Manifestation of Novel Social Challenges of the European

















- Slides: 45

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Dr. Judit Pongrácz Three dimensional tissue cultures and tissue engineering – Lecture 3 STEM CELLS (2)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cord blood stem cells • Approx. 130 million babies born yearly – the umbilical cord blood is the largest potential source of stem cells for regenerative medicine • In the past 36 yrs 10000 patients were treated for over 80 different diseases

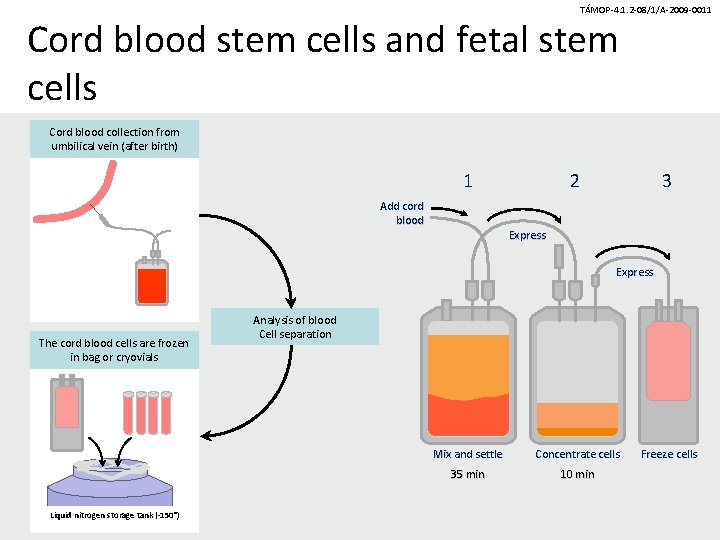
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cord blood stem cells and fetal stem cells Cord blood collection from umbilical vein (after birth) 1 Add cord blood 2 3 Express The cord blood cells are frozen in bag or cryovials Liquid nitrogen storage tank (-150°) Analysis of blood Cell separation Mix and settle Concentrate cells 35 min 10 min Freeze cells

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cryopreservation • Cryopreservation of primary cells is possible for long term (so far 20 yrs). • The low-temperature is maintained at -150 -196 o. C in liquid nitrogen.

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cord blood processing 1. Red cell depletion (using Ficoll, Hetastarch, Lymphoprep, Prepacyte) 2. Depletion of plasma for smaller storage size 3. Testing of the final cell pool (infection, volume, cellularity, stem cell content, CD 34+)

Cord blood processing and cryopreservation TÁMOP-4. 1. 2 -08/1/A-2009 -0011 • Cord blood is primarily useful in hematological disorders • Cord blood is collected at birth • Either processed or just simply frozen in DMSO

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cord blood banking • Cord blood banks should be set up in every metropolitan city with HLY specification and linked to an international computer network • Keeping cord blood for a considerable length of time is costly

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Pluripotenciy of cord blood stem cells Cord Blood Purification Stem cells CBE MSC Endodermal Mesodermal Ectodermal Hepato-Biliary Blood Neural

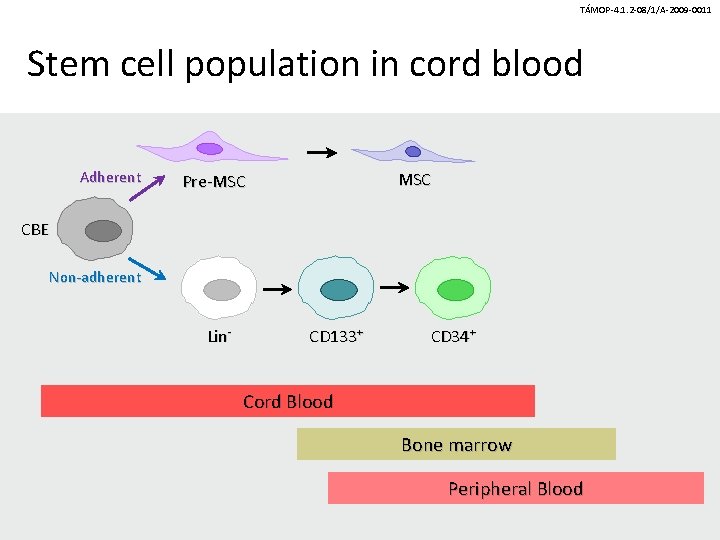
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Stem cell population in cord blood Adherent MSC Pre-MSC CBE Non-adherent Lin- CD 133+ CD 34+ Cord Blood Bone marrow Peripheral Blood

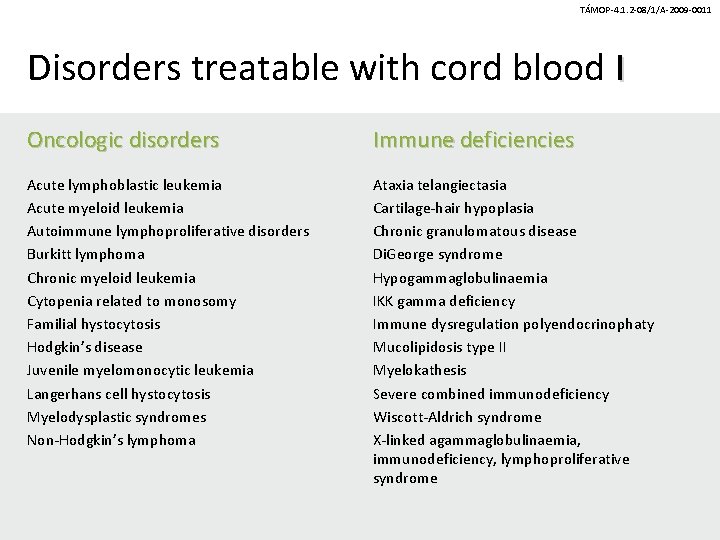
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Disorders treatable with cord blood I Oncologic disorders Immune deficiencies Acute lymphoblastic leukemia Acute myeloid leukemia Autoimmune lymphoproliferative disorders Burkitt lymphoma Chronic myeloid leukemia Cytopenia related to monosomy Familial hystocytosis Hodgkin’s disease Juvenile myelomonocytic leukemia Langerhans cell hystocytosis Myelodysplastic syndromes Non-Hodgkin’s lymphoma Ataxia telangiectasia Cartilage-hair hypoplasia Chronic granulomatous disease Di. George syndrome Hypogammaglobulinaemia IKK gamma deficiency Immune dysregulation polyendocrinophaty Mucolipidosis type II Myelokathesis Severe combined immunodeficiency Wiscott-Aldrich syndrome X-linked agammaglobulinaemia, immunodeficiency, lymphoproliferative syndrome

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Disorders treatable with cord blood II Hematological disorders Metabolic disorders Autoimmune neutropenia Cyclic neutropenia Diamond Blackfran anemia Evan’s syndrome Red cell aplasia Refractory anemia Severe aplastic anemia Sickle cell disease Thalassaemia Fanconi’s anemia Galnzmann’s disease Congenital sideroblastic anemia Juvenile dermatomyositis and xanthogranulomas Adrenoleukodystrophy Alpha mannosidosis Type I diabetes Gaucher’s disease Gunther disease Hermansky-Pudlak syndrome Hurler-Scheie syndrome Krabbe’s disease Maroteau-lamy syndrome Metachromatic leukodystrophy Mucolipidosis Types II, III Neimann Pick syndrome, Types A and B Sandoff syndrome Sanfilippo syndrome Tay Sachs disease

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Fat stem cells (ASC) Fat or adipose tissue stem cells (ASC): • • • Easily obtainable Consistent immunophenotype Similar to BMSC Multipotent Manipulation by genetic engineering

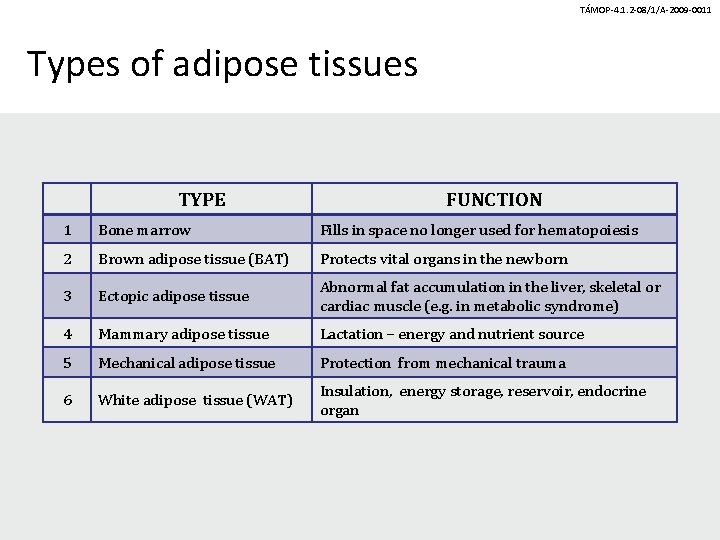
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Types of adipose tissues TYPE FUNCTION 1 Bone marrow Fills in space no longer used for hematopoiesis 2 Brown adipose tissue (BAT) Protects vital organs in the newborn 3 Ectopic adipose tissue Abnormal fat accumulation in the liver, skeletal or cardiac muscle (e. g. in metabolic syndrome) 4 Mammary adipose tissue Lactation – energy and nutrient source 5 Mechanical adipose tissue Protection from mechanical trauma 6 White adipose tissue (WAT) Insulation, energy storage, reservoir, endocrine organ

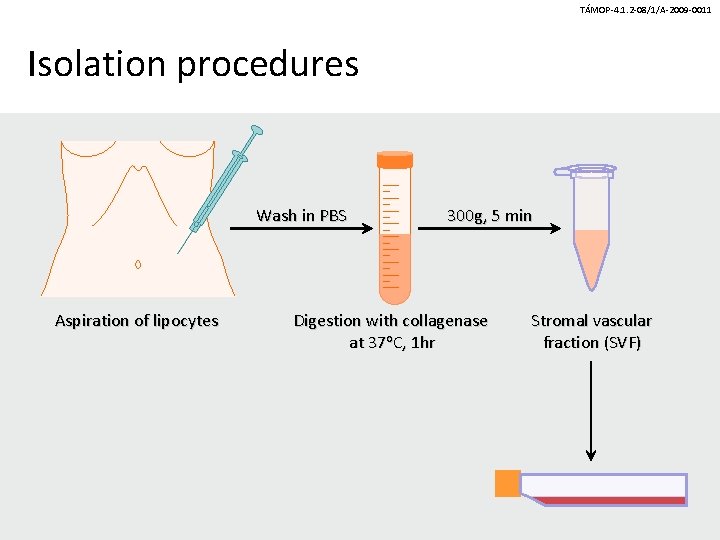
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Isolation procedures Wash in PBS Aspiration of lipocytes 300 g, 5 min Digestion with collagenase at 37 o. C, 1 hr Stromal vascular fraction (SVF)

