General Chemistry M R NaimiJamal Faculty of Chemistry































- Slides: 61

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology




CHEMISTRY: Chemistry is the study of the properties, composition, and structure of matter, the physical and chemical changes it undergoes, and the energy liberated or absorbed during those changes.

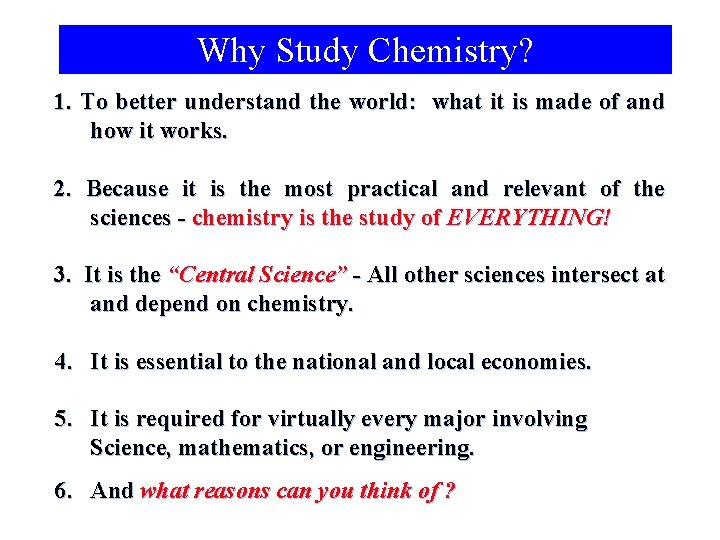
Why Study Chemistry? 1. To better understand the world: what it is made of and how it works. 2. Because it is the most practical and relevant of the sciences - chemistry is the study of EVERYTHING! 3. It is the “Central Science” - All other sciences intersect at and depend on chemistry. 4. It is essential to the national and local economies. 5. It is required for virtually every major involving Science, mathematics, or engineering. 6. And what reasons can you think of ?

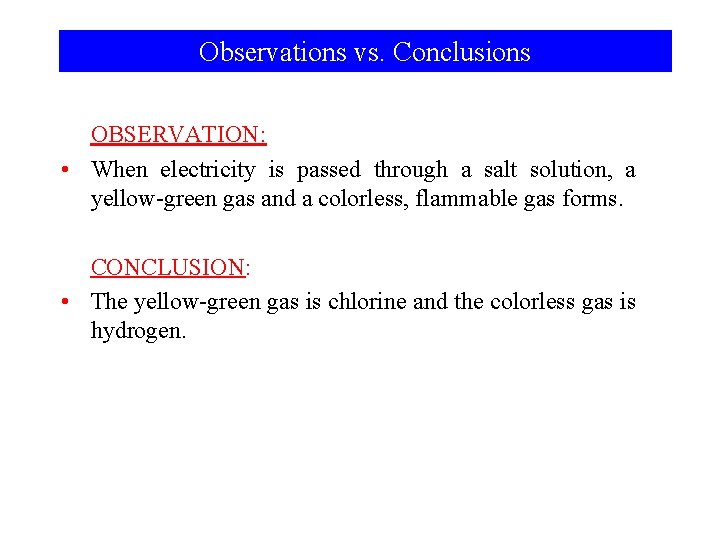
Chemistry is an Observational Science ► Observation: Using the five senses to “see” what is and happens around you. ► Conclusion: An explanation of the cause or causes for one or more observations.

Observations vs. Conclusions OBSERVATION: • When electricity is passed through a salt solution, a yellow-green gas and a colorless, flammable gas forms. CONCLUSION: • The yellow-green gas is chlorine and the colorless gas is hydrogen.

The Scientific Method 1. Collect Facts or Data (Observe!!) 2. Search for Generalizations or Laws to Summarize the Facts. 3. Freely Use Your Imagination to Construct Theories or Models of Nature that Will Account for the Laws. 4. Test Theories/Hypotheses for Accuracy. 5. Modify Theories/Hypotheses as Necessary Based on Your Test Results.





The Law of Conservation of Matter: Matter is neither created nor destroyed during a chemical reaction or a physical change. The Law of Conservation of Energy: Energy is neither created nor destroyed during a chemical reaction or a physical change. It can only be changed from one form into another. The Law of Conservation of Matter/Energy: The combined amount of matter and energy in the universe is constant. E = mc 2


ﺣﺎﻻﺕ ﻣﺎﺩﻩ Solid: has a rigid shape and a fixed volume that changes very little with temperature and pressure Liquid: like solids have a fixed volume but no definite shape (take on the shape of the container) Gas: no fixed volume - volume determined by the size of the container - the volume of a gas varies greatly with temperature and pressure


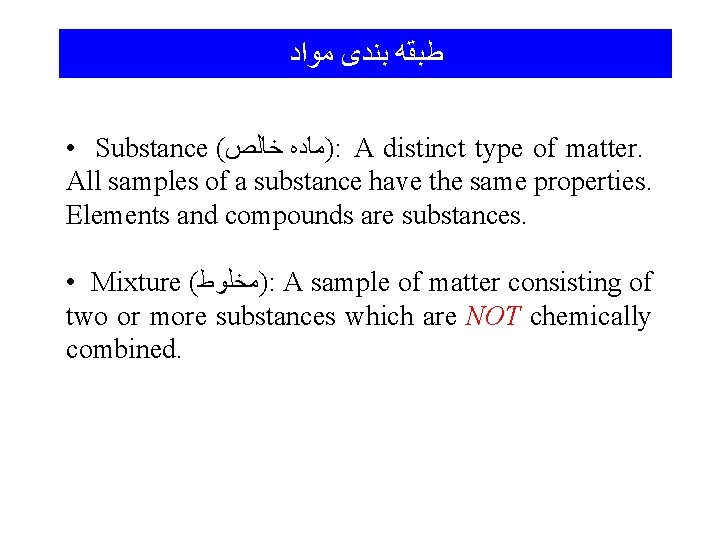
ﻃﺒﻘﻪ ﺑﻨﺪی ﻣﻮﺍﺩ • Substance ( )ﻣﺎﺩﻩ ﺧﺎﻟﺺ : A distinct type of matter. All samples of a substance have the same properties. Elements and compounds are substances. • Mixture ( )ﻣﺨﻠﻮﻁ : A sample of matter consisting of two or more substances which are NOT chemically combined.

