestation names
Manifestation names Clinical manifestation of hepatitis
Clinical manifestation of hepatitis Manifestation of god name
Manifestation of god name Hemorrhagic
Hemorrhagic Vocabulaire manifestation
Vocabulaire manifestation How should sunnatullah be interpreted
How should sunnatullah be interpreted Friday manifestation
Friday manifestation Bbl lotion for scabies
Bbl lotion for scabies Social thinking adalah
Social thinking adalah Social thinking social influence social relations
Social thinking social influence social relations Social challenges
Social challenges Venizelos efthymiou
Venizelos efthymiou Federation of european social employers
Federation of european social employers European social structure
European social structure European social fund plus
European social fund plus European social fund plus
European social fund plus Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế