General Chemistry M R NaimiJamal Faculty of Chemistry




![Example: What is the concentration at 100 s? [H 2 O 2]i = 2. Example: What is the concentration at 100 s? [H 2 O 2]i = 2.](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-12.jpg)


![Example: R 3 = k [Hg. Cl 2]3 m[C 2 O 42 -]3 n Example: R 3 = k [Hg. Cl 2]3 m[C 2 O 42 -]3 n](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-18.jpg)
![Example: R 2 = k[Hg. Cl 2]21[C 2 O 42 -]2 n = k(0. Example: R 2 = k[Hg. Cl 2]21[C 2 O 42 -]2 n = k(0.](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-19.jpg)
![Example: R = k [Hg. Cl 2] [C 2 O 42 -] 2 First Example: R = k [Hg. Cl 2] [C 2 O 42 -] 2 First](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-20.jpg)
![15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k 15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-21.jpg)
![Integrated Rate Law -Δ[A] Δt =k Move to the -d[A] infinitesimal dt =k And Integrated Rate Law -Δ[A] Δt =k Move to the -d[A] infinitesimal dt =k And](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-22.jpg)
![Second-Order Reaction 1 1 = kt + [A]t [A]0 Second-Order Reaction 1 1 = kt + [A]t [A]0](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-29.jpg)
![Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-31.jpg)

- Slides: 46

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


Contents 14 -1 The Rate of a Chemical Reaction 14 -2 Measuring Reaction Rates 14 -3 Effect of Concentration on Reaction Rates: The Rate Law 14 -4 Zero-Order Reactions 14 -5 First-Order Reactions 14 -6 Second-Order Reactions 14 -7 Reaction Kinetics: A Summary

Contents 14 -8 Theoretical Models for Chemical Kinetics 14 -9 The Effect of Temperature on Reaction Rates 14 -10 Reaction Mechanisms 14 -11 Catalysis Focus On Combustion and Explosions



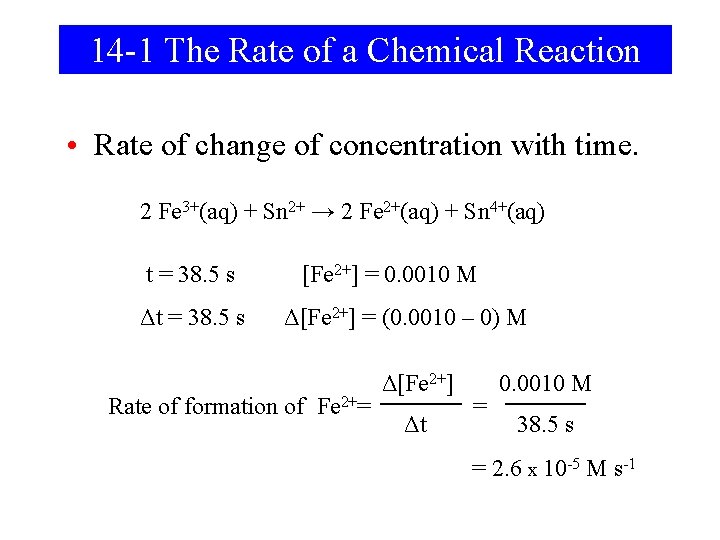
14 -1 The Rate of a Chemical Reaction • Rate of change of concentration with time. 2 Fe 3+(aq) + Sn 2+ → 2 Fe 2+(aq) + Sn 4+(aq) t = 38. 5 s Δt = 38. 5 s [Fe 2+] = 0. 0010 M Δ[Fe 2+] = (0. 0010 – 0) M Rate of formation of Fe 2+= Δ[Fe 2+] Δt = 0. 0010 M 38. 5 s = 2. 6 x 10 -5 M s-1

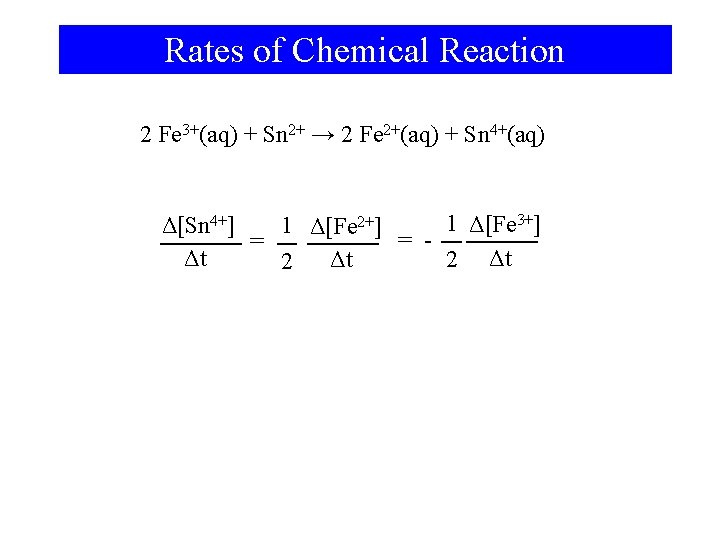
Rates of Chemical Reaction 2 Fe 3+(aq) + Sn 2+ → 2 Fe 2+(aq) + Sn 4+(aq) 1 Δ[Fe 3+] Δ[Sn 4+] 1 Δ[Fe 2+] = = Δt 2 Δt Δt 2

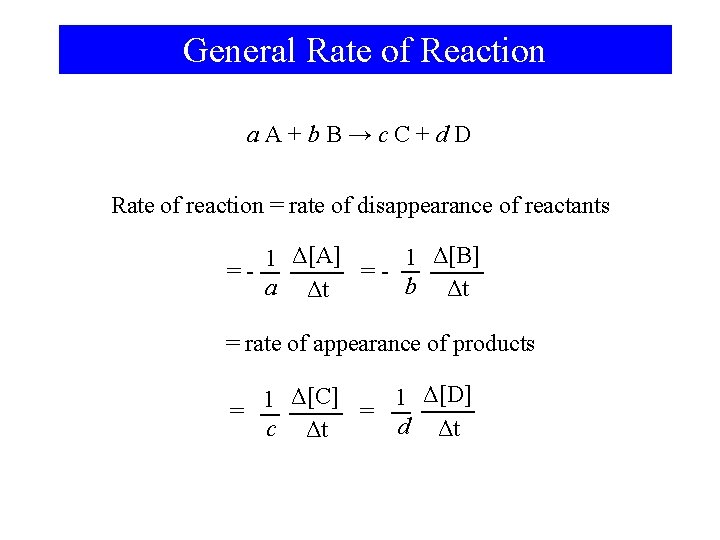
General Rate of Reaction a. A+b. B→c. C+d. D Rate of reaction = rate of disappearance of reactants 1 Δ[B] 1 Δ[A] ==b Δt a Δt = rate of appearance of products 1 Δ[D] 1 Δ[C] = = d Δt c Δt

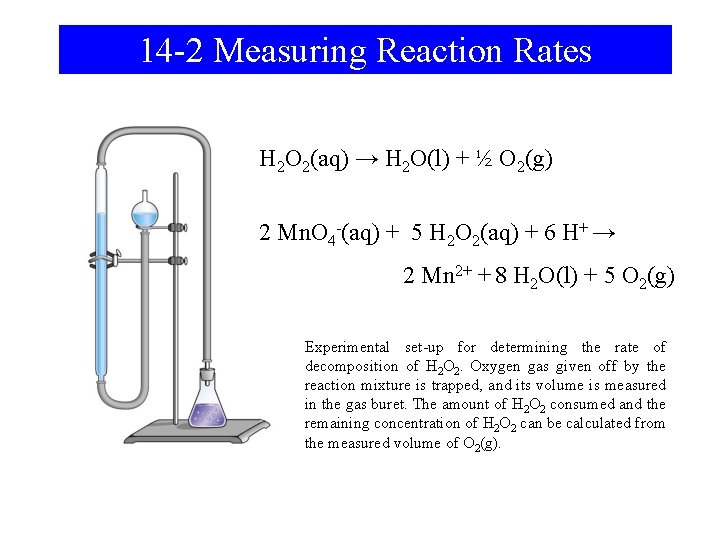
14 -2 Measuring Reaction Rates H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) 2 Mn. O 4 -(aq) + 5 H 2 O 2(aq) + 6 H+ → 2 Mn 2+ + 8 H 2 O(l) + 5 O 2(g) Experimental set-up for determining the rate of decomposition of H 2 O 2. Oxygen gas given off by the reaction mixture is trapped, and its volume is measured in the gas buret. The amount of H 2 O 2 consumed and the remaining concentration of H 2 O 2 can be calculated from the measured volume of O 2(g).

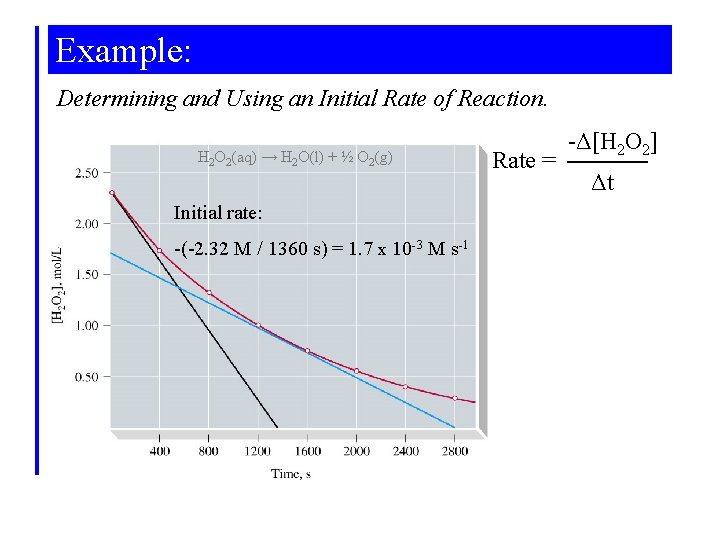
Example: Determining and Using an Initial Rate of Reaction. H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) Initial rate: -(-2. 32 M / 1360 s) = 1. 7 x 10 -3 M s-1 Rate = -Δ[H 2 O 2] Δt
![Example What is the concentration at 100 s H 2 O 2i 2 Example: What is the concentration at 100 s? [H 2 O 2]i = 2.](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-12.jpg)
Example: What is the concentration at 100 s? [H 2 O 2]i = 2. 32 M Rate = 1. 7 x 10 -3 M s-1 = - Δ[H 2 O 2] Δt -Δ[H 2 O 2] = -([H 2 O 2]f - [H 2 O 2]i) = 1. 7 x 10 -3 M s-1 x Δt [H 2 O 2]100 s – 2. 32 M = -1. 7 x 10 -3 M s-1 x 100 s [H 2 O 2]100 s = 2. 32 M - 0. 17 M = 2. 17 M

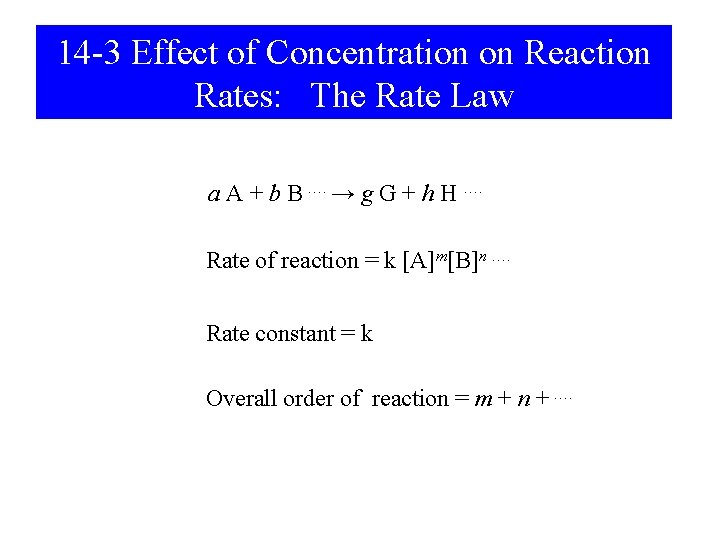
14 -3 Effect of Concentration on Reaction Rates: The Rate Law a A + b B …. → g G + h H …. Rate of reaction = k [A]m[B]n …. Rate constant = k Overall order of reaction = m + n + ….



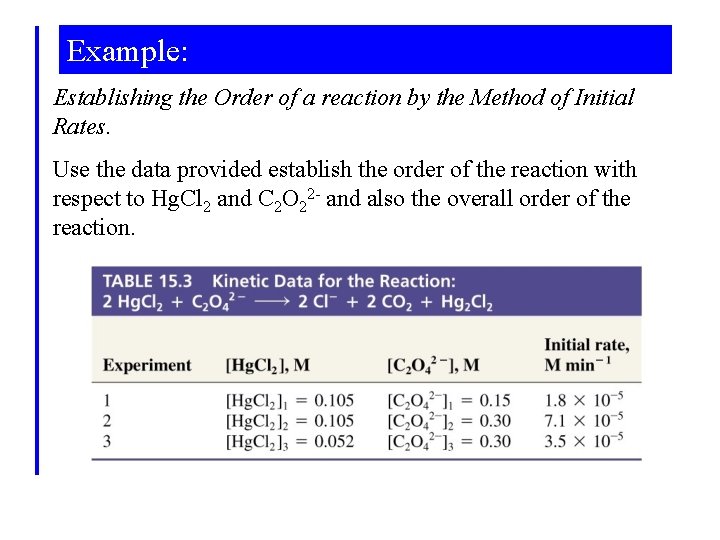
Example: Establishing the Order of a reaction by the Method of Initial Rates. Use the data provided establish the order of the reaction with respect to Hg. Cl 2 and C 2 O 22 - and also the overall order of the reaction.

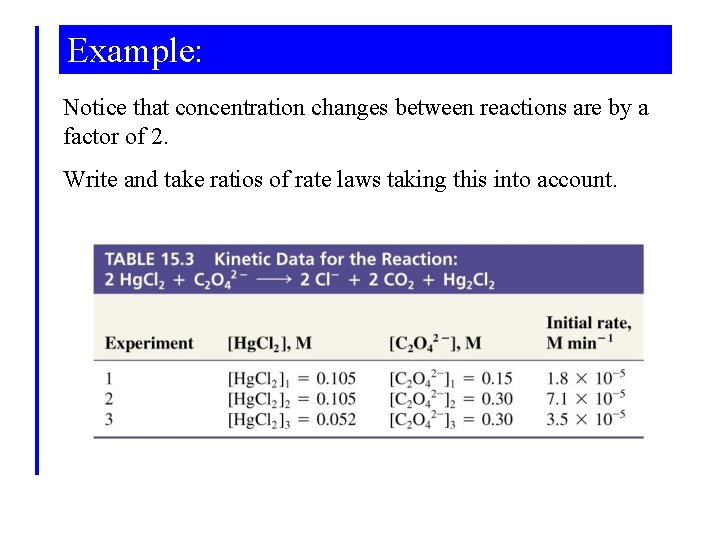
Example: Notice that concentration changes between reactions are by a factor of 2. Write and take ratios of rate laws taking this into account.
![Example R 3 k Hg Cl 23 mC 2 O 42 3 n Example: R 3 = k [Hg. Cl 2]3 m[C 2 O 42 -]3 n](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-18.jpg)
Example: R 3 = k [Hg. Cl 2]3 m[C 2 O 42 -]3 n R 2 = k[Hg. Cl 2]2 m[C 2 O 42 -]2 n k (0. 105)m [C 2 O 42 -]2 n R 2 = R 3 k (0. 052)m [C 2 O 42 -]3 n -5 7. 1 x 10 R 2 = 2 m = 3. 5 x 10 -5 R 3 2 m = 2. 0 therefore m = 1. 0
![Example R 2 kHg Cl 221C 2 O 42 2 n k0 Example: R 2 = k[Hg. Cl 2]21[C 2 O 42 -]2 n = k(0.](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-19.jpg)
Example: R 2 = k[Hg. Cl 2]21[C 2 O 42 -]2 n = k(0. 105)(0. 30)n R 1 = k[Hg. Cl 2]11[C 2 O 42 -]1 n = k(0. 105)(0. 15)n R 2 = R 1 k(0. 105)(0. 30)n k(0. 105)(0. 15)n -5 (0. 30)n 7. 1 x 10 n = = 2 (0. 15)n 1. 8 x 10 -5 2 n = 3. 98 therefore n = 2. 0 = 3. 94
![Example R k Hg Cl 2 C 2 O 42 2 First Example: R = k [Hg. Cl 2] [C 2 O 42 -] 2 First](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-20.jpg)
Example: R = k [Hg. Cl 2] [C 2 O 42 -] 2 First order + Second order = Third Order
![15 4 ZeroOrder Reactions A products Rrxn k A0 Rrxn k 15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-21.jpg)
15 -4 Zero-Order Reactions A → products Rrxn = k [A]0 Rrxn = k [k] = mol L-1 s-1
![Integrated Rate Law ΔA Δt k Move to the dA infinitesimal dt k And Integrated Rate Law -Δ[A] Δt =k Move to the -d[A] infinitesimal dt =k And](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-22.jpg)
Integrated Rate Law -Δ[A] Δt =k Move to the -d[A] infinitesimal dt =k And integrate from 0 to time t t [A]t -∫ d[A] [A]0 = ∫ k dt 0 -[A]t + [A]0 = kt [A]t = [A]0 - kt

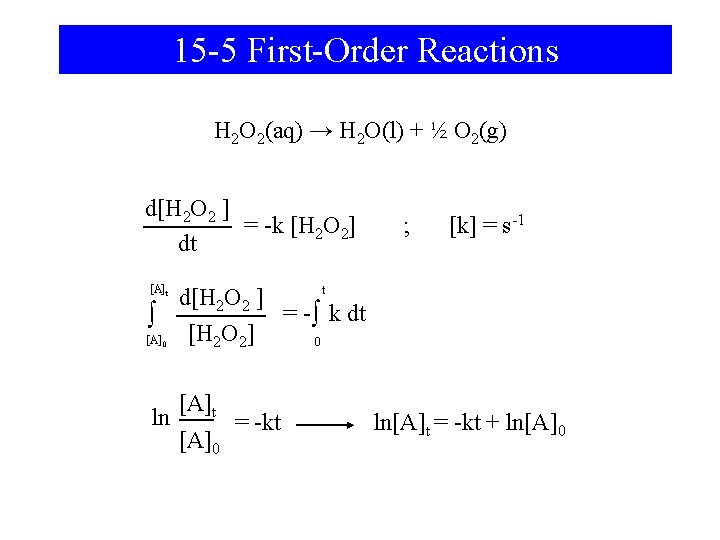
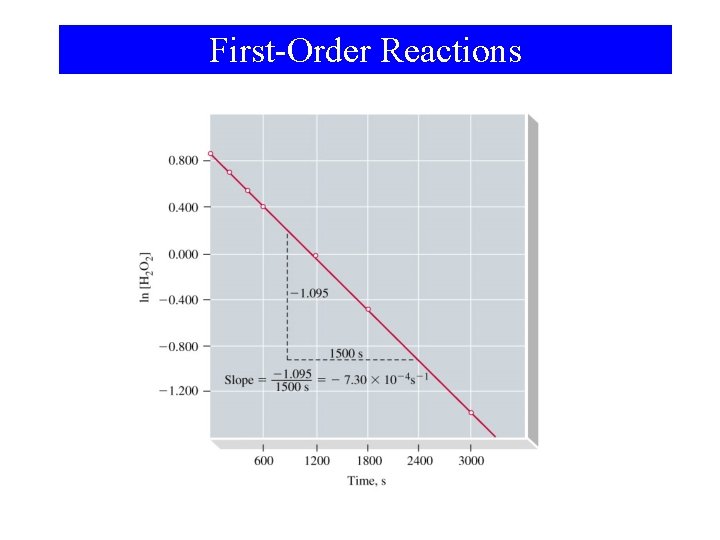
15 -5 First-Order Reactions H 2 O 2(aq) → H 2 O(l) + ½ O 2(g) d[H 2 O 2 ] = -k [H 2 O 2] dt ; [k] = s-1 [A]t t d[H 2 O 2 ] = - ∫ k dt ∫ [H 2 O 2] [A] 0 0 ln [A]t [A]0 = -kt ln[A]t = -kt + ln[A]0

First-Order Reactions

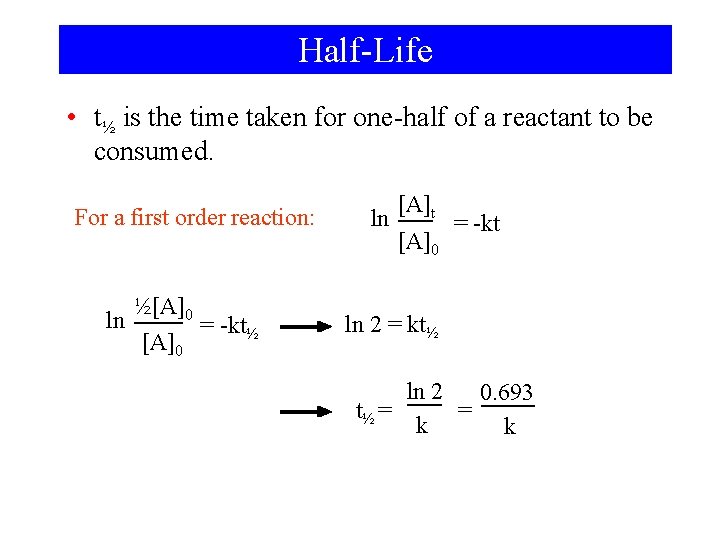
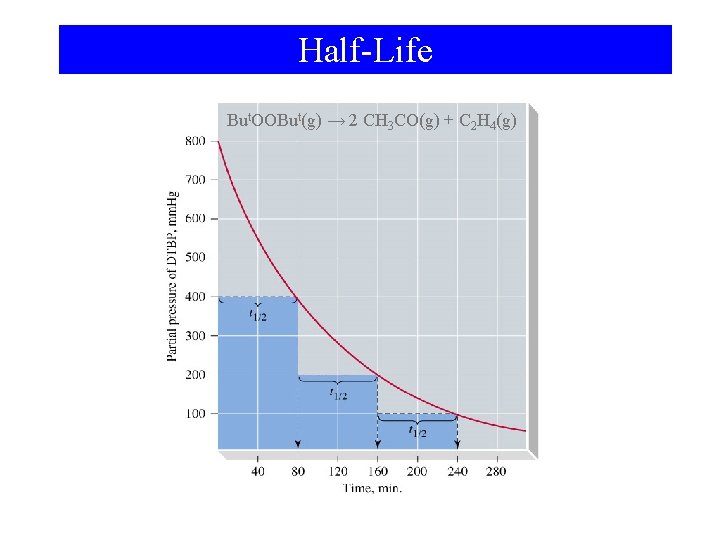
Half-Life • t½ is the time taken for one-half of a reactant to be consumed. For a first order reaction: ½[A]0 ln = -kt½ [A]0 ln [A]t [A]0 = -kt ln 2 = kt½ ln 2 0. 693 t½ = = k k

Half-Life But. OOBut(g) → 2 CH 3 CO(g) + C 2 H 4(g)

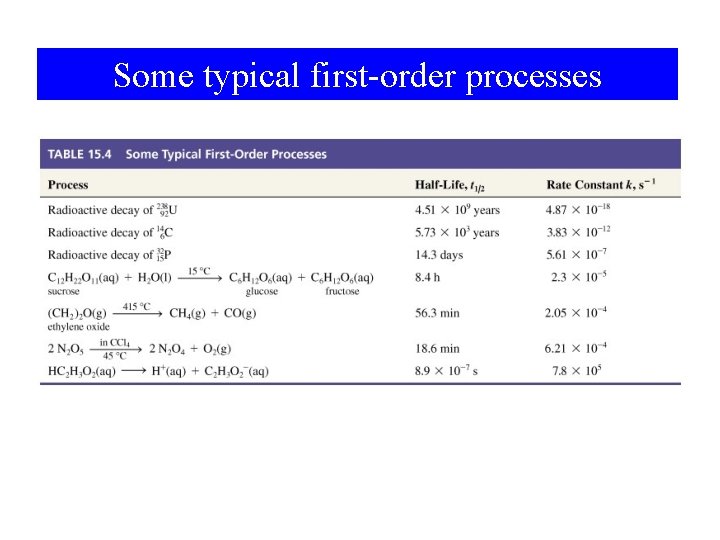
Some Typical First-Order Processes Some typical first-order processes

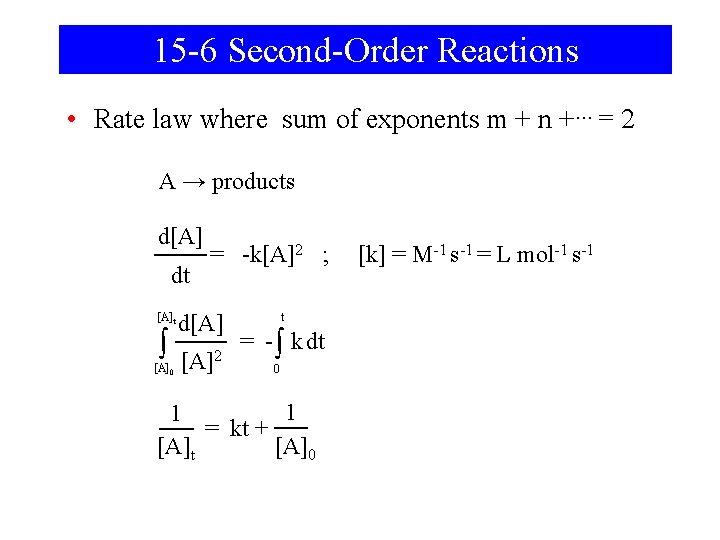
15 -6 Second-Order Reactions • Rate law where sum of exponents m + n +… = 2 A → products d[A] dt [A]t ∫ [A]0 = -k[A]2 ; d[A] [A]2 t = - ∫ k dt 0 1 1 = kt + [A]t [A]0 [k] = M-1 s-1 = L mol-1 s-1
![SecondOrder Reaction 1 1 kt At A0 Second-Order Reaction 1 1 = kt + [A]t [A]0](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-29.jpg)
Second-Order Reaction 1 1 = kt + [A]t [A]0

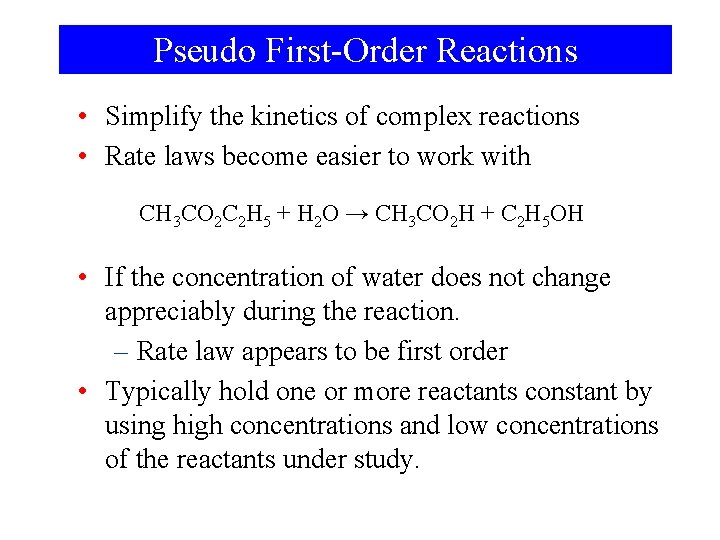
Pseudo First-Order Reactions • Simplify the kinetics of complex reactions • Rate laws become easier to work with CH 3 CO 2 C 2 H 5 + H 2 O → CH 3 CO 2 H + C 2 H 5 OH • If the concentration of water does not change appreciably during the reaction. – Rate law appears to be first order • Typically hold one or more reactants constant by using high concentrations and low concentrations of the reactants under study.
![Testing for a Rate Law Plot A vs t Plot lnA vs t Plot Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot](https://slidetodoc.com/presentation_image_h2/7bd70c3048c0fb2762d172b8b0709d13/image-31.jpg)
Testing for a Rate Law Plot [A] vs t. Plot ln[A] vs t. Plot 1/[A] vs t. 2 nd order

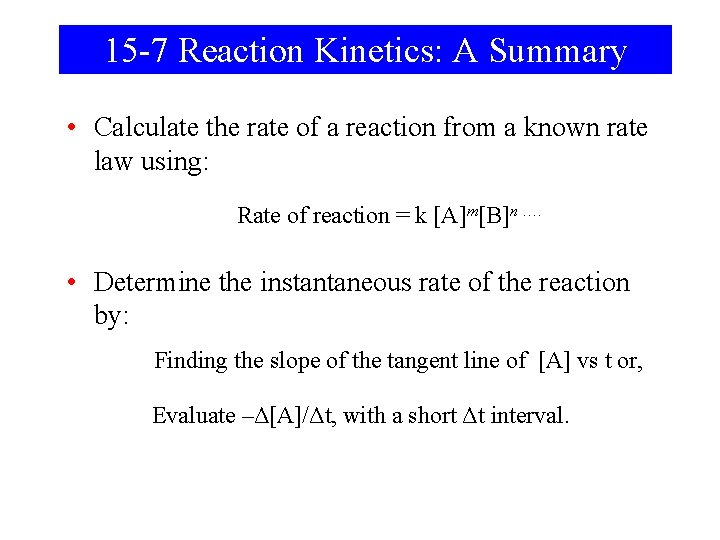
15 -7 Reaction Kinetics: A Summary • Calculate the rate of a reaction from a known rate law using: Rate of reaction = k [A]m[B]n …. • Determine the instantaneous rate of the reaction by: Finding the slope of the tangent line of [A] vs t or, Evaluate –Δ[A]/Δt, with a short Δt interval.

Summary of Kinetics • Determine the order of reaction by: Using the method of initial rates Find the graph that yields a straight line Test for the half-life to find first order reactions Substitute data into integrated rate laws to find the rate law that gives a consistent value of k.

Summary of Kinetics • Find the rate constant k by: Determining the slope of a straight line graph. Evaluating k with the integrated rate law. Measuring the half life of first-order reactions. • Find reactant concentrations or times for certain conditions using the integrated rate law after determining k.

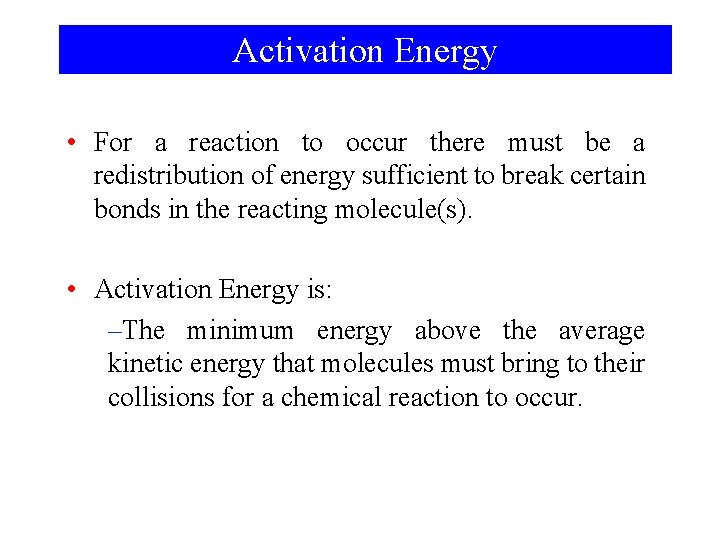
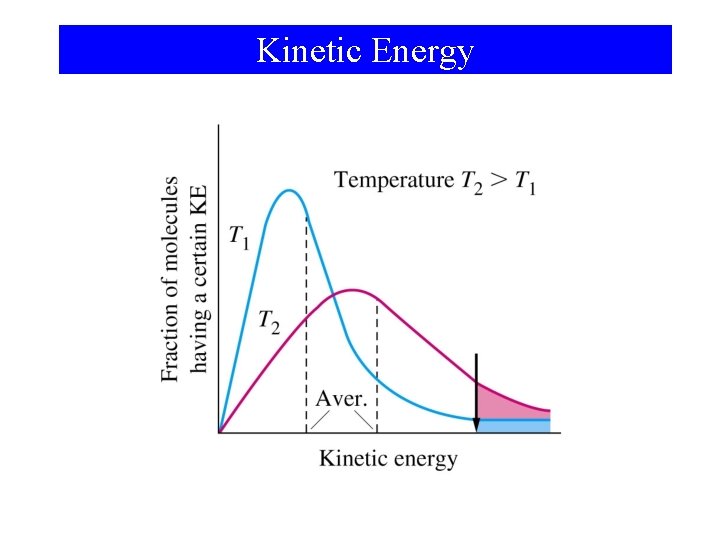
Activation Energy • For a reaction to occur there must be a redistribution of energy sufficient to break certain bonds in the reacting molecule(s). • Activation Energy is: –The minimum energy above the average kinetic energy that molecules must bring to their collisions for a chemical reaction to occur.

Activation Energy

Kinetic Energy

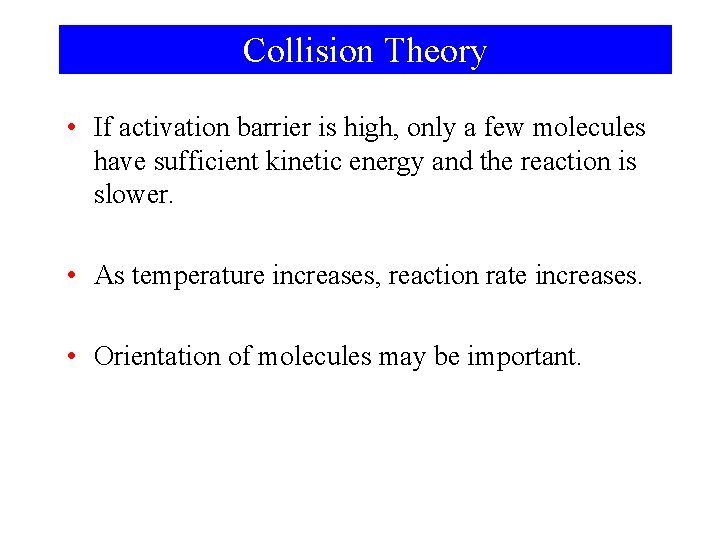
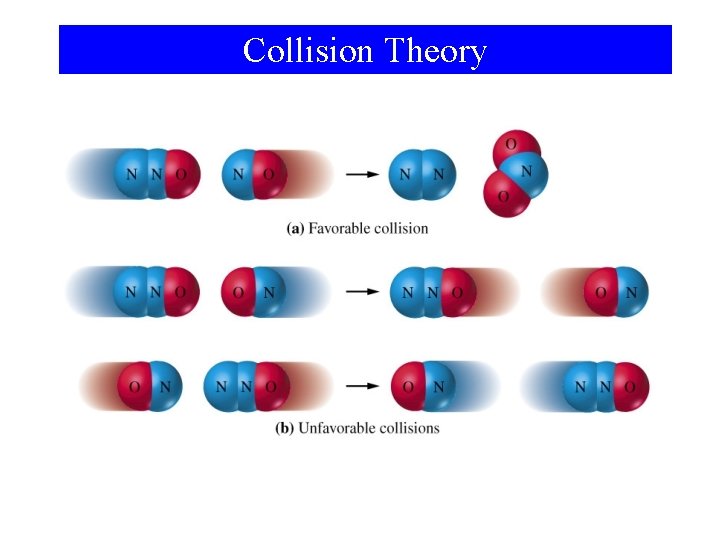
Collision Theory • If activation barrier is high, only a few molecules have sufficient kinetic energy and the reaction is slower. • As temperature increases, reaction rate increases. • Orientation of molecules may be important.

Collision Theory

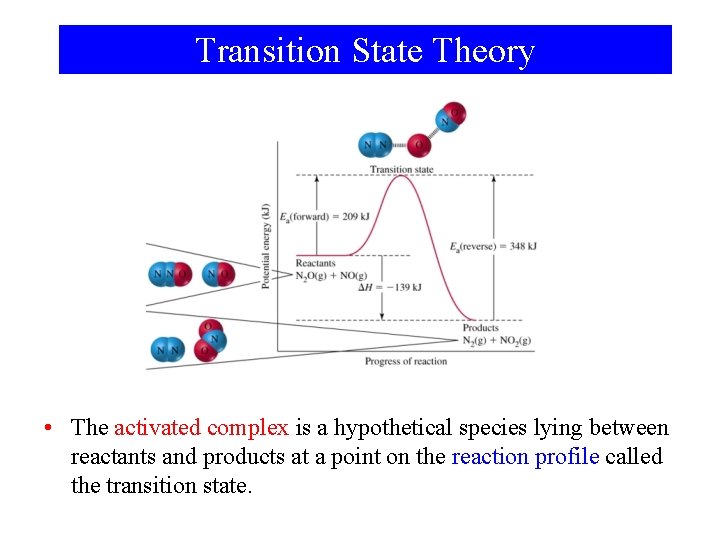
Transition State Theory • The activated complex is a hypothetical species lying between reactants and products at a point on the reaction profile called the transition state.

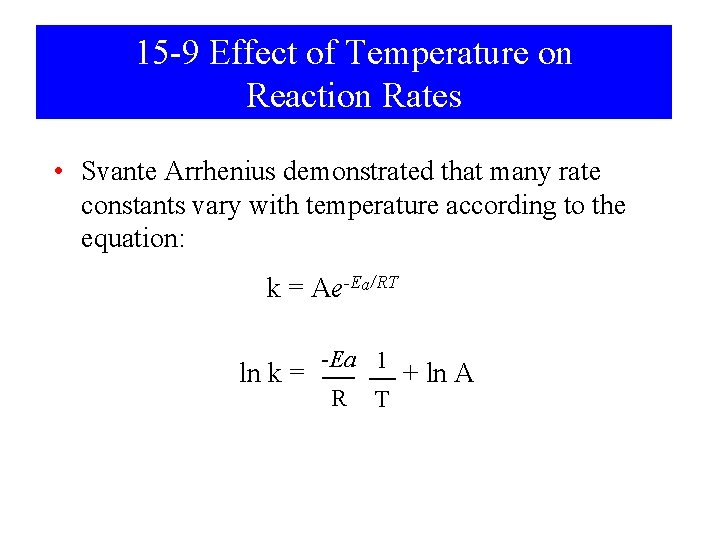
