General Chemistry M R NaimiJamal Faculty of Chemistry



















- Slides: 48

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


Contents 3 -1 3 -2 3 -3 Molecular and Ionic Compounds Molecular Mass Composition

Molecules, Compounds, and Formulas • Molecule – smallest identifiable unit in which a pure substance can be divided and still retain the composition and chemical properties of the substance

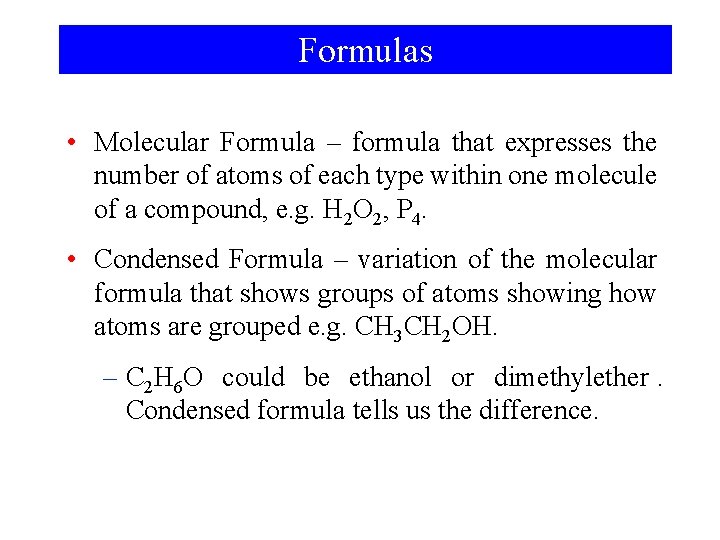
Formulas • Molecular Formula – formula that expresses the number of atoms of each type within one molecule of a compound, e. g. H 2 O 2, P 4. • Condensed Formula – variation of the molecular formula that shows groups of atoms showing how atoms are grouped e. g. CH 3 CH 2 OH. – C 2 H 6 O could be ethanol or dimethylether. Condensed formula tells us the difference.

Formulas • Structural Formula – shows how the atoms in a compound are connected – Lines between atoms represent chemical bonds • A chemical bond is the interaction between two or more atoms that holds them together


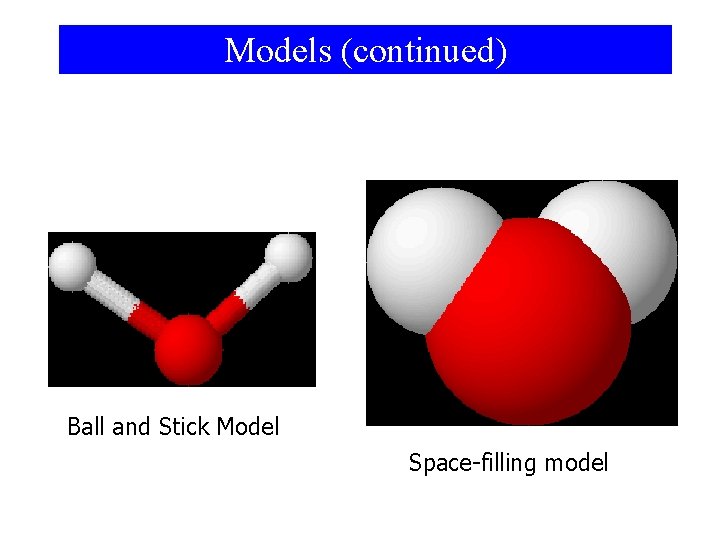
Models • Since the particulate level is to small to be seen, we use models to represent particles – Models are a representation of something else • Two common models – Ball-and-stick model • Atoms are balls • Electrons are sticks that connect atoms – Space-filling model • Shows outer boundries of the particle in 3 D space

Models (continued) Ball and Stick Model Space-filling model

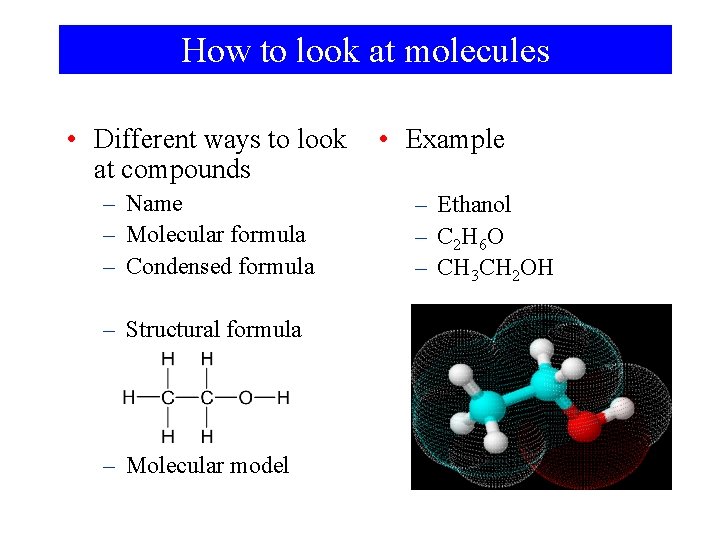
How to look at molecules • Different ways to look at compounds – Name – Molecular formula – Condensed formula – Structural formula – Molecular model • Example – Ethanol – C 2 H 6 O – CH 3 CH 2 OH