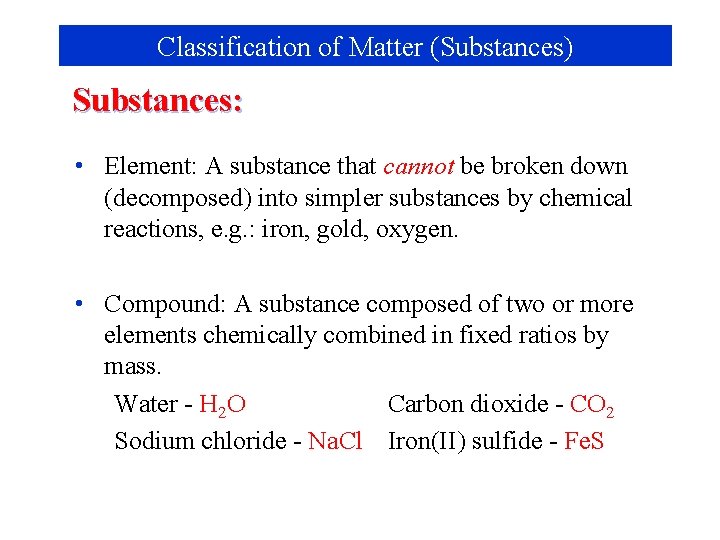
Classification of Matter (Substances) Substances: • Element: A substance that cannot be broken down (decomposed) into simpler substances by chemical reactions, e. g. : iron, gold, oxygen. • Compound: A substance composed of two or more elements chemically combined in fixed ratios by mass. Water - H 2 O Carbon dioxide - CO 2 Sodium chloride - Na. Cl Iron(II) sulfide - Fe. S

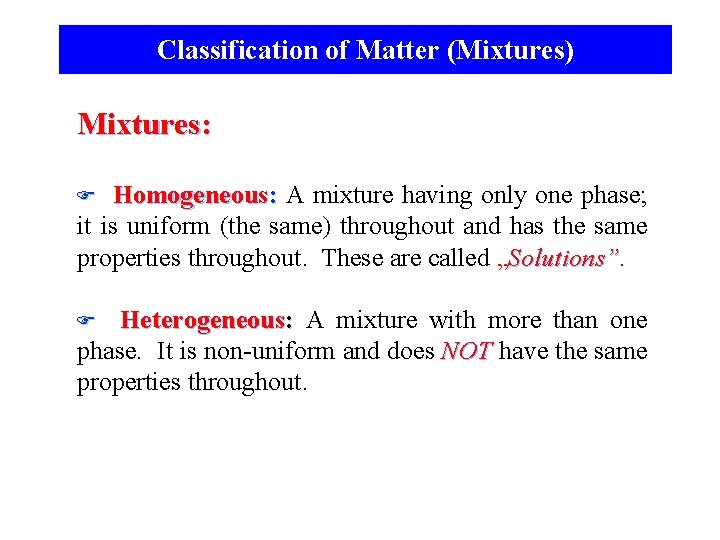
Classification of Matter (Mixtures) Mixtures: Homogeneous: A mixture having only one phase; it is uniform (the same) throughout and has the same properties throughout. These are called „Solutions” F Heterogeneous: A mixture with more than one phase. It is non-uniform and does NOT have the same properties throughout. F

Matter and Change • Phase - A sample of matter that is uniform in composition and physical state and is separated from other phases by a definite boundary.

• Physical Change: A change in which each substance involved in the change retains its original identity and no new elements or compounds are formed. H 2 O (s) melting H 2 O (l)

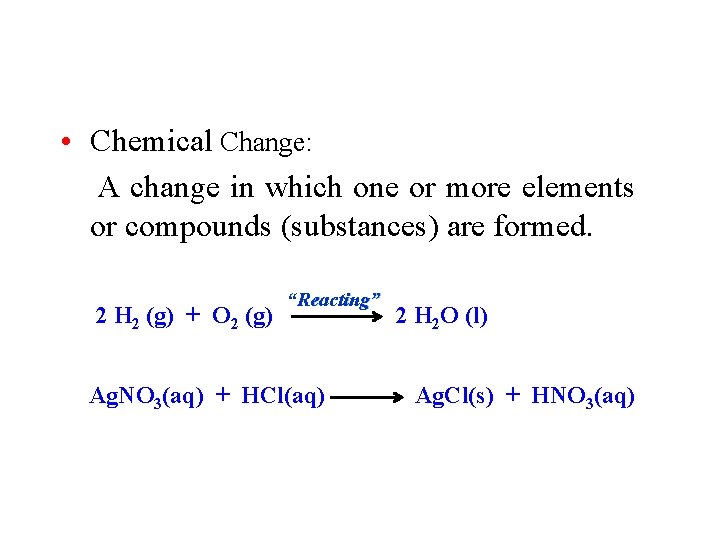
Matter and Change • Chemical Change: A change in which one or more elements or compounds (substances) are formed. 2 H 2 (g) + O 2 (g) “Reacting” Ag. NO 3(aq) + HCl(aq) 2 H 2 O (l) Ag. Cl(s) + HNO 3(aq)

n Chemical and physical change



n Paper Chromatography

Measurement • Chemistry is an Observational science. • Chemistry is a Quantitative science. • Measurement - A quantitative observation.

