Manifestation of Novel Social Challenges of the European





- Slides: 14

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Tímea Berki and Ferenc Boldizsár Signal transduction SIGNALING IN THE NERVOUS SYSTEM

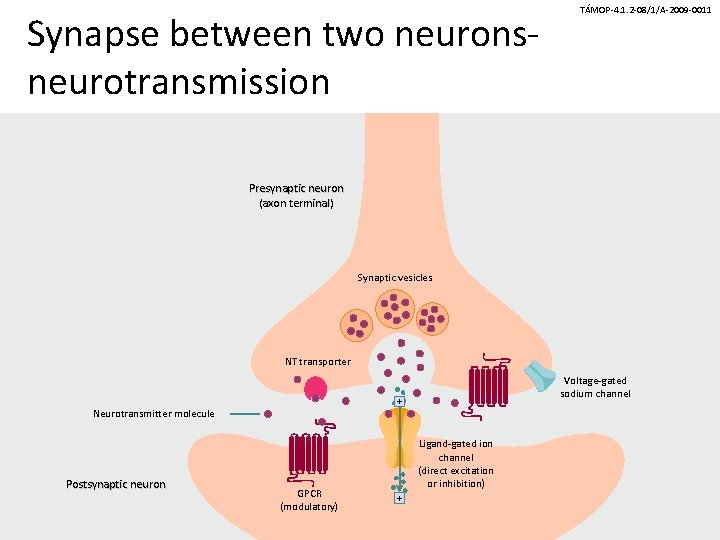
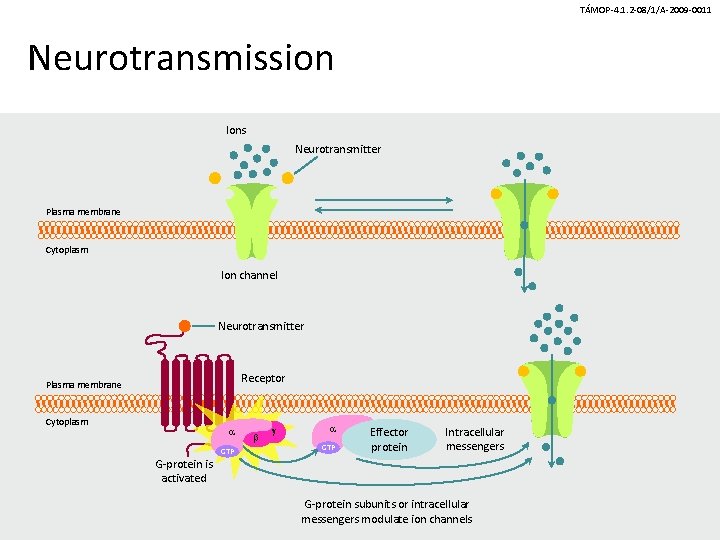
Synapse between two neuronsneurotransmission TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Presynaptic neuron (axon terminal) Synaptic vesicles NT transporter + Neurotransmitter molecule Postsynaptic neuron Voltage-gated sodium channel GPCR (modulatory) Ligand-gated ion channel (direct excitation or inhibition) +

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mechanism of neurotransmission • Synaptic vesicles contain a neurotransmitter (NT) and release it when their membranes fuse with the outer cell membrane. • Neurotransmitter molecules cross the synaptic cleft and bind to receptors known as ligand-gated ion channels (LGICs) and Gprotein–coupled receptors (GPCRs) on the postsynaptic neuron. • GPCRs on the presynaptic neuron’s axon terminal alter the function of voltage-gated ion channels and modulate neurotransmitter release. • Neurotransmitter transporters remove neurotransmitter molecules from the synaptic cleft so that they can be repackaged into vesicles

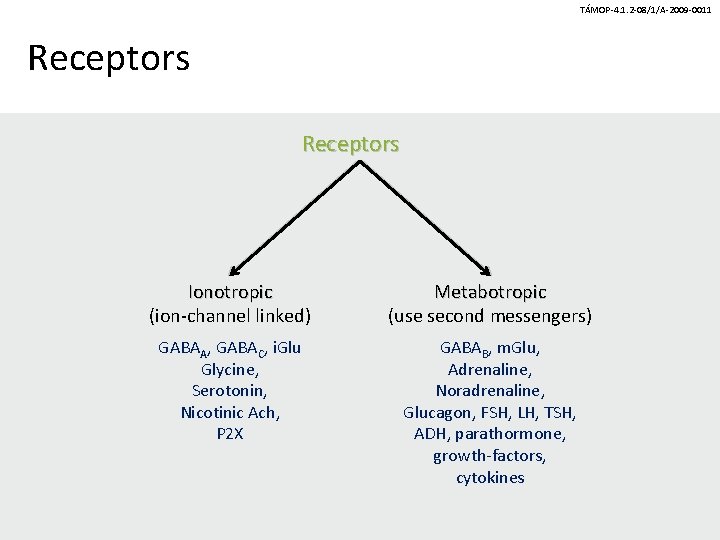
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Receptors Ionotropic (ion-channel linked) Metabotropic (use second messengers) GABAA, GABAC, i. Glu Glycine, Serotonin, Nicotinic Ach, P 2 X GABAB, m. Glu, Adrenaline, Noradrenaline, Glucagon, FSH, LH, TSH, ADH, parathormone, growth-factors, cytokines

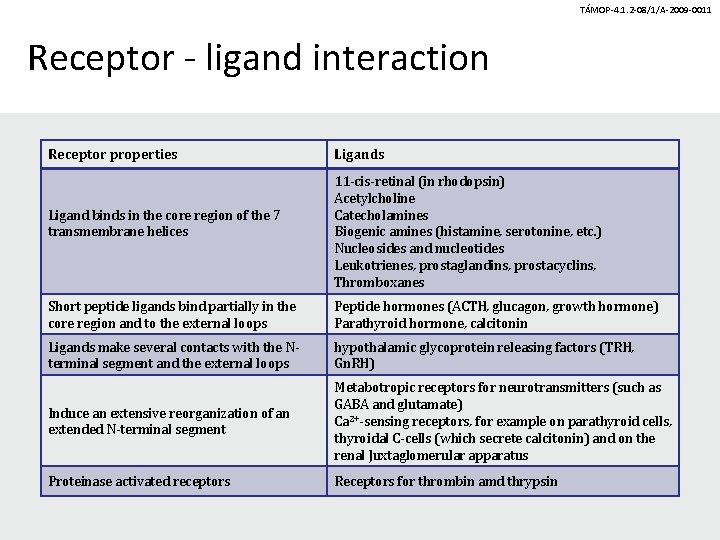
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Receptor - ligand interaction Receptor properties Ligand binds in the core region of the 7 transmembrane helices Ligands 11 -cis-retinal (in rhodopsin) Acetylcholine Catecholamines Biogenic amines (histamine, serotonine, etc. ) Nucleosides and nucleotides Leukotrienes, prostaglandins, prostacyclins, Thromboxanes Short peptide ligands bind partially in the core region and to the external loops Peptide hormones (ACTH, glucagon, growth hormone) Parathyroid hormone, calcitonin Ligands make several contacts with the Nterminal segment and the external loops hypothalamic glycoprotein releasing factors (TRH, Gn. RH) Induce an extensive reorganization of an extended N-terminal segment Metabotropic receptors for neurotransmitters (such as GABA and glutamate) Ca 2+-sensing receptors, for example on parathyroid cells, thyroidal C-cells (which secrete calcitonin) and on the renal Juxtaglomerular apparatus Proteinase activated receptors Receptors for thrombin amd thrypsin

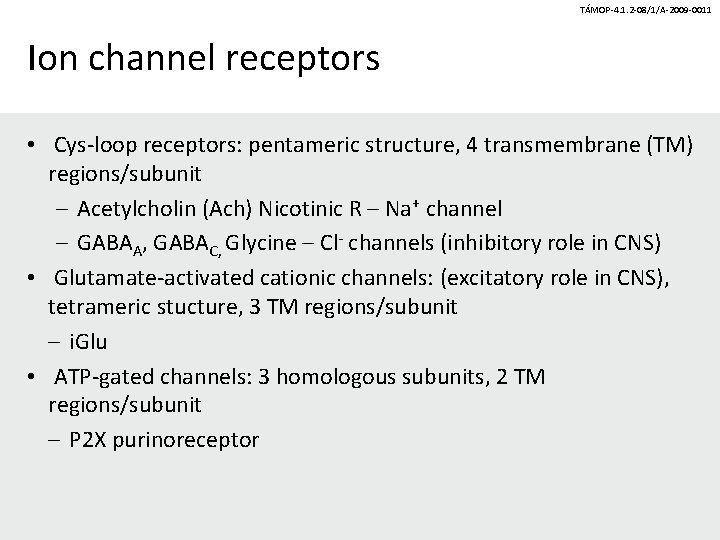
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Ion channel receptors • Cys-loop receptors: pentameric structure, 4 transmembrane (TM) regions/subunit – Acetylcholin (Ach) Nicotinic R – Na+ channel – GABAA, GABAC, Glycine – Cl- channels (inhibitory role in CNS) • Glutamate-activated cationic channels: (excitatory role in CNS), tetrameric stucture, 3 TM regions/subunit – i. Glu • ATP-gated channels: 3 homologous subunits, 2 TM regions/subunit – P 2 X purinoreceptor