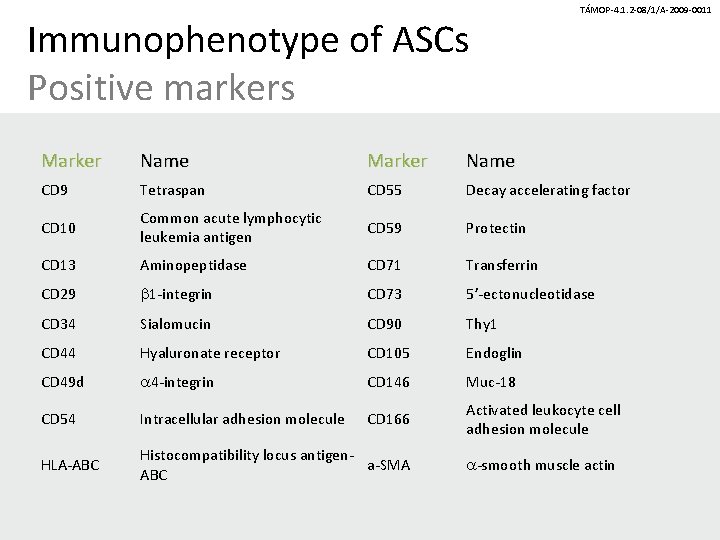
Immunophenotype of ASCs Positive markers TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Marker Name CD 9 Tetraspan CD 55 Decay accelerating factor CD 10 Common acute lymphocytic leukemia antigen CD 59 Protectin CD 13 Aminopeptidase CD 71 Transferrin CD 29 b 1 -integrin CD 73 5’-ectonucleotidase CD 34 Sialomucin CD 90 Thy 1 CD 44 Hyaluronate receptor CD 105 Endoglin CD 49 d a 4 -integrin CD 146 Muc-18 CD 54 Intracellular adhesion molecule CD 166 Activated leukocyte cell adhesion molecule HLA-ABC Histocompatibility locus antigena-SMA ABC a-smooth muscle actin

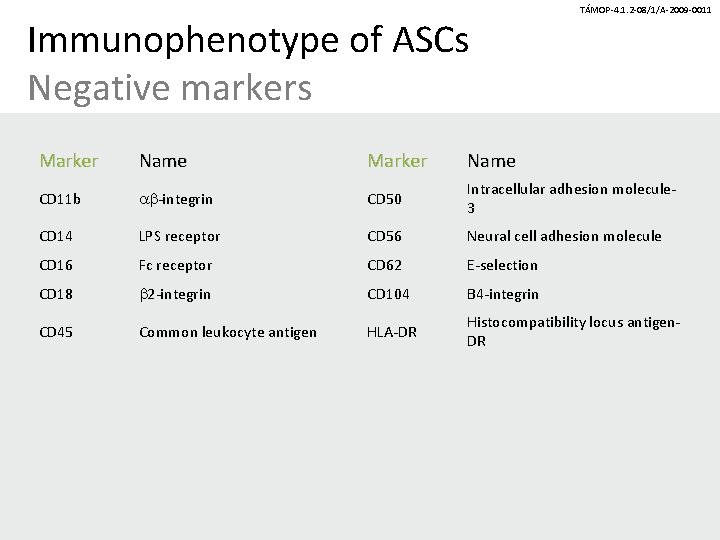
Immunophenotype of ASCs Negative markers TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Marker Name CD 11 b ab-integrin CD 50 Intracellular adhesion molecule 3 CD 14 LPS receptor CD 56 Neural cell adhesion molecule CD 16 Fc receptor CD 62 E-selection CD 18 b 2 -integrin CD 104 B 4 -integrin CD 45 Common leukocyte antigen HLA-DR Histocompatibility locus antigen. DR

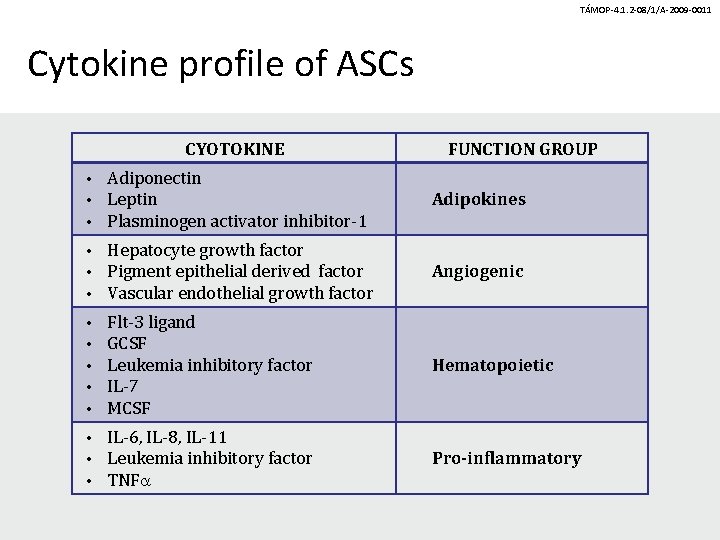
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cytokine profile of ASCs CYOTOKINE FUNCTION GROUP • • • Adiponectin Leptin Plasminogen activator inhibitor-1 Adipokines • • • Hepatocyte growth factor Pigment epithelial derived factor Vascular endothelial growth factor Angiogenic • • • Flt-3 ligand GCSF Leukemia inhibitory factor IL-7 MCSF Hematopoietic • • • IL-6, IL-8, IL-11 Leukemia inhibitory factor TNFa Pro-inflammatory

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Immunogenecity of ASCs • Lack of immunogenicity is linked to the absence of the major histocompatibility class II antigens (HLA-DR) on their surface. • Their immunosuppressive properties are linked to prostaglandin E 2 production.