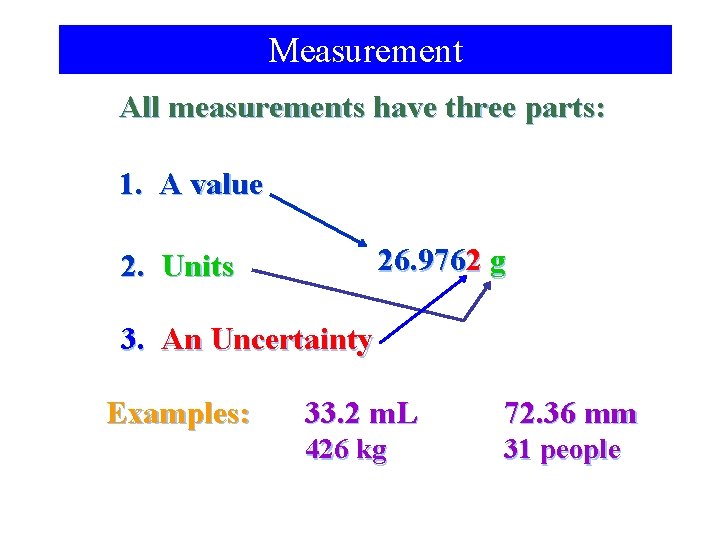
Measurement All measurements have three parts: 1. A value 26. 9762 g 2. Units 3. An Uncertainty Examples: 33. 2 m. L 72. 36 mm 426 kg 31 people

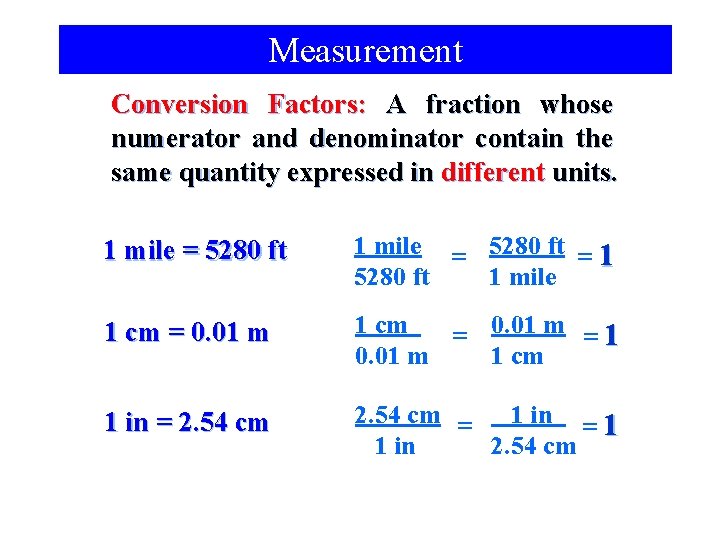
Measurement Conversion Factors: A fraction whose numerator and denominator contain the same quantity expressed in different units. 1 mile = 5280 ft = 1 5280 ft 1 mile 1 cm = 0. 01 m = 1 0. 01 m 1 cm 1 in = 2. 54 cm = 1 in = 1 1 in 2. 54 cm

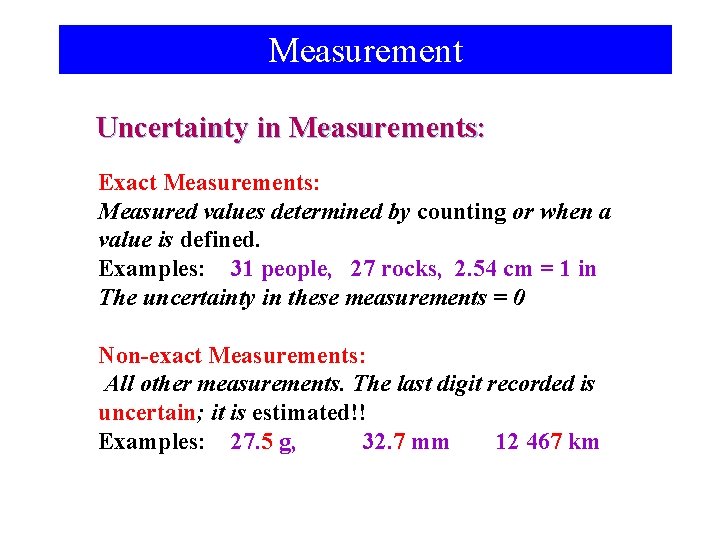
Measurement Uncertainty in Measurements: Exact Measurements: Measured values determined by counting or when a value is defined. Examples: 31 people, 27 rocks, 2. 54 cm = 1 in The uncertainty in these measurements = 0 Non-exact Measurements: All other measurements. The last digit recorded is uncertain; it is estimated!! Examples: 27. 5 g, 32. 7 mm 12 467 km

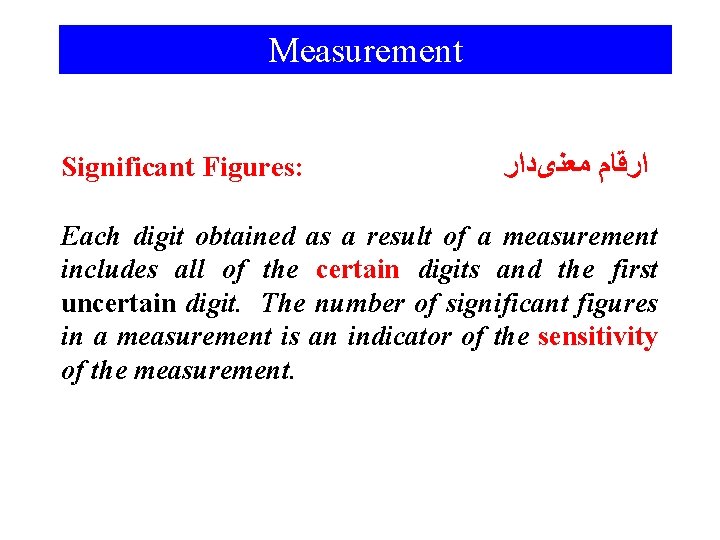
Measurement Significant Figures: ﺍﺭﻗﺎﻡ ﻣﻌﻨیﺩﺍﺭ Each digit obtained as a result of a measurement includes all of the certain digits and the first uncertain digit. The number of significant figures in a measurement is an indicator of the sensitivity of the measurement.

