Manifestation of Novel Social Challenges of the European











- Slides: 20

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011

Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Zoltan Balajthy Molecular Therapies- Lecture 12 CELL CYCLE AND CANCER

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Learning objectives of chapter 12 and 13. The purpose of this chapter is to describe the processes and regulations of both cell cycle and cell death, explain the unregulated cell division, and to point out therapeutic intervention in cancer at molecular levels. Topics in chapter 12. 1. Interpretation of cell cycle Constitutive and Inducible Cell Cycle Kinase Inhibitors 12. 2. Mitogen-activated Protein (MAP) Kinase Cascade Transcriptional Events in G 1 Phase of Cell-cycle Mechanisms of Gene-suppression by the Retinoblastoma Protein 12. 3. Biochemical Events of Cell-cycle – in M Phase 12. 4. Cancer Causing Genes in Mitotic Signal Pathway Proto-oncogenes and Oncogenes 12. 5. Different Families of Receptor Tyrosine Kinases (RTK) Proto-oncogenes are normal genes that can become oncogenes 12. 6. Therapeutic Targets Monoclonal antibodies and specific inhibitors

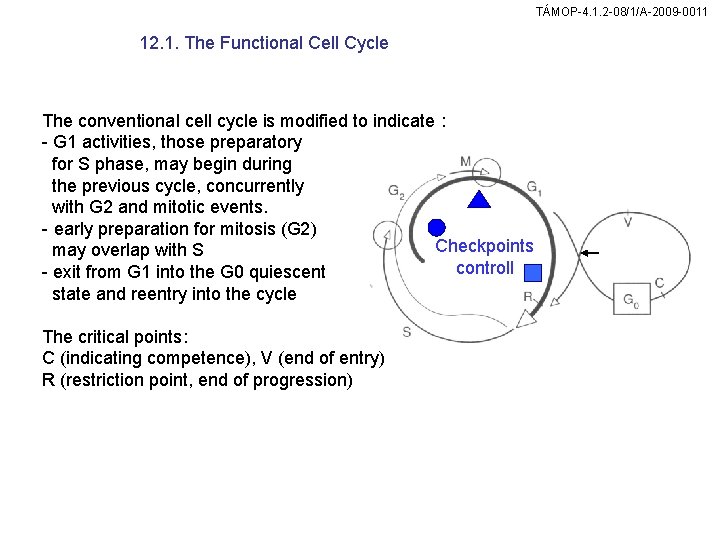
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 12. 1. The Functional Cell Cycle The conventional cell cycle is modified to indicate : - G 1 activities, those preparatory for S phase, may begin during the previous cycle, concurrently with G 2 and mitotic events. - early preparation for mitosis (G 2) Checkpoints may overlap with S controll - exit from G 1 into the G 0 quiescent state and reentry into the cycle The critical points: C (indicating competence), V (end of entry) R (restriction point, end of progression)

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Cyclin-CDK Regulators of Cell-Cycle

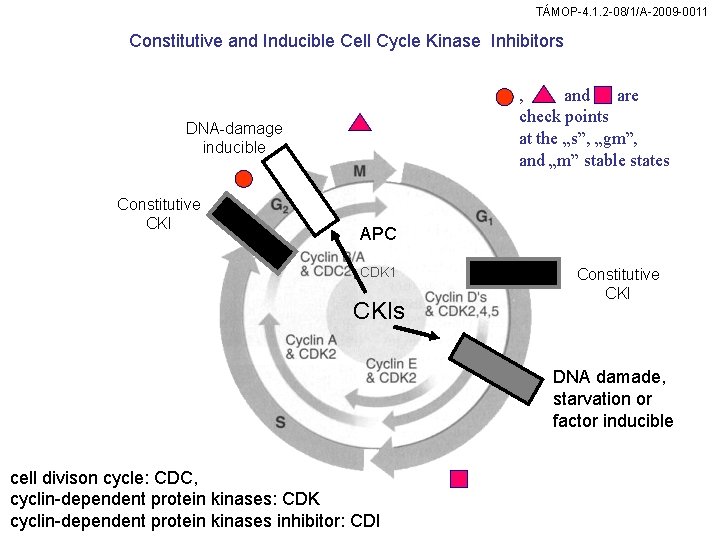
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Constitutive and Inducible Cell Cycle Kinase Inhibitors , and are check points at the „s”, „gm”, and „m” stable states DNA-damage inducible Constitutive CKI APC , CDK 1 CKIs Constitutive CKI DNA damade, starvation or factor inducible cell divison cycle: CDC, cyclin-dependent protein kinases: CDK cyclin-dependent protein kinases inhibitor: CDI