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation potential of ASCs • • • Adipocyte Cardiac myocytes Chondrocyte Endodermal and ectodermal lineages Endothelial and smooth muscle cells Hematopoietic support Neuronal lineage Osteoblast Skeletal myocytes

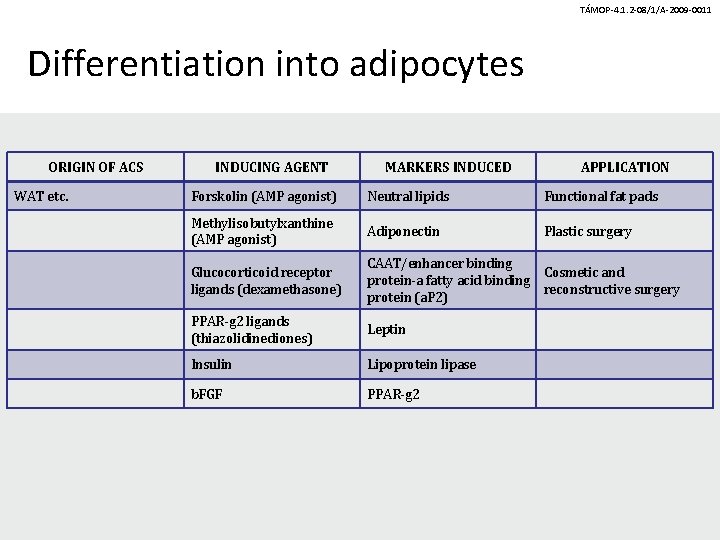
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into adipocytes ORIGIN OF ACS WAT etc. INDUCING AGENT MARKERS INDUCED APPLICATION Forskolin (AMP agonist) Neutral lipids Functional fat pads Methylisobutylxanthine (AMP agonist) Adiponectin Plastic surgery Glucocorticoid receptor ligands (dexamethasone) CAAT/enhancer binding Cosmetic and protein-a fatty acid binding reconstructive surgery protein (a. P 2) PPAR-g 2 ligands (thiazolidinediones) Leptin Insulin Lipoprotein lipase b. FGF PPAR-g 2

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into cardiac myocytes ORIGIN OF ACS INDUCING AGENT MARKERS INDUCED BAT 5 -azacytadine Sarcomeric actinin WAT Cardiomyocyte extract Connexin-43 Desmin troponin-I APPLICATION Repair injured cardiac tissue after ischemic injury

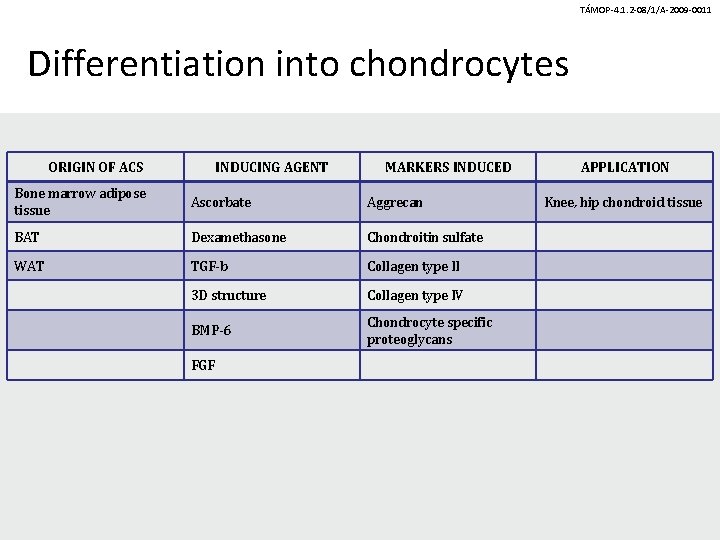
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into chondrocytes ORIGIN OF ACS INDUCING AGENT MARKERS INDUCED Bone marrow adipose tissue Ascorbate Aggrecan BAT Dexamethasone Chondroitin sulfate WAT TGF-b Collagen type II 3 D structure Collagen type IV BMP-6 Chondrocyte specific proteoglycans FGF APPLICATION Knee, hip chondroid tissue

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into osteocytes ORIGIN OF ACS INDUCING AGENT MARKERS INDUCED APPLICATION WAT Ascorbate Osteocalcin Bone implantation Bone marrow adipose tissue Dexamethasone DMP-1 Bone fracture repair 1, 25 -dihydroxy vitamin D 3 Osteoadherin B-glycerophosphate BMP-2 BMP-7 Runx 2

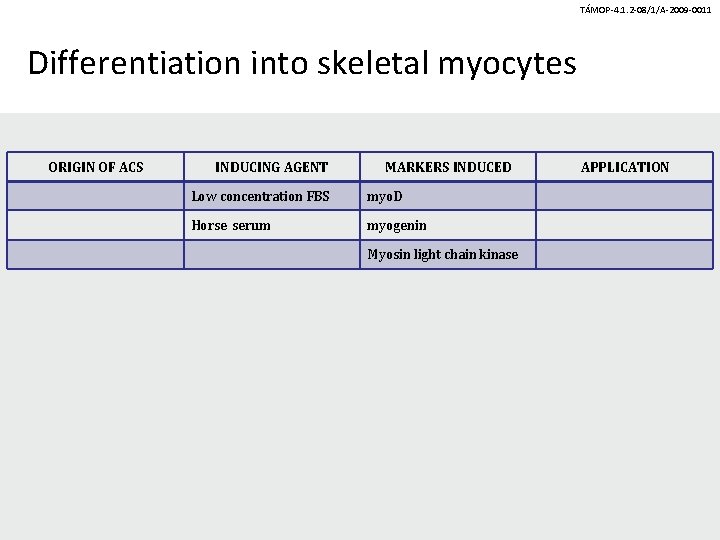
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into skeletal myocytes ORIGIN OF ACS INDUCING AGENT MARKERS INDUCED Low concentration FBS myo. D Horse serum myogenin Myosin light chain kinase APPLICATION