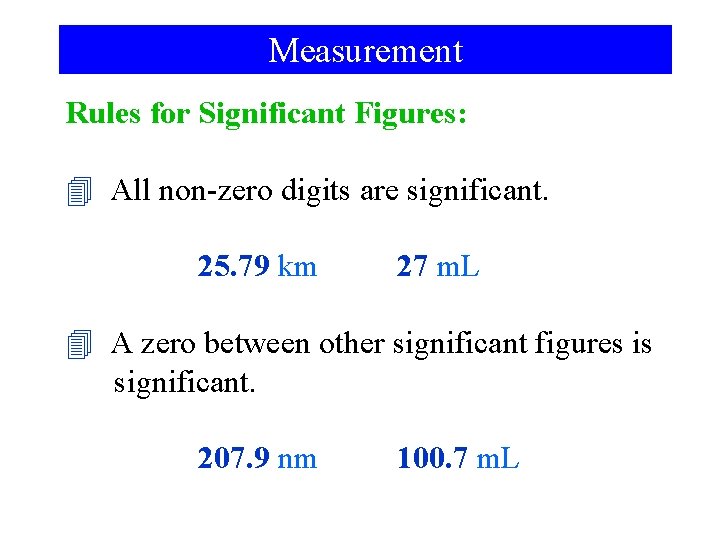
Measurement Rules for Significant Figures: 4 All non-zero digits are significant. 25. 79 km 27 m. L 4 A zero between other significant figures is significant. 207. 9 nm 100. 7 m. L

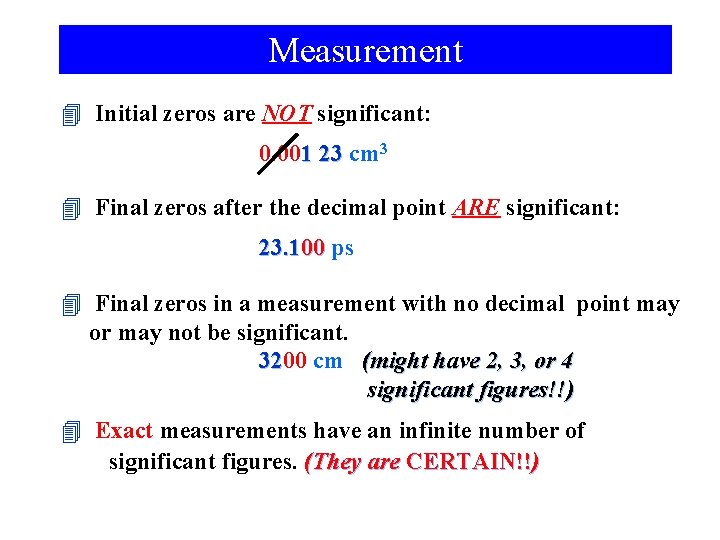
Measurement 4 Initial zeros are NOT significant: 0. 001 23 cm 3 4 Final zeros after the decimal point ARE significant: 23. 100 ps 4 Final zeros in a measurement with no decimal point may or may not be significant. 3200 32 cm (might have 2, 3, or 4 significant figures!!) 4 Exact measurements have an infinite number of significant figures. (They are CERTAIN!!)

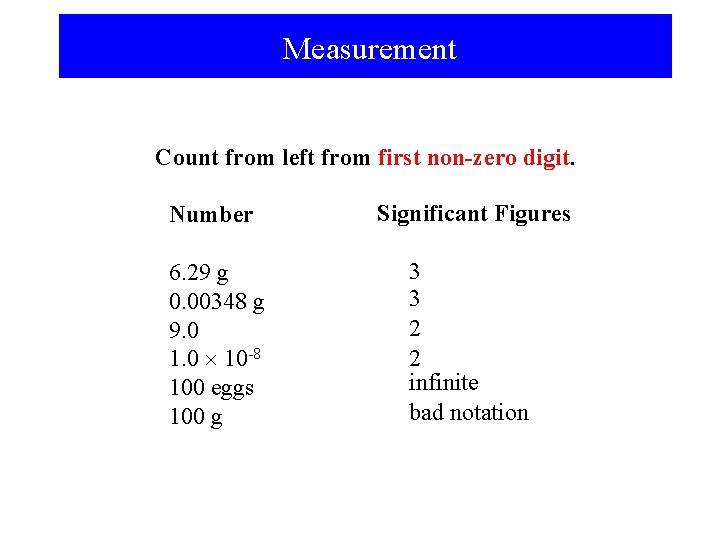
Measurement Count from left from first non-zero digit. Number Significant Figures 6. 29 g 0. 00348 g 9. 0 1. 0 10 -8 100 eggs 100 g 3 3 2 2 infinite bad notation

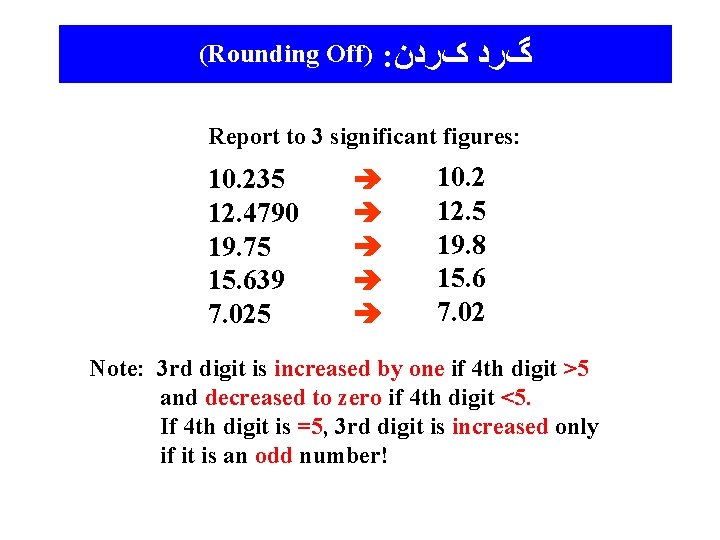
(Rounding Off) : گﺮﺩ کﺮﺩﻥ Report to 3 significant figures: 10. 235 12. 4790 19. 75 15. 639 7. 025 10. 2 12. 5 19. 8 15. 6 7. 02 Note: 3 rd digit is increased by one if 4 th digit >5 and decreased to zero if 4 th digit <5. If 4 th digit is =5, 3 rd digit is increased only if it is an odd number!