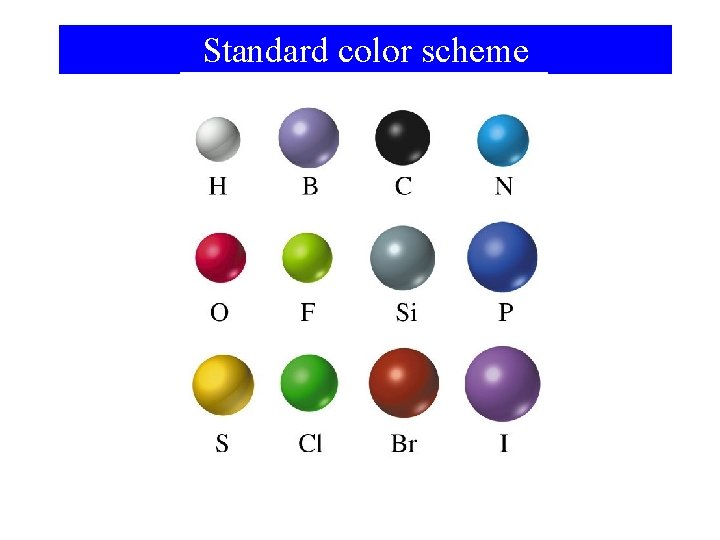
Standard color scheme

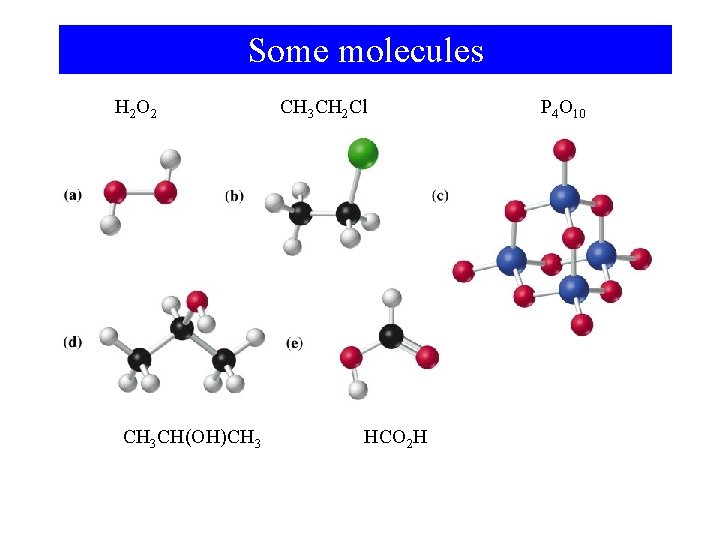
Some molecules H 2 O 2 CH 3 CH(OH)CH 3 CH 2 Cl HCO 2 H P 4 O 10

Inorganic molecules S 8 P 4

Ions and Ionic Compounds • An atom that either gains or loses electron(s) is an ion. • There is no change in the number of protons or neutrons in the nucleus of the atom. • Cation – has a positive charge from loss of electron(s). • Anion – has a negative charge from gain of electron(s).

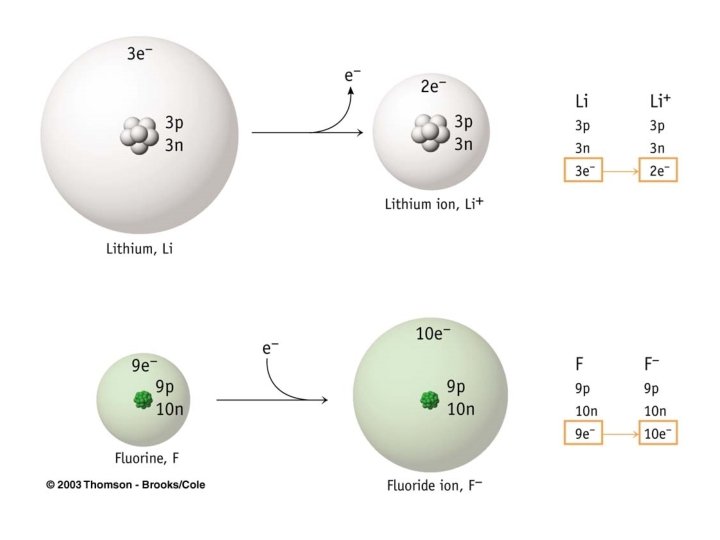
Cations • If an atom loses an electron (the electron is transferred to an atom of another element in a reaction), the atom has one more proton than electrons. – When this happens, a cation is formed – Cation is the positively charged ion – Li 1 e- + Li+

Anions • If an atom gains an electron (the electron is transferred to the atom from another element in a reaction), the atom has one more electron than protons. – When this happens, an anion is formed – Anion is the negatively charged ion – O + 2 e. O 2 -

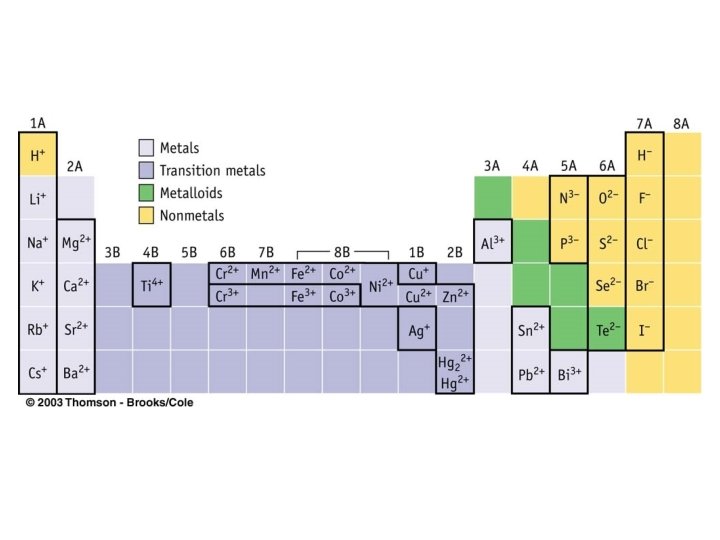
How to determine if atom will gain or lose electrons. • Metals generally lose electrons in the course of their reactions to form cations • Nonmetals frequently gain one or more electrons to form anions in the course of their chemical reactions.


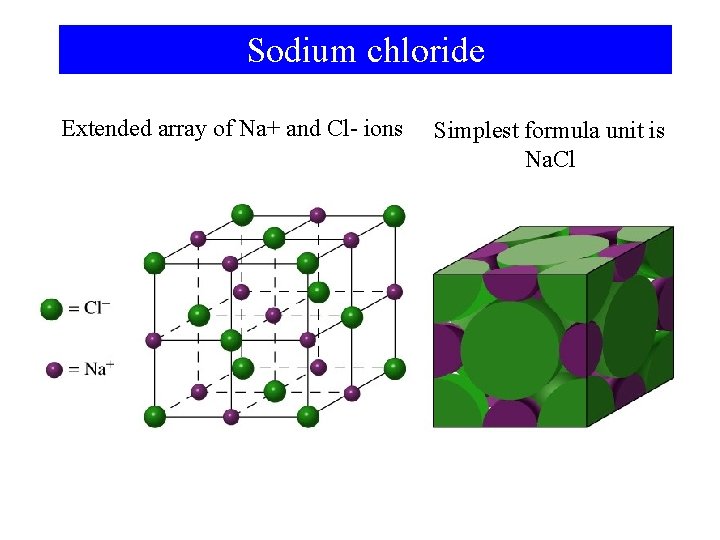
Sodium chloride Extended array of Na+ and Cl- ions Simplest formula unit is Na. Cl

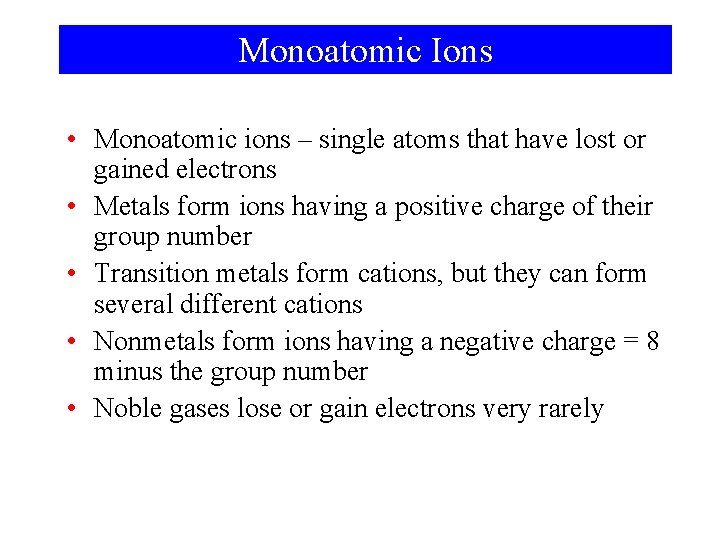
Monoatomic Ions • Monoatomic ions – single atoms that have lost or gained electrons • Metals form ions having a positive charge of their group number • Transition metals form cations, but they can form several different cations • Nonmetals form ions having a negative charge = 8 minus the group number • Noble gases lose or gain electrons very rarely


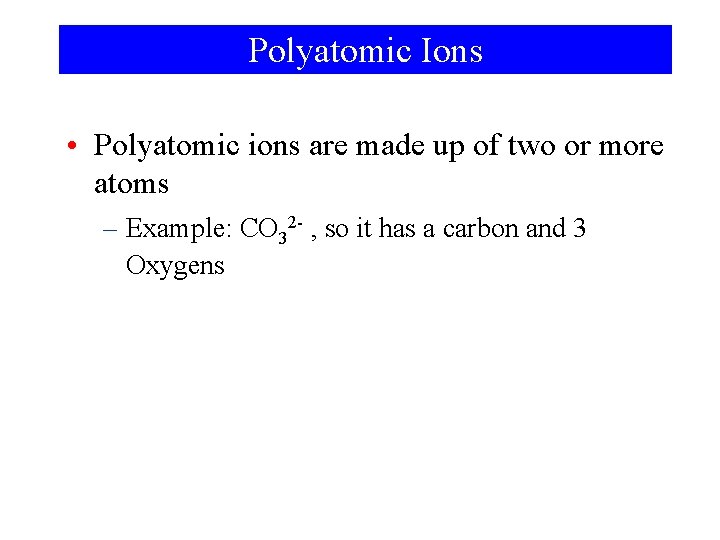
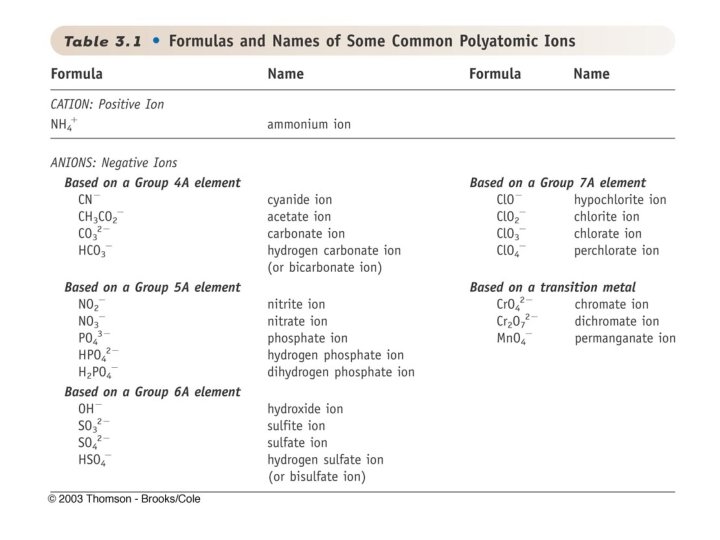
Polyatomic Ions • Polyatomic ions are made up of two or more atoms – Example: CO 32 - , so it has a carbon and 3 Oxygens


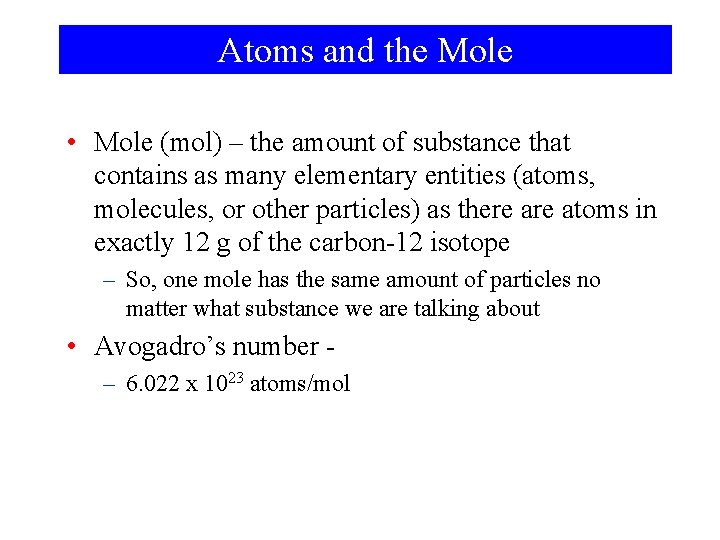
Atoms and the Mole • Mole (mol) – the amount of substance that contains as many elementary entities (atoms, molecules, or other particles) as there atoms in exactly 12 g of the carbon-12 isotope – So, one mole has the same amount of particles no matter what substance we are talking about • Avogadro’s number – 6. 022 x 1023 atoms/mol

Molar Mass • Molar mass – mass in grams of one mole of atoms – Sodium (Na) – 22. 99 g/mol = 6. 022 x 1023 Na atoms – Lead (Pb) – 207. 2 g/mol = 6. 022 x 1023 Pb atoms

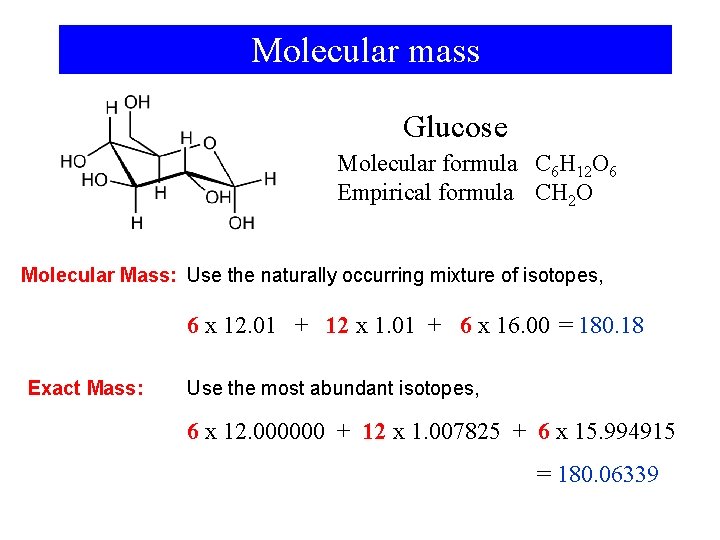
Molecular mass Glucose Molecular formula C 6 H 12 O 6 Empirical formula CH 2 O Molecular Mass: Use the naturally occurring mixture of isotopes, 6 x 12. 01 + 12 x 1. 01 + 6 x 16. 00 = 180. 18 Exact Mass: Use the most abundant isotopes, 6 x 12. 000000 + 12 x 1. 007825 + 6 x 15. 994915 = 180. 06339

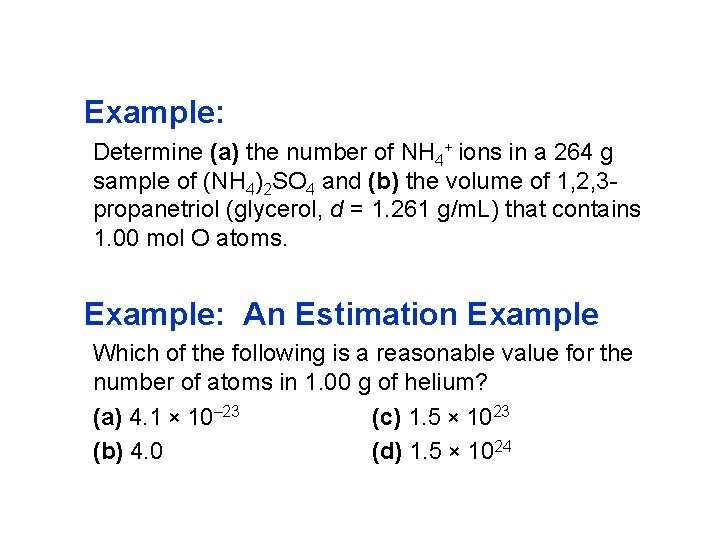
Example: Determine (a) the number of NH 4+ ions in a 264 g sample of (NH 4)2 SO 4 and (b) the volume of 1, 2, 3 propanetriol (glycerol, d = 1. 261 g/m. L) that contains 1. 00 mol O atoms. Example: An Estimation Example Which of the following is a reasonable value for the number of atoms in 1. 00 g of helium? (a) 4. 1 × 10– 23 (c) 1. 5 × 1023 (b) 4. 0 (d) 1. 5 × 1024

Percent Composition • Law of constant composition – any sample of a pure compound always consists of the same elements combined in the same proportion by mass • Three ways to express this – Number of atoms of each type per molecule – Mass of each element per mole of compound – Mass percent - mass of each element in the compound relative to the total mass • Percent Composition – mass percent of each element

Mass Percent Composition from Chemical Formulas The mass percent composition of a compound refers to the proportion of the constituent elements, expressed as the number of grams of each element per 100 grams of the compound. In other words … g element X % element X = ––––––– OR … 100 g compound g element % element = –––––– × 100 g compound

Percentage Composition of Butane

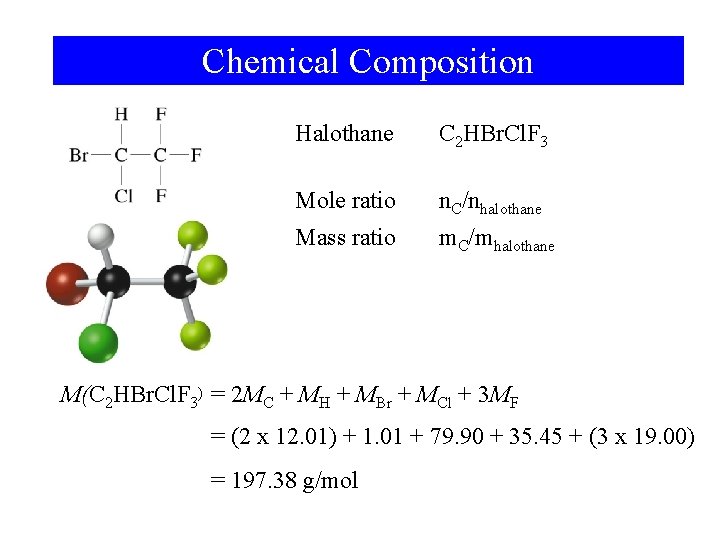
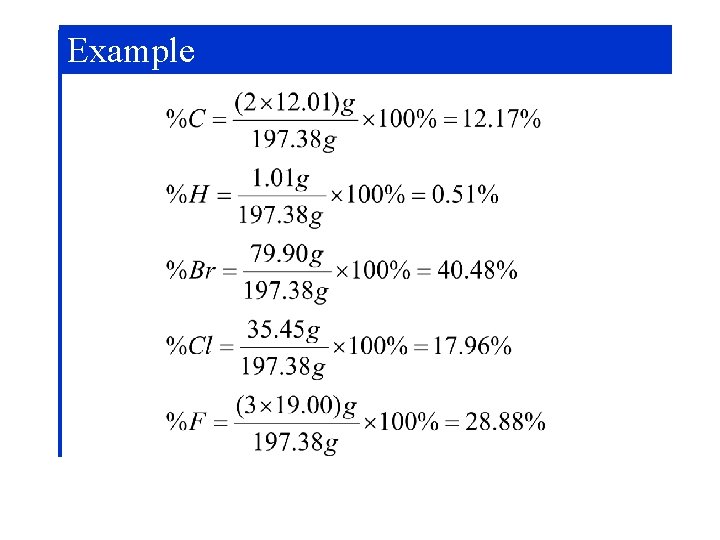
Chemical Composition Halothane C 2 HBr. Cl. F 3 Mole ratio n. C/nhalothane Mass ratio m. C/mhalothane M(C 2 HBr. Cl. F 3) = 2 MC + MH + MBr + MCl + 3 MF = (2 x 12. 01) + 1. 01 + 79. 90 + 35. 45 + (3 x 19. 00) = 197. 38 g/mol

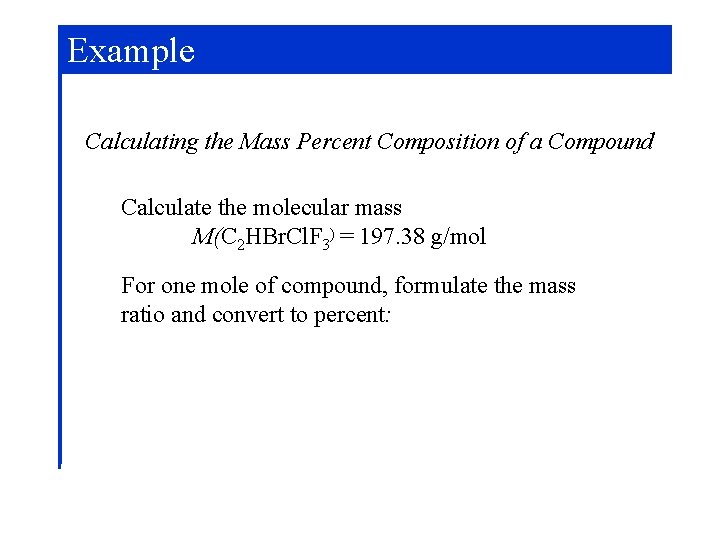
Example Calculating the Mass Percent Composition of a Compound Calculate the molecular mass M(C 2 HBr. Cl. F 3) = 197. 38 g/mol For one mole of compound, formulate the mass ratio and convert to percent:

Example

Example: Calculate, to four significant figures, the mass percent of each element in ammonium nitrate. Example: How many grams of nitrogen are present in 46. 34 g ammonium nitrate? Example: An Estimation Example Without doing detailed calculations, determine which of these compounds contains the greatest mass of sulfur per gram of compound: barium sulfate, lithium sulfate, sodium sulfate, or lead sulfate.

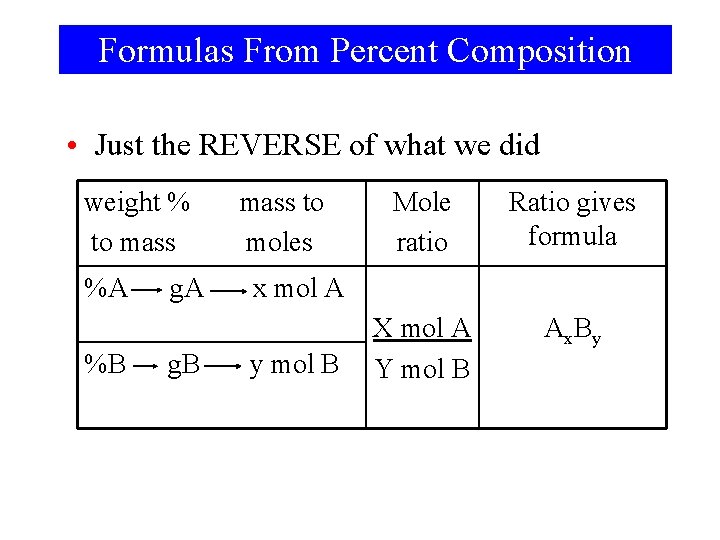
Formulas From Percent Composition • Just the REVERSE of what we did weight % to mass %A %B g. A g. B mass to moles Mole ratio Ratio gives formula X mol A Y mol B Ax. By x mol A y mol B

Formulas From Percent Composition • This tells us the ratio not the total number of atoms • In a molecular formula, we must know the total number of atoms • So, NH 2 is the empirical formula of hydrazine – Hydrazine could be NH 2, N 2 H 4, N 3 H 6 …. – If given, molar mass of hydrazine is 32. 0 g/mol, we know the correct molecular formula is N 2 H 4

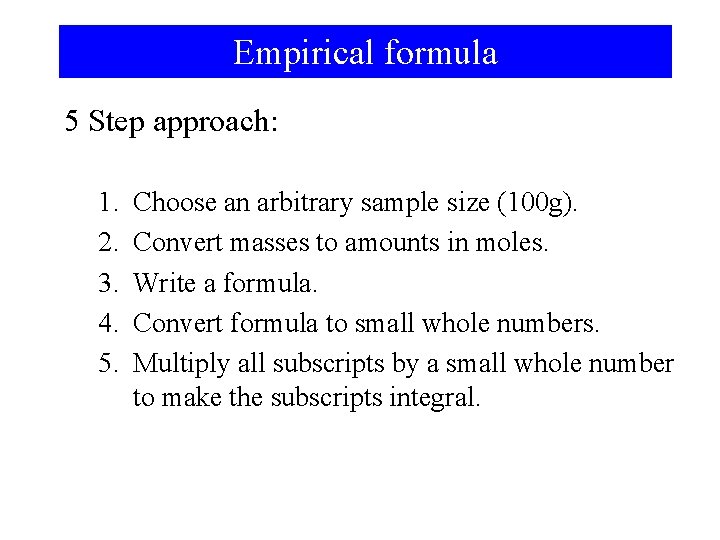
Empirical formula 5 Step approach: 1. 2. 3. 4. 5. Choose an arbitrary sample size (100 g). Convert masses to amounts in moles. Write a formula. Convert formula to small whole numbers. Multiply all subscripts by a small whole number to make the subscripts integral.