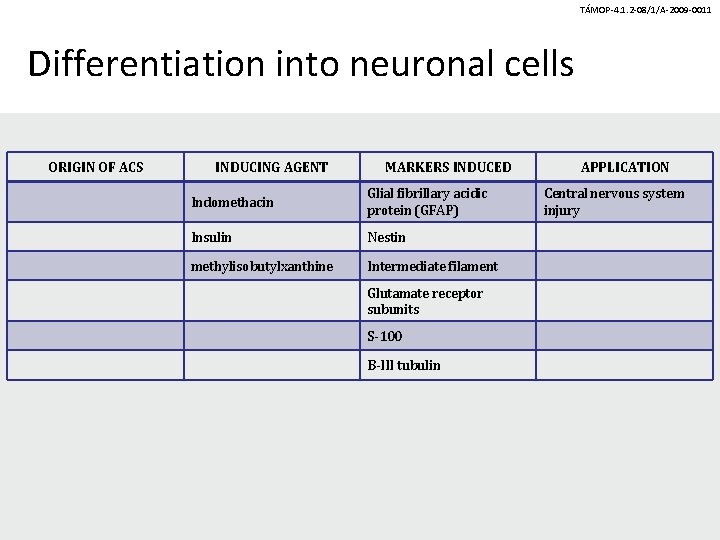
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into neuronal cells ORIGIN OF ACS INDUCING AGENT MARKERS INDUCED Indomethacin Glial fibrillary acidic protein (GFAP) Insulin Nestin methylisobutylxanthine Intermediate filament Glutamate receptor subunits S-100 B-III tubulin APPLICATION Central nervous system injury

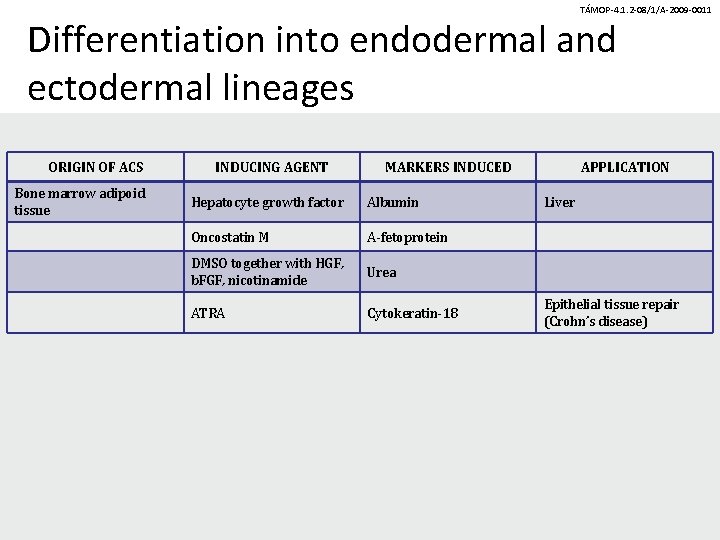
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into endodermal and ectodermal lineages ORIGIN OF ACS Bone marrow adipoid tissue INDUCING AGENT MARKERS INDUCED Hepatocyte growth factor Albumin Oncostatin M A-fetoprotein DMSO together with HGF, b. FGF, nicotinamide Urea ATRA Cytokeratin-18 APPLICATION Liver Epithelial tissue repair (Crohn’s disease)

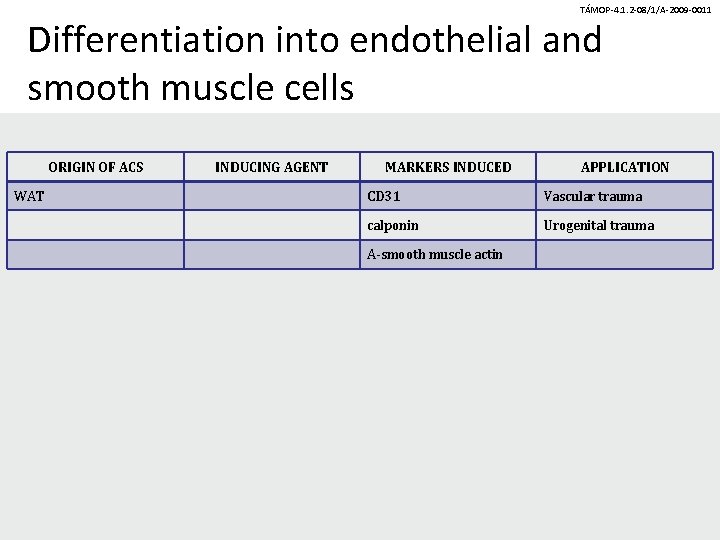
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation into endothelial and smooth muscle cells ORIGIN OF ACS WAT INDUCING AGENT MARKERS INDUCED APPLICATION CD 31 Vascular trauma calponin Urogenital trauma A-smooth muscle actin