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 12. 1. Mitogen-activated Protein (MAP) Kinase Cascade

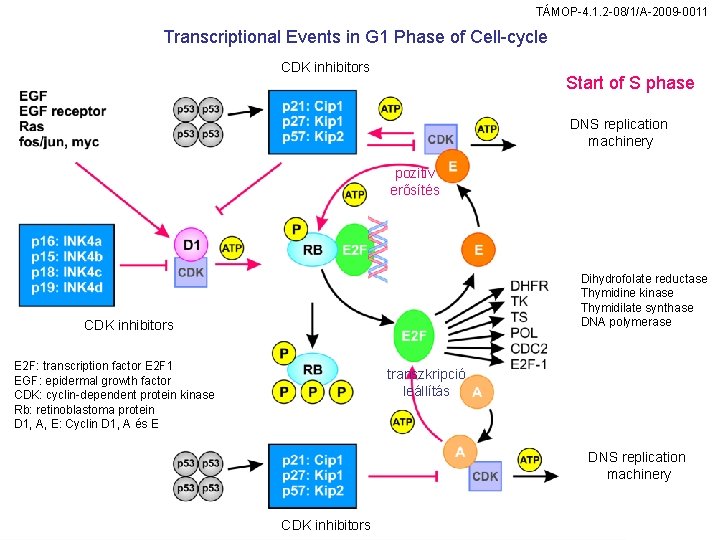
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Transcriptional Events in G 1 Phase of Cell-cycle CDK inhibitors Start of S phase DNS replication machinery pozitív erősítés Dihydrofolate reductase Thymidine kinase Thymidilate synthase DNA polymerase CDK inhibitors E 2 F: transcription factor E 2 F 1 EGF: epidermal growth factor CDK: cyclin-dependent protein kinase Rb: retinoblastoma protein D 1, A, E: Cyclin D 1, A és E transzkripció leállítás DNS replication machinery CDK inhibitors

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Mechanisms of Gene-suppression by the Retinoblastoma Protein

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 11. 3. Biochemical Events of Cell-cycle – in M Phase

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 12. 4. Cancer Causing Genes in Mitotic Signal Pathway

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Proto-oncogenes and Oncogenes

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Possible Biochemical Mechanisms of Protooncogene - oncogene Conversion - promoter insertion - enhancer insertion - chromosomal translocation - gene amplification - single point mutation or deletion Out the 5 mechanisms described above, the first 4 involve an increase in amount of the product of an oncogene due to increased transcription but no alteration of the structure of the product of the oncogene. Thus it appears that increased amounts of the product of an oncogene may be sufficient to push a cell becoming malignant. The fifth mechanism, single point mutation, involves a change in the structure of the product of the oncogene but not necessarily any change in its amount. This implies that the presence of a structurally abnormal key regulatory protein in a cell may be sufficient to tip the scale toward malignancy.

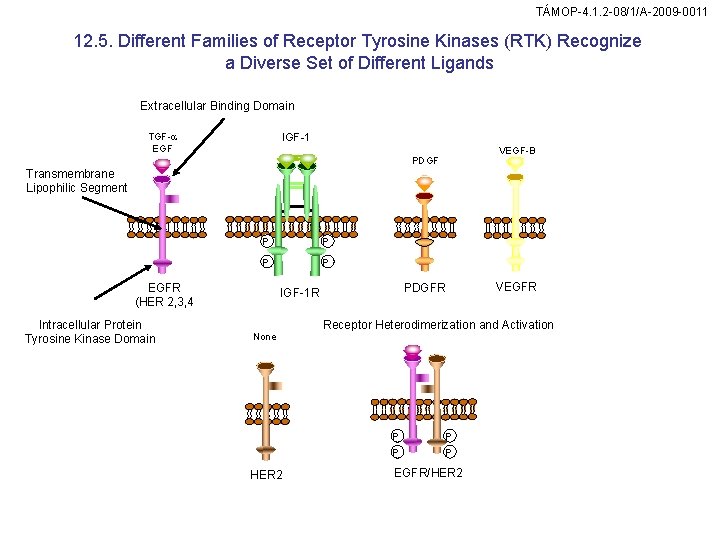
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 12. 5. Different Families of Receptor Tyrosine Kinases (RTK) Recognize a Diverse Set of Different Ligands Extracellular Binding Domain IGF-1 IGF-2 TGF-a EGF VEGF-A VEGF-B PDGF Transmembrane Lipophilic Segment P P EGFR (HER 2, 3, 4 Intracellular Protein Tyrosine Kinase Domain PDGFR IGF-1 R None HER 2 VEGFR Receptor Heterodimerization and Activation P P EGFR/HER 2

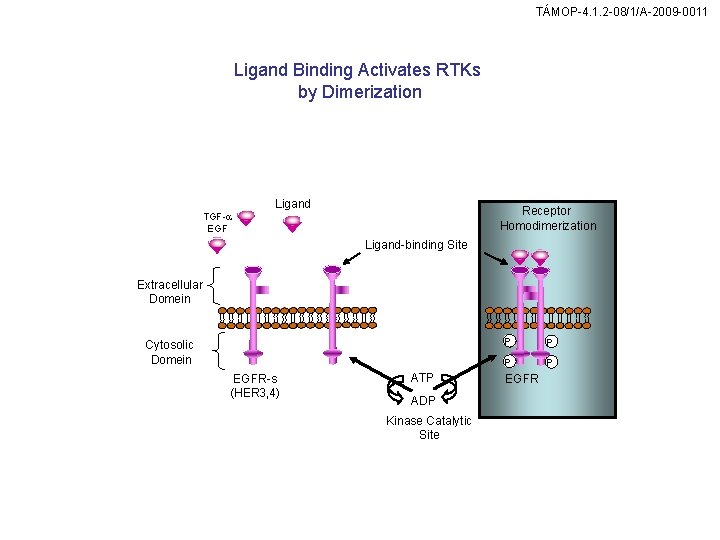
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Ligand Binding Activates RTKs by Dimerization Ligand Receptor Homodimerization TGF-a EGF Ligand-binding Site Extracellular Domein Cytosolic Domein EGFR-s (HER 3, 4) ATP ADP Kinase Catalytic Site P P EGFR

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Proto-oncogenes are Normal Genes That Can Become Oncogenes

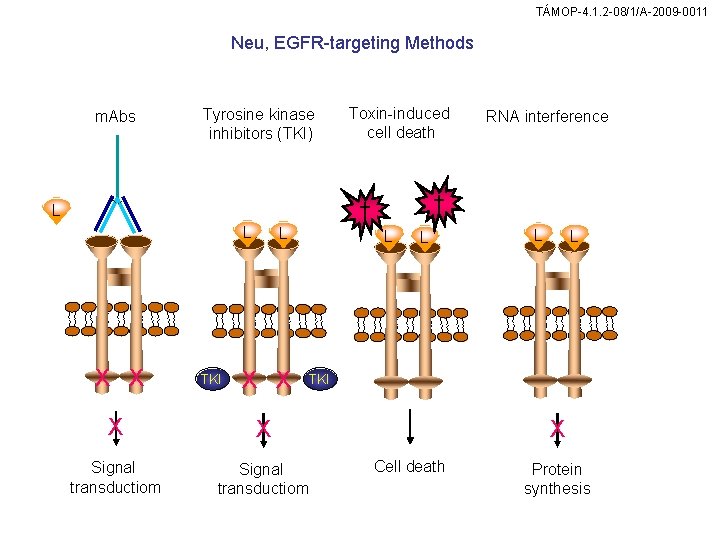
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Neu, EGFR-targeting Methods m. Abs Tyrosine kinase inhibitors (TKI) Toxin-induced cell death ✝ ✝ L L X X TKI L X X RNA interference L L TKI X X Signal transductiom X Cell death Protein synthesis