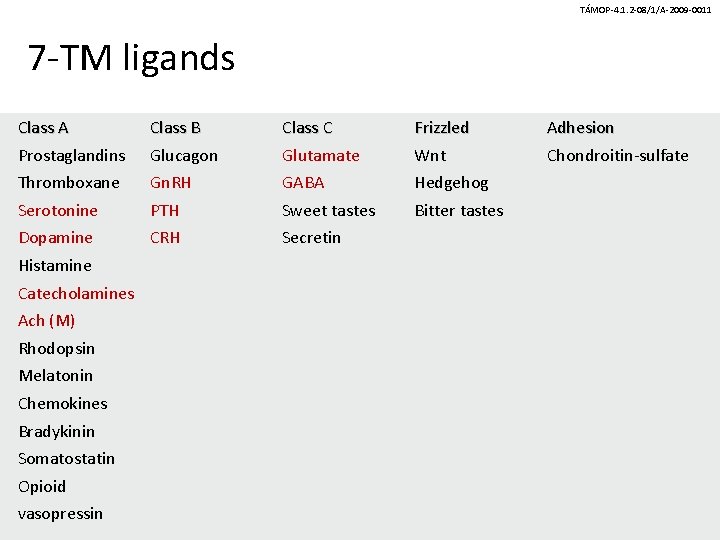
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 7 -transmembrane-spanning receptors (7 -TM) • Class A: Rhodopsin-like • Class B: Secretin family • Class C: Glutamate and GABA (metabotropic) • Frizzled • Adhesion family

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 7 -TM ligands Class A Prostaglandins Class B Glucagon Class C Glutamate Frizzled Wnt Thromboxane Gn. RH GABA Hedgehog Serotonine PTH Sweet tastes Bitter tastes Dopamine CRH Secretin Histamine Catecholamines Ach (M) Rhodopsin Melatonin Chemokines Bradykinin Somatostatin Opioid vasopressin Adhesion Chondroitin-sulfate

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Nicotinic Ach receptor • Pore formed from 5 subunits: 2 , , g, d • Opening: the 2 a units are distorted • Desensitization: in the open conformation the , g, d subunits become phosphorylated by Protein kinase A and C

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Neurotransmission Ions Neurotransmitter Plasma membrane Cytoplasm Ion channel Neurotransmitter Receptor Plasma membrane Cytoplasm GTP g GTP Effector protein Intracellular messengers G-protein is activated G-protein subunits or intracellular messengers modulate ion channels

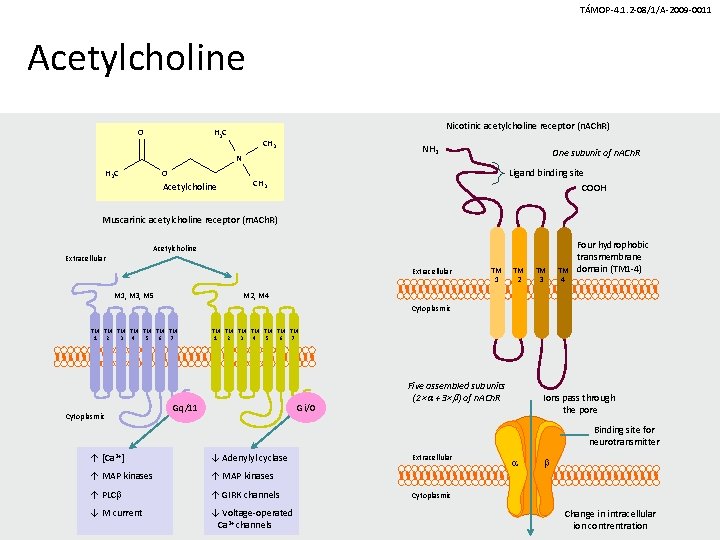
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Acetylcholine O Nicotinic acetylcholine receptor (n. ACh. R) H 3 C CH 3 NH 2 N O H 3 C Acetylcholine One subunit of n. ACh. R Ligand binding site CH 3 COOH Muscarinic acetylcholine receptor (m. ACh. R) Acetylcholine Extracellular M 1, M 3, M 5 TM 1 TM 2 TM 3 TM 4 Four hydrophobic transmembrane domain (TM 1 -4) M 2, M 4 Cytoplasmic TM TM 2 4 6 1 3 5 7 Cytoplasmic TM TM 1 3 5 7 2 4 6 Gq/11 Gi/0 Five assembled subunits (2× + 3× ) of n. ACh. R Ions pass through the pore Binding site for neurotransmitter ↑ [Ca 2+] ↓ Adenylyl cyclase ↑ MAP kinases ↑ PLC ↑ GIRK channels ↓ M current ↓ Voltage-operated Ca 2+ channels Extracellular Cytoplasmic Change in intracellular ion contrentration

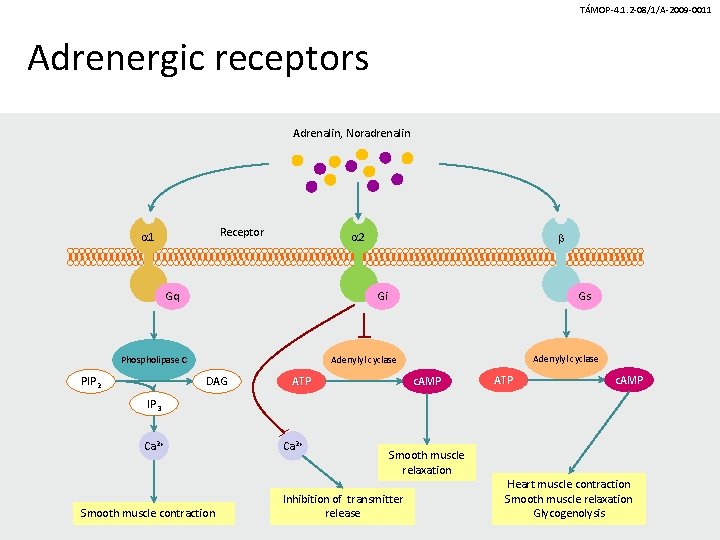
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Adrenergic receptors Adrenalin, Noradrenalin Receptor 1 2 Gq Gi Phospholipase C PIP 2 Gs Adenylyl cyclase DAG ATP c. AMP IP 3 Ca 2+ Smooth muscle contraction Ca 2+ Smooth muscle relaxation Inhibition of transmitter release Heart muscle contraction Smooth muscle relaxation Glycogenolysis

Blocking the neuromuscular transmission TÁMOP-4. 1. 2 -08/1/A-2009 -0011 a-Bungarotoxin: • Snake venom (Bungarus multicinctus) • Binds to the N-Ach receptor and inactivates Curare (tubocurarin): • In South American plants Strychnos toxifera and Chondrodendron tomentosum • Indians use as arrow poison • Curare binds to the same place on the N-Ach receptor than Achetylcholin BUT channel doesn’t open • Causes paralysis of breathing muscles • Used as muscle relaxant in anaesthesia • Antidote: Acetylcholinesterase inhibitors
 Newer drug delivery system
Newer drug delivery system Vocabulaire de l'étonnement
Vocabulaire de l'étonnement Manifestation commerciale exemple
Manifestation commerciale exemple Manifestation meeting for 504
Manifestation meeting for 504 Manifestation determination flowchart
Manifestation determination flowchart Manifestation determination flowchart
Manifestation determination flowchart Manifestation shamballa
Manifestation shamballa The manifestation of the holy spirit
The manifestation of the holy spirit Virginia henderson les besoins fondamentaux
Virginia henderson les besoins fondamentaux Manifestation determination definition
Manifestation determination definition A failure is a manifestation of an
A failure is a manifestation of an Manifestation names
Manifestation names Hepatomogaly
Hepatomogaly Manifestation names
Manifestation names Hemorrhagic
Hemorrhagic



