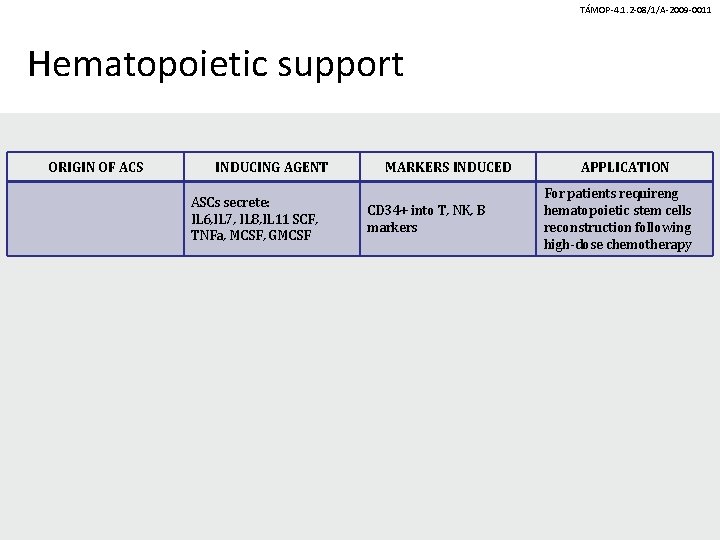
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Hematopoietic support ORIGIN OF ACS INDUCING AGENT ASCs secrete: IL 6, IL 7, IL 8, IL 11 SCF, TNFa, MCSF, GMCSF MARKERS INDUCED CD 34+ into T, NK, B markers APPLICATION For patients requireng hematopoietic stem cells reconstruction following high-dose chemotherapy

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Dr. Judit Pongrácz Three dimensional tissue cultures and tissue engineering – Lecture 4 STEM CELLS (3)

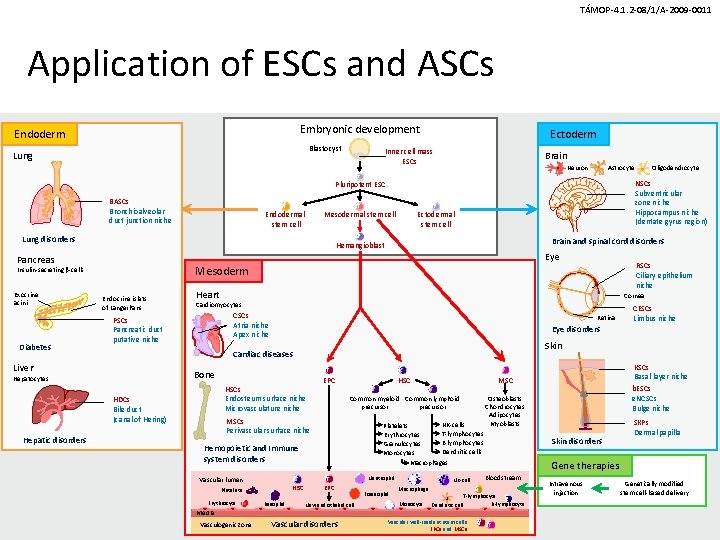
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Application of ESCs and ASCs Embryonic development Endoderm Blastocyst Lung Ectoderm Inner cell mass ESCs Brain Neuron Astrocyte NSCs Subventricular zone niche Hippocampus niche (dentate gyrus region) Pluripotent ESC BASCs Bronchioalveolar duct junction niche Endodermal stem cell Lung disorders Endocrine islets of Langerhans RSCs Ciliary epithelium niche Heart Cornea Cardiomyocytes CSCs Atria niche Apex niche PSCs Pancreatic duct putative niche Retina Skin Bone Hepatocytes HSCs Endosteum surface niche Microvasculature niche HDCs Bile duct (canal of Hering) EPC HSC MSC Common myeloid Common lymphoid precursor Osteoblasts Chondrocytes Adipocytes Myoblasts MSCs Perivascular surface niche NK-cells Platelets T-lymphocytes Erythrocytes B-lymphocytes Granulocytes Dendritic cells Monocytes Macrophages Hemopoietic and Immune system disorders Neutrophil Vascular lumen HSC Platelets Erythrocyte Basophil EPC New endothelial cell Media Vasculogenic zone CESCs Limbus niche Eye disorders Cardiac diseases Liver Hepatic disorders Eye Mesoderm Insulin-secreting b-cells Diabetes Brain and spinal cord disorders Hemangioblast Pancreas Exocrine acini Ectodermal stem cell Mesodermal stem cell Oligodendrocyte Vascular disorders Eosinophil NK-cell Macrophage Monocyte Skin disorders SKPs Dermal papilla Gene therapies Bloodstream T-lymphocyte B-lymphocyte Dendritic cell Vascular wall-resident stem cells EPCs and MSCs KSCs Basal layer niche b. ESCs e. NCSCs Bulge niche Intravenous injection Genetically modified stem cell-based delivery

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Genetic engineering and gene delivery using ASCs • Lentiviral vectors can transduce ASCs • Other recombinant viral vectors • Nucleofection

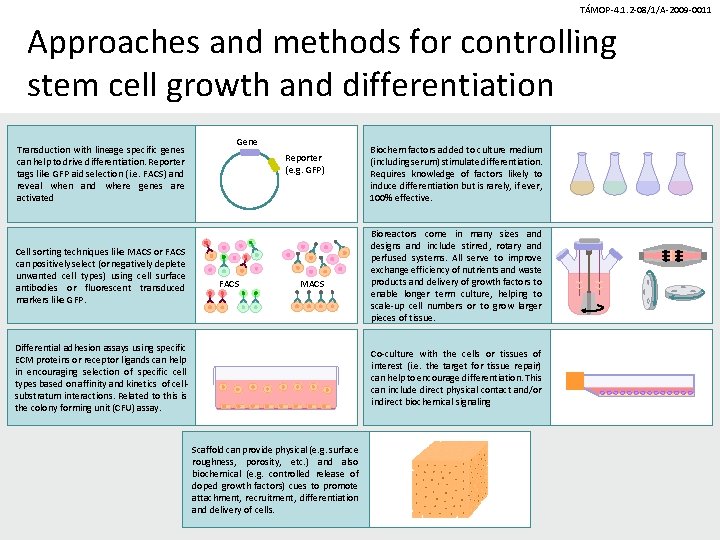
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Approaches and methods for controlling stem cell growth and differentiation Transduction with lineage specific genes can help to drive differentiation. Reporter tags like GFP aid selection (i. e. FACS) and reveal when and where genes are activated Cell sorting techniques like MACS or FACS can positively select (or negatively deplete unwanted cell types) using cell surface antibodies or fluorescent transduced markers like GFP. Gene Reporter (e. g. GFP) FACS MACS Differential adhesion assays using specific ECM proteins or receptor ligands can help in encouraging selection of specific cell types based on affinity and kinetics of cellsubstratum interactions. Related to this is the colony forming unit (CFU) assay. Biochem factors added to culture medium (including serum) stimulate differentiation. Requires knowledge of factors likely to induce differentiation but is rarely, if ever, 100% effective. Bioreactors come in many sizes and designs and include stirred, rotary and perfused systems. All serve to improve exchange efficiency of nutrients and waste products and delivery of growth factors to enable longer term culture, helping to scale-up cell numbers or to grow larger pieces of tissue. Co-culture with the cells or tissues of interest (i. e. the target for tissue repair) can help to encourage differentiation. This can include direct physical contact and/or indirect biochemical signaling Scaffold can provide physical (e. g. surface roughness, porosity, etc. ) and also biochemical (e. g. controlled release of doped growth factors) cues to promote attachment, recruitment, differentiation and delivery of cells.