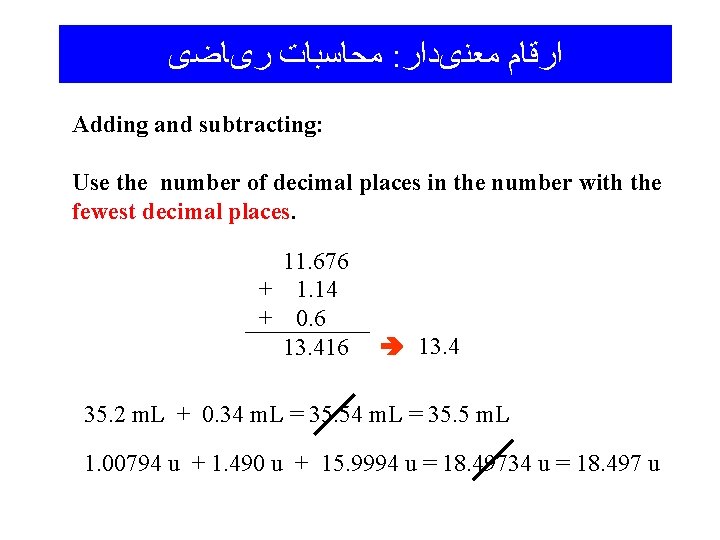
ﻣﺤﺎﺳﺒﺎﺕ ﺭیﺎﺿی : ﺍﺭﻗﺎﻡ ﻣﻌﻨیﺩﺍﺭ Adding and subtracting: Use the number of decimal places in the number with the fewest decimal places. 11. 676 + 1. 14 + 0. 6 13. 416 13. 4 35. 2 m. L + 0. 34 m. L = 35. 5 m. L 1. 00794 u + 1. 490 u + 15. 9994 u = 18. 49734 u = 18. 497 u

ﻣﺤﺎﺳﺒﺎﺕ ﺭیﺎﺿی : ﺍﺭﻗﺎﻡ ﻣﻌﻨیﺩﺍﺭ Multiplying and dividing: Use the fewest significant figures. 4 3 0. 01208 0. 236 = 0. 51186 = 5. 12 10 -3 (9. 5760 g)/(12. 2 m. L) = 0. 785 g/m. L 5 3


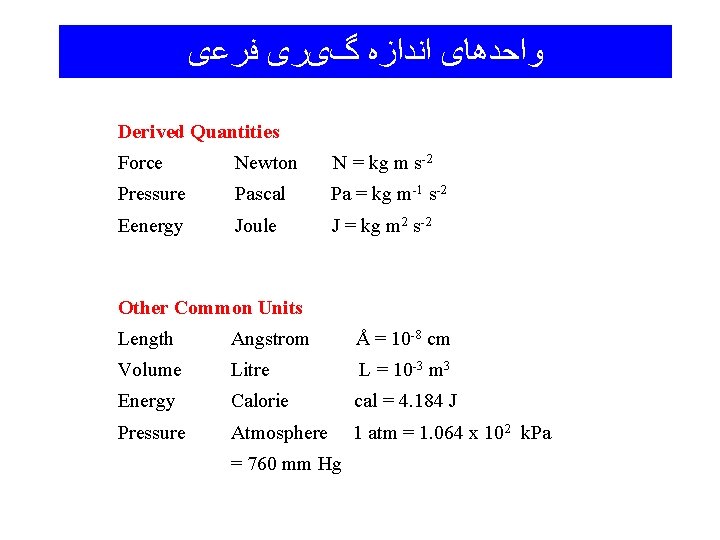
ﻭﺍﺣﺪﻫﺎی ﺍﻧﺪﺍﺯﻩ گیﺮی ﻓﺮﻋی Derived Quantities Force Newton N = kg m s-2 Pressure Pascal Pa = kg m-1 s-2 Eenergy Joule J = kg m 2 s-2 Other Common Units Length Angstrom Å = 10 -8 cm Volume Litre L = 10 -3 m 3 Energy Calorie cal = 4. 184 J Pressure Atmosphere 1 atm = 1. 064 x 102 k. Pa = 760 mm Hg


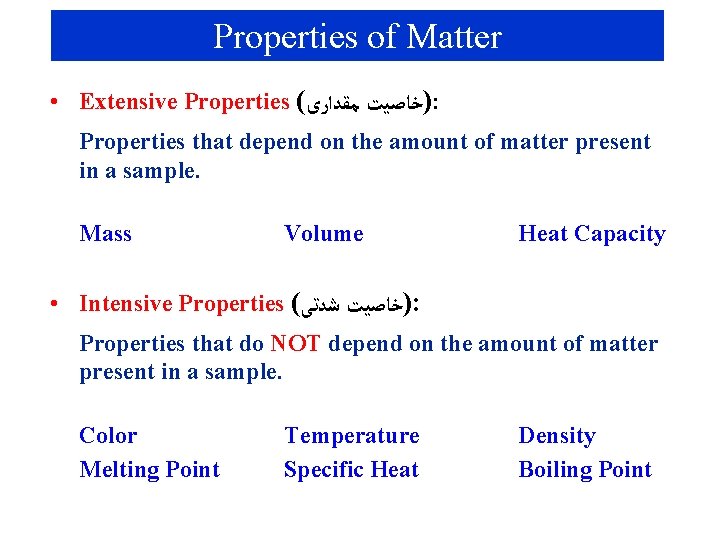
Properties of Matter • Extensive Properties ( )ﺧﺎﺻﻴﺖ ﻣﻘﺪﺍﺭﻯ : Properties that depend on the amount of matter present in a sample. Mass Volume Heat Capacity • Intensive Properties ( )ﺧﺎﺻﻴﺖ ﺷﺪﺗﻰ : Properties that do NOT depend on the amount of matter present in a sample. Color Melting Point Temperature Specific Heat Density Boiling Point

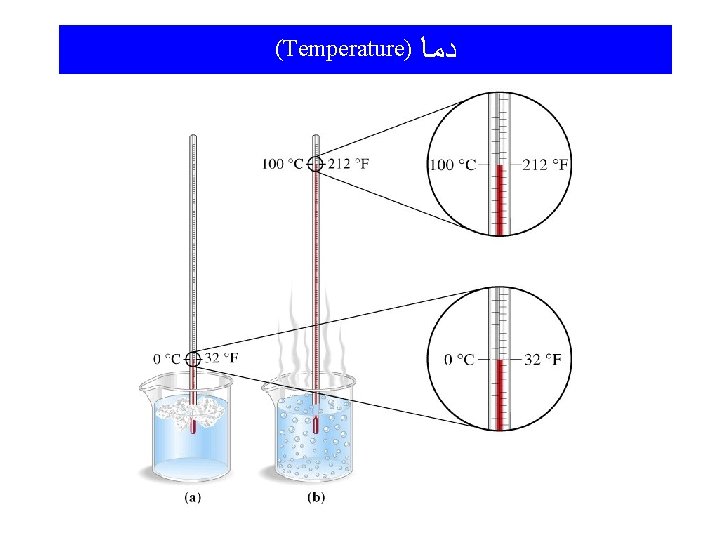
Temperature and Thermal Energy Temperature: A measure of the “hotness”and “coldness” of an object; a measure of the average kinetic energy of the atoms and molecules of the object. The higher the temperature, the more kinetic energy the atoms and/or molecules have. Thermal Energy: Often called “heat”, it is the form of energy toward which all other forms tend to go.

(Temperature) ﺩﻣﺎ



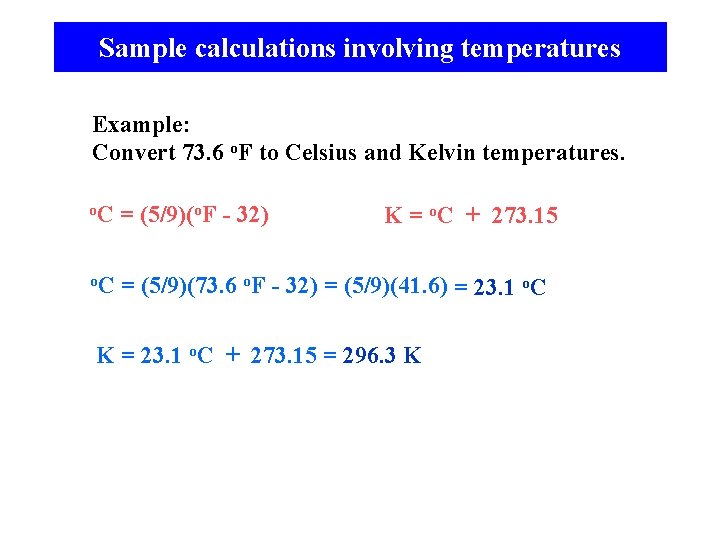
Sample calculations involving temperatures Example: Convert 73. 6 o. F to Celsius and Kelvin temperatures. o. C = (5/9)(o. F - 32) o. C = (5/9)(73. 6 o. F - 32) = (5/9)(41. 6) = 23. 1 o. C K = o. C + 273. 15 K = 23. 1 o. C + 273. 15 = 296. 3 K



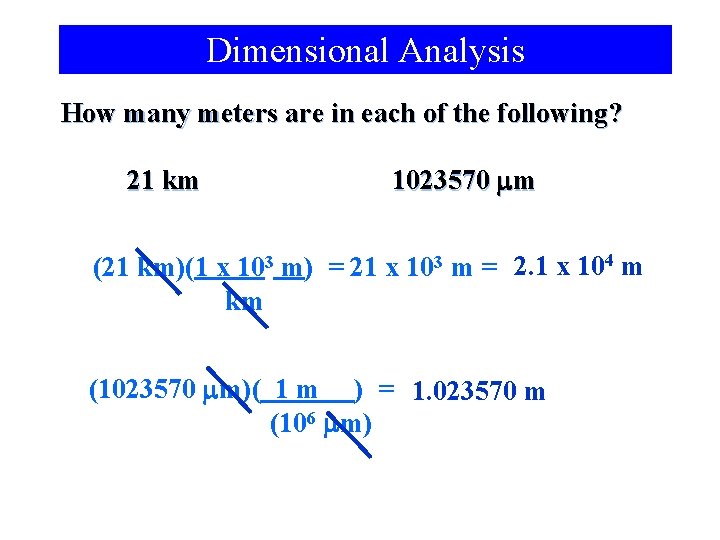
Dimensional Analysis How many meters are in each of the following? 21 km 1023570 mm (21 km)(1 x 103 m) = 21 x 103 m = 2. 1 x 104 m km (1023570 mm)( 1 m ) = 1. 023570 m (106 mm)

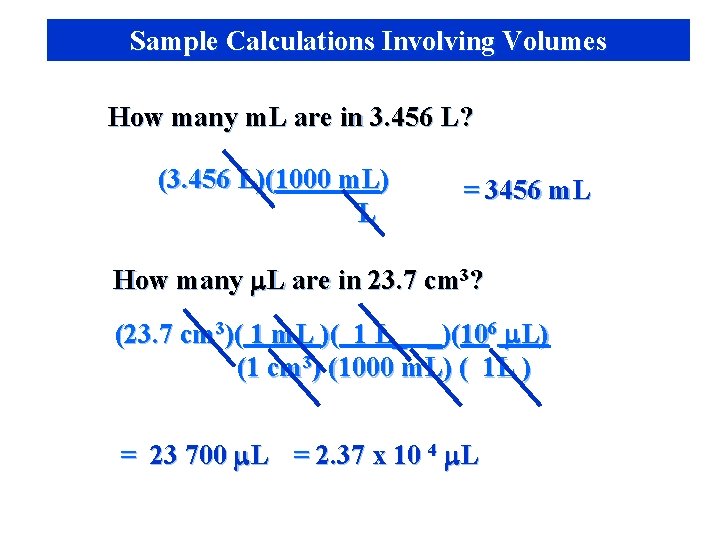
Sample Calculations Involving Volumes How many m. L are in 3. 456 L? (3. 456 L)(1000 m. L) L = 3456 m. L How many m. L are in 23. 7 cm 3? (23. 7 cm 3)( 1 m. L )( 1 L_ _)(106 m. L) (1 cm 3) (1000 m. L) ( 1 L ) = 23 700 m. L = 2. 37 x 10 4 m. L