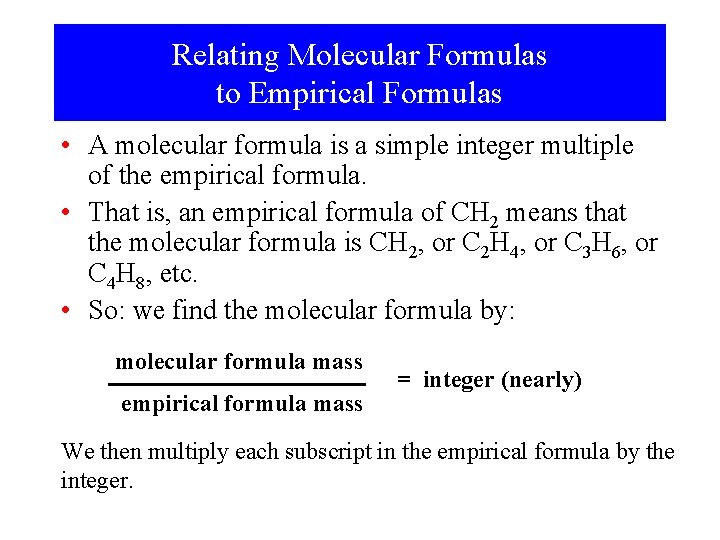
Relating Molecular Formulas to Empirical Formulas • A molecular formula is a simple integer multiple of the empirical formula. • That is, an empirical formula of CH 2 means that the molecular formula is CH 2, or C 2 H 4, or C 3 H 6, or C 4 H 8, etc. • So: we find the molecular formula by: molecular formula mass empirical formula mass = integer (nearly) We then multiply each subscript in the empirical formula by the integer.

Example 3. 11 The empirical formula of hydroquinone, a chemical used in photography, is C 3 H 3 O, and its molecular mass is 110 u. What is its molecular formula?

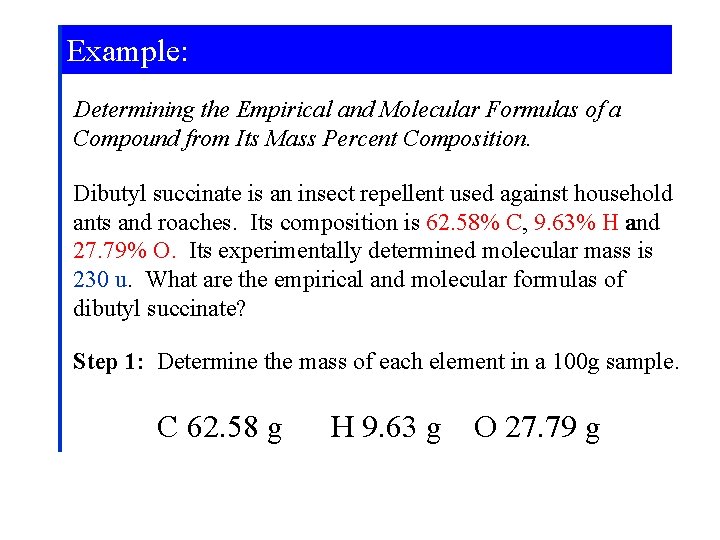
Example: Determining the Empirical and Molecular Formulas of a Compound from Its Mass Percent Composition. Dibutyl succinate is an insect repellent used against household ants and roaches. Its composition is 62. 58% C, 9. 63% H and 27. 79% O. Its experimentally determined molecular mass is 230 u. What are the empirical and molecular formulas of dibutyl succinate? Step 1: Determine the mass of each element in a 100 g sample. C 62. 58 g H 9. 63 g O 27. 79 g

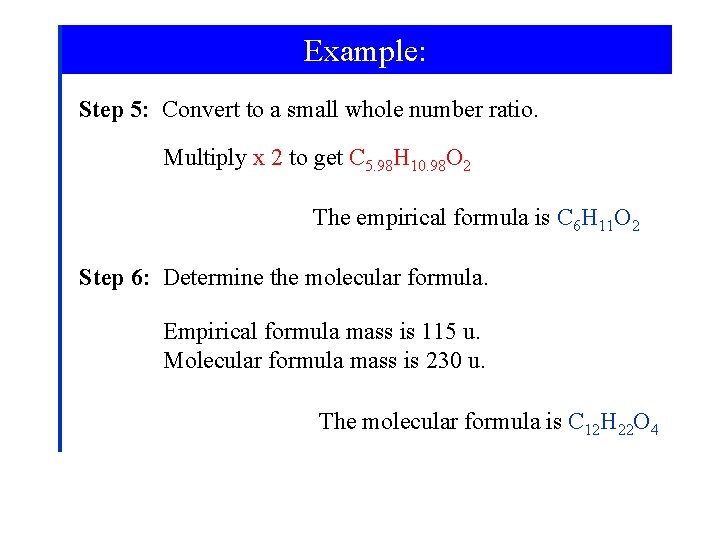
Example: Step 2: Convert masses to amounts in moles. Step 3: Write a tentative formula. C 5. 21 H 9. 55 O 1. 74 Step 4: Convert to small whole numbers. C 2. 99 H 5. 49 O

Example: Step 5: Convert to a small whole number ratio. Multiply x 2 to get C 5. 98 H 10. 98 O 2 The empirical formula is C 6 H 11 O 2 Step 6: Determine the molecular formula. Empirical formula mass is 115 u. Molecular formula mass is 230 u. The molecular formula is C 12 H 22 O 4

Example: Phenol, a general disinfectant, has the composition 76. 57% C, 6. 43% H, and 17. 00% O by mass. Determine its empirical formula. Example: Diethylene glycol, used in antifreeze, as a softening agent for textile fibers and some leathers, and as a moistening agent for glues and paper, has the composition 45. 27% C, 9. 50% H, and 45. 23% O by mass. Determine its empirical formula.

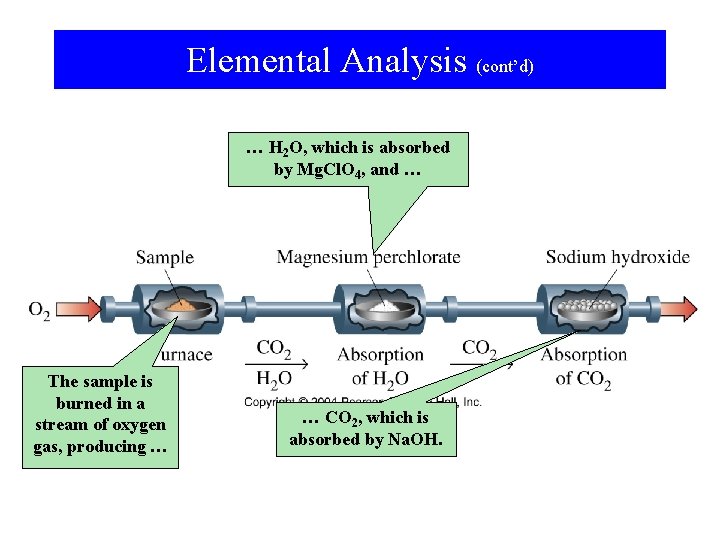
Elemental Analysis … • … is one method of determining empirical formulas in the laboratory. • This method is used primarily for simple organic compounds (that contain carbon, hydrogen, oxygen). – The organic compound is burned in oxygen. – The products of combustion (usually CO 2 and H 2 O) are weighed. – The amount of each element is determined from the mass of products.

Elemental Analysis (cont’d) … H 2 O, which is absorbed by Mg. Cl. O 4, and … The sample is burned in a stream of oxygen gas, producing … … CO 2, which is absorbed by Na. OH.

Elemental Analysis (cont’d) If our sample were CH 3 OH, every two molecules of CH 3 OH … … would give two molecules of CO 2 … … and four molecules of H 2 O.

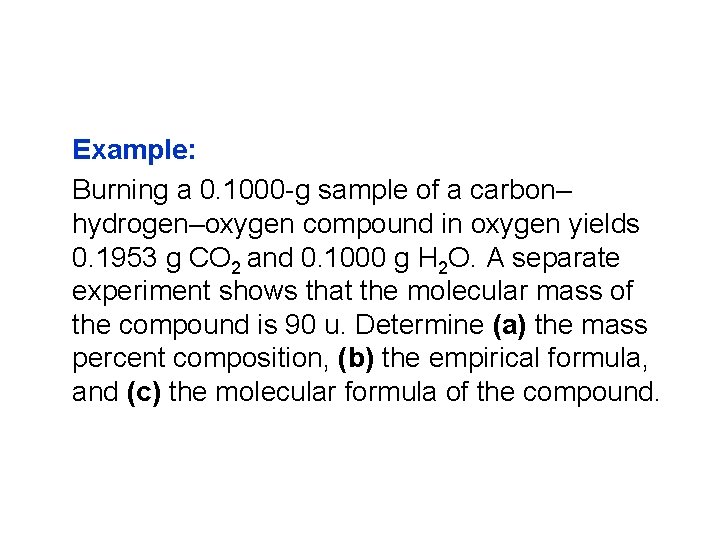
Example: Burning a 0. 1000 -g sample of a carbon– hydrogen–oxygen compound in oxygen yields 0. 1953 g CO 2 and 0. 1000 g H 2 O. A separate experiment shows that the molecular mass of the compound is 90 u. Determine (a) the mass percent composition, (b) the empirical formula, and (c) the molecular formula of the compound.

Chapter 3 Questions 1, 4, 19, 30, 35, 40, 41, 46, 54, 57, 60, 69
 Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 Klorpentan
Klorpentan General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science faculty
University of bridgeport computer science faculty University of bridgeport computer science
University of bridgeport computer science Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Http://www-bcf.usc.edu/~gareth/isl/advertising.csv
Http://www-bcf.usc.edu/~gareth/isl/advertising.csv Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Penn state neurosurgery
Penn state neurosurgery Mercy faculty forward
Mercy faculty forward Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Faculty kutztown carelli
Faculty kutztown carelli Florida state university computer science
Florida state university computer science Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd ece faculty
Umd ece faculty Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Ecu faculty 180
Ecu faculty 180 Faculty of engineering shoubra
Faculty of engineering shoubra Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Brad karp ucl
Brad karp ucl Exam queries scdl
Exam queries scdl Training and development metrics
Training and development metrics