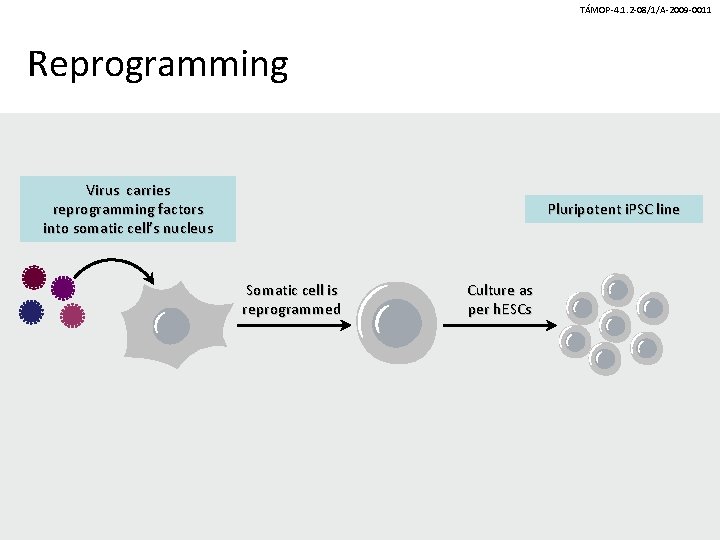
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Reprogramming Virus carries reprogramming factors into somatic cell’s nucleus Pluripotent i. PSC line Somatic cell is reprogrammed Culture as per h. ESCs

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation of Cells I Precursor cell Regulatory protein 1 Cell division Regulatory protein 2 Regulatory protein 3 Cell A Cell B Cell C Regulatory protein 2 Regulatory protein 3 Cell D Cell E Regulatory protein 3 Cell F Cell G Regulatory protein 3 Cell H

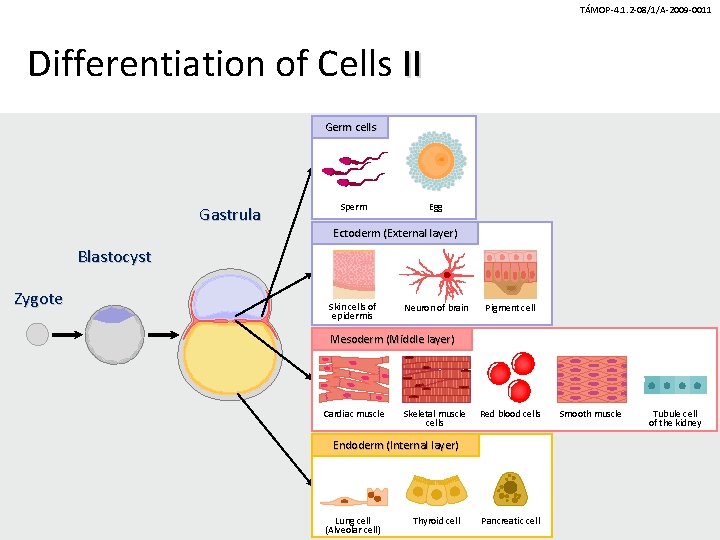
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation of Cells II Germ cells Gastrula Sperm Egg Ectoderm (External layer) Blastocyst Zygote Skin cells of epidermis Neuron of brain Pigment cell Mesoderm (Middle layer) Cardiac muscle Skeletal muscle cells Red blood cells Endoderm (Internal layer) Lung cell (Alveolar cell) Thyroid cell Pancreatic cell Smooth muscle Tubule cell of the kidney

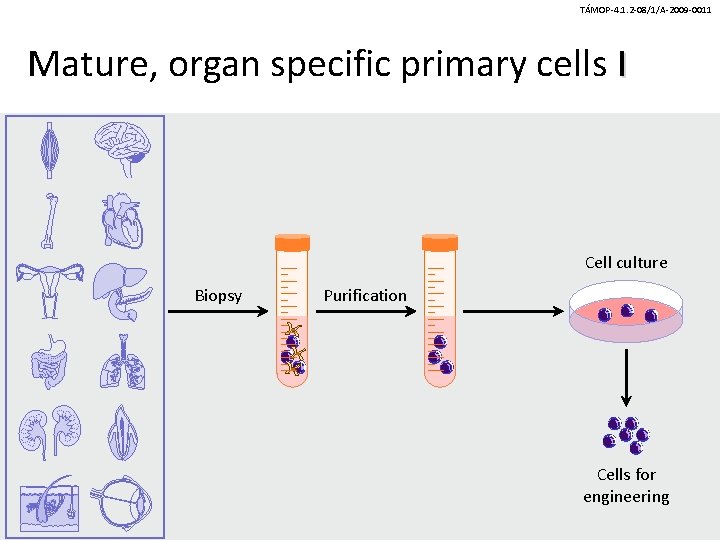
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mature, organ specific primary cells I Cell culture Biopsy Purification Cells for engineering