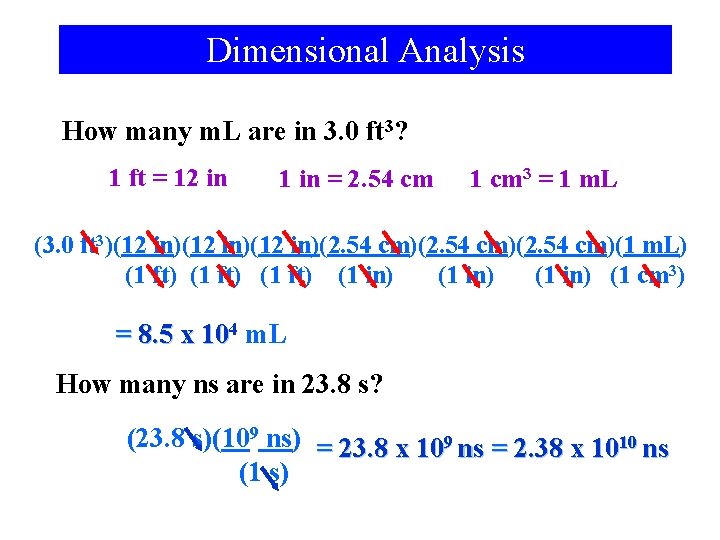
Dimensional Analysis How many m. L are in 3. 0 ft 3? 1 ft = 12 in 1 in = 2. 54 cm 1 cm 3 = 1 m. L (3. 0 ft 3)(12 in)(12 in)(2. 54 cm)(2. 54 cm)(1 m. L) (1 ft) (1 in) (1 cm 3) = 8. 5 x 104 m. L How many ns are in 23. 8 s? (23. 8 s)(109 ns) = 23. 8 x 109 ns = 2. 38 x 1010 ns (1 s)

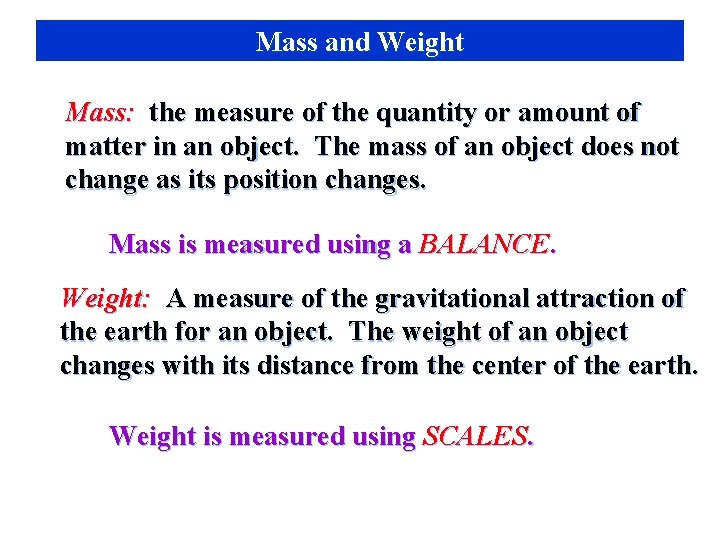
Mass and Weight Mass: the measure of the quantity or amount of matter in an object. The mass of an object does not change as its position changes. Mass is measured using a BALANCE. Weight: A measure of the gravitational attraction of the earth for an object. The weight of an object changes with its distance from the center of the earth. Weight is measured using SCALES.

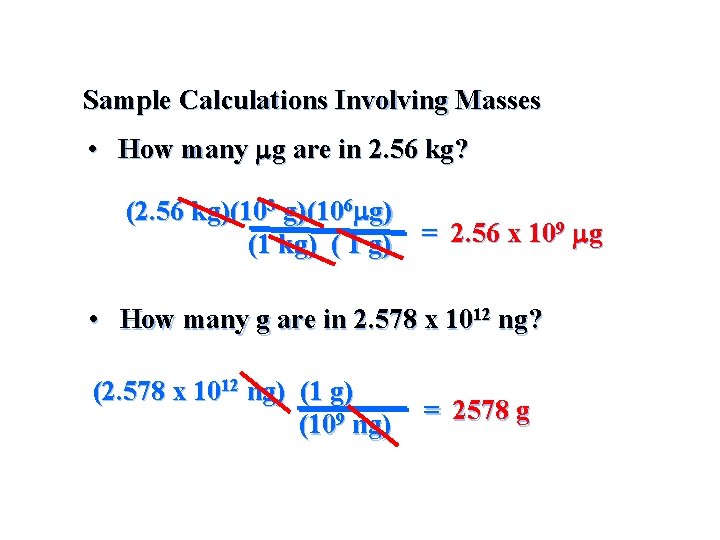
Sample Calculations Involving Masses • How many mg are in 2. 56 kg? (2. 56 kg)(103 g)(106 mg) 9 mg = 2. 56 x 10 (1 kg) ( 1 g) • How many g are in 2. 578 x 1012 ng? (2. 578 x 1012 ng) (109 ng) = 2578 g

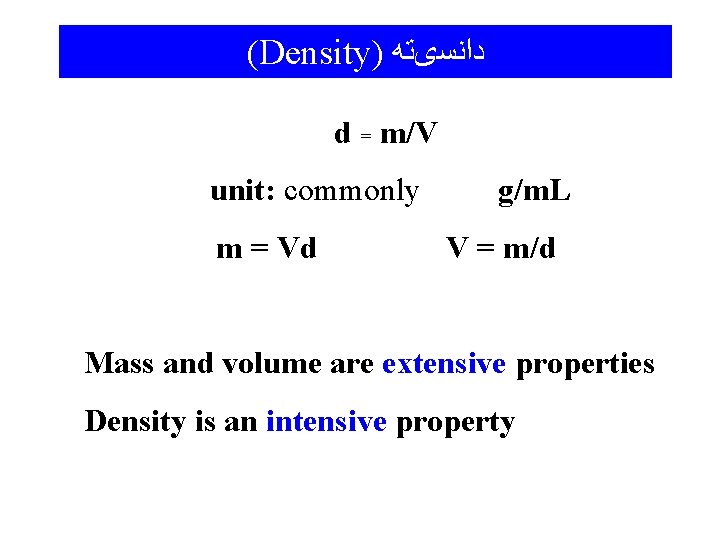
(Density) ﺩﺍﻧﺴیﺘﻪ d = m/V unit: commonly m = Vd g/m. L V = m/d Mass and volume are extensive properties Density is an intensive property

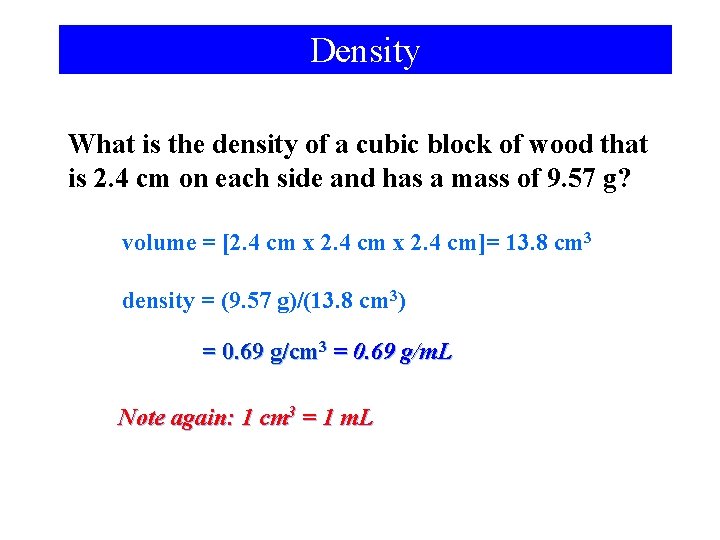
Density What is the density of a cubic block of wood that is 2. 4 cm on each side and has a mass of 9. 57 g? volume = [2. 4 cm x 2. 4 cm]= 13. 8 cm 3 density = (9. 57 g)/(13. 8 cm 3) = 0. 69 g/cm 3 = 0. 69 g/m. L Note again: 1 cm 3 = 1 m. L

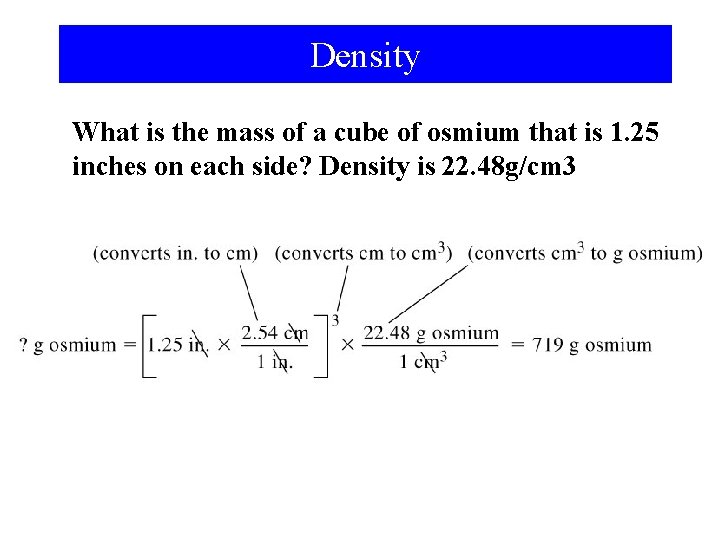
Density What is the mass of a cube of osmium that is 1. 25 inches on each side? Density is 22. 48 g/cm 3

Wrong units

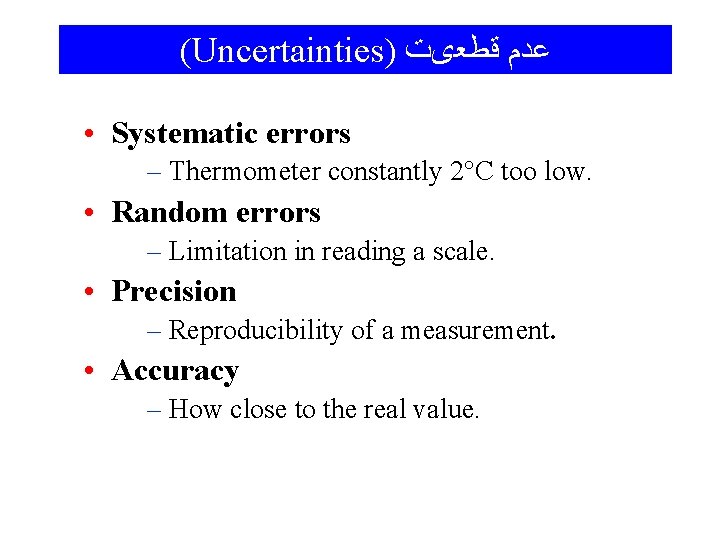
(Uncertainties) ﻋﺪﻡ ﻗﻄﻌیﺖ • Systematic errors – Thermometer constantly 2°C too low. • Random errors – Limitation in reading a scale. • Precision – Reproducibility of a measurement. • Accuracy – How close to the real value.


Chapter 1, Questions 3, 5, 12, 14, 17, 30, 38, 43, 49, 59, 60
 Nit calicut chemistry
Nit calicut chemistry Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 Organik kimya adlandırma öncelik sırası
Organik kimya adlandırma öncelik sırası General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science faculty
University of bridgeport computer science faculty University of bridgeport engineering
University of bridgeport engineering Alamo colleges faculty salary schedule
Alamo colleges faculty salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Faculty marshall usc advertising csv
Faculty marshall usc advertising csv Hyperparathyreosis
Hyperparathyreosis Penn state neurosurgery faculty
Penn state neurosurgery faculty Mercy faculty forward
Mercy faculty forward Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Faculty kutztown carelli
Faculty kutztown carelli Florida state university masters in computer science
Florida state university masters in computer science Faculty of business and economics mendel university in brno
Faculty of business and economics mendel university in brno Umd ee
Umd ee Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Ecu faculty manual
Ecu faculty manual Faculty of engineering shoubra
Faculty of engineering shoubra Singularity executive program
Singularity executive program Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Ucl cs
Ucl cs Symbiosis centre for distance learning (scdl)
Symbiosis centre for distance learning (scdl) Training and development process
Training and development process Https://faculty.washington.edu/chudler/stm0.html
Https://faculty.washington.edu/chudler/stm0.html Territorial matrix vs interterritorial matrix
Territorial matrix vs interterritorial matrix Parson kutztown
Parson kutztown Student faculty ratio for nba
Student faculty ratio for nba Kfupm faculty housing pictures
Kfupm faculty housing pictures Masaryk university medical faculty
Masaryk university medical faculty Faculty model of training
Faculty model of training Jey veerasamy
Jey veerasamy Fulbright faculty development program
Fulbright faculty development program Feup university of porto
Feup university of porto Faculty of organizational sciences
Faculty of organizational sciences Ldap cuni
Ldap cuni Electrotechnical faculty belgrade
Electrotechnical faculty belgrade