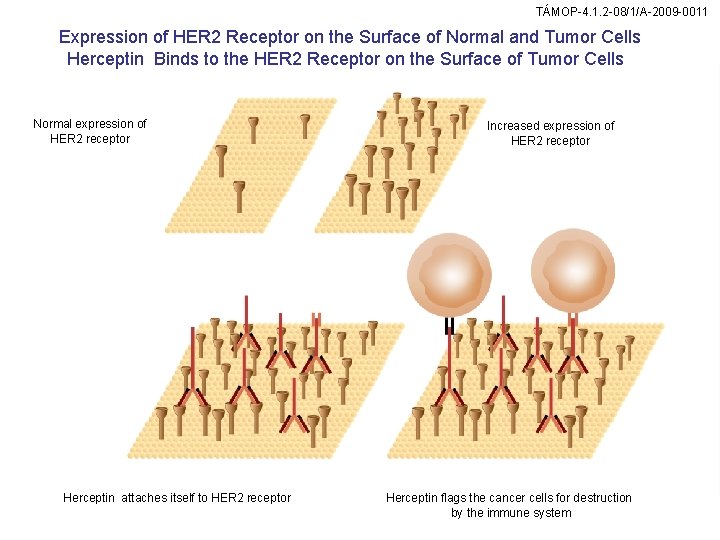
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Expression of HER 2 Receptor on the Surface of Normal and Tumor Cells Herceptin Binds to the HER 2 Receptor on the Surface of Tumor Cells Normal expression of HER 2 receptor Herceptin attaches itself to HER 2 receptor Increased expression of HER 2 receptor Herceptin flags the cancer cells for destruction by the immune system

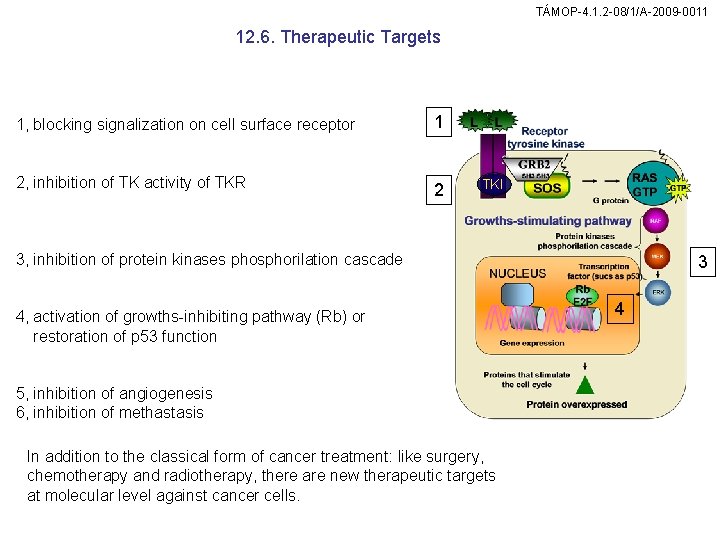
TÁMOP-4. 1. 2 -08/1/A-2009 -0011 12. 6. Therapeutic Targets 1, blocking signalization on cell surface receptor 1 2, inhibition of TK activity of TKR 2 TKI 3, inhibition of protein kinases phosphorilation cascade 4, activation of growths-inhibiting pathway (Rb) or restoration of p 53 function 5, inhibition of angiogenesis 6, inhibition of methastasis In addition to the classical form of cancer treatment: like surgery, chemotherapy and radiotherapy, there are new therapeutic targets at molecular level against cancer cells. 3 4

TÁMOP-4. 1. 2 -08/1/A-2009 -0011 Blocking of Oncoproteins of EGFR and Mitogen-activated Protein Kinase Signalization via Monoclonal abs. and Specific Inhibitors
 Newer drug delivery system
Newer drug delivery system Les sentiments de joie
Les sentiments de joie Manifestation commerciale exemple
Manifestation commerciale exemple Manifestation meeting for 504
Manifestation meeting for 504 Canvas.aps
Canvas.aps Manifestation determination flowchart
Manifestation determination flowchart Rayon rubis doré
Rayon rubis doré The manifestation of the holy spirit
The manifestation of the holy spirit La dépendance selon virginia henderson
La dépendance selon virginia henderson Manifestation determination process
Manifestation determination process Fault is manifestation of defects.
Fault is manifestation of defects. Manifestation names
Manifestation names Hepatomogaly
Hepatomogaly Colossians 1:17-19
Colossians 1:17-19 Clinical manifestation of epistaxis
Clinical manifestation of epistaxis Vocabulaire manifestation
Vocabulaire manifestation The concept of sunnatullah
The concept of sunnatullah Friday manifestation
Friday manifestation Bbl lotion for scabies
Bbl lotion for scabies Social thinking and social influence in psychology
Social thinking and social influence in psychology Social thinking social influence social relations
Social thinking social influence social relations







