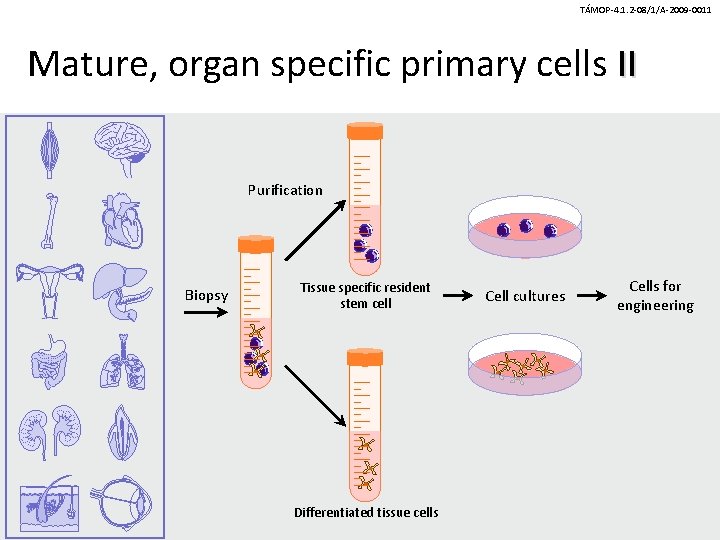
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mature, organ specific primary cells II Purification Biopsy Tissue specific resident stem cell Differentiated tissue cells Cell cultures Cells for engineering

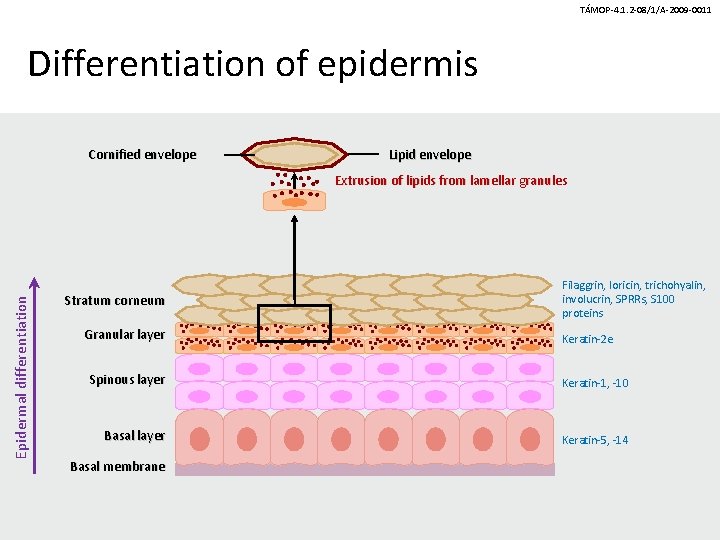
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Differentiation of epidermis Cornified envelope Lipid envelope Epidermal differentiation Extrusion of lipids from lamellar granules Stratum corneum Granular layer Filaggrin, loricin, trichohyalin, involucrin, SPRRs, S 100 proteins Keratin-2 e Spinous layer Keratin-1, -10 Basal layer Keratin-5, -14 Basal membrane

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mature tissue specific cells in tissue engineering • • Biopsy or resection Purification Regaining proliferation capacity in cell culture Re-differentiation

Regulatory issues I Cells • GLP • GMP • Permit to work on ES TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Regulatory issues II Animals • Permission to work on animals • UK: Home Office Licence 1986 • EC 1394/2007 TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Regulatory issues III Human Embryonic Stem Cells TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Ethical issues of using human embryos as sources of stem cells

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Regenerative medicine • Organ regeneration by inducing self-regenerative biochemical and cellular processes • Organ regeneration by addition of in vitro generated full organs or specific tissues of an organ

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Organ failure • Organ failure due to disease, accident or aging requires full organ replacement or regeneration • Ideally, one’s own tissues (autologue) should provide the necessary biomaterial for generation of such organs
 New drug delivery system
New drug delivery system Manifestation physique
Manifestation physique Manifestation commerciale exemple
Manifestation commerciale exemple Manifestation meeting for 504
Manifestation meeting for 504 Canvas.aps
Canvas.aps Manifestation determination flowchart
Manifestation determination flowchart Rayon rubis doré
Rayon rubis doré The manifestation of the holy spirit
The manifestation of the holy spirit Définition besoin fondamental selon virginia henderson
Définition besoin fondamental selon virginia henderson Manifestation determination definition
Manifestation determination definition Fault is manifestation of which defects
Fault is manifestation of which defects Manifestation names
Manifestation names Clinical manifestation of hepatitis
Clinical manifestation of hepatitis Manifestation names
Manifestation names Clinical manifestation of epistaxis
Clinical manifestation of epistaxis Vocabulaire manifestation
Vocabulaire manifestation Meaning of sunnatullah
Meaning of sunnatullah Friday manifestation
Friday manifestation Bbl lotion for scabies
Bbl lotion for scabies Social thinking and social influence
Social thinking and social influence Social thinking social influence social relations
Social thinking social influence social relations Social challenges
Social challenges Venizelos efthymiou
Venizelos efthymiou Federation of european social employers
Federation of european social employers European social structure
European social structure European social fund plus
European social fund plus European social fund plus
European social fund plus Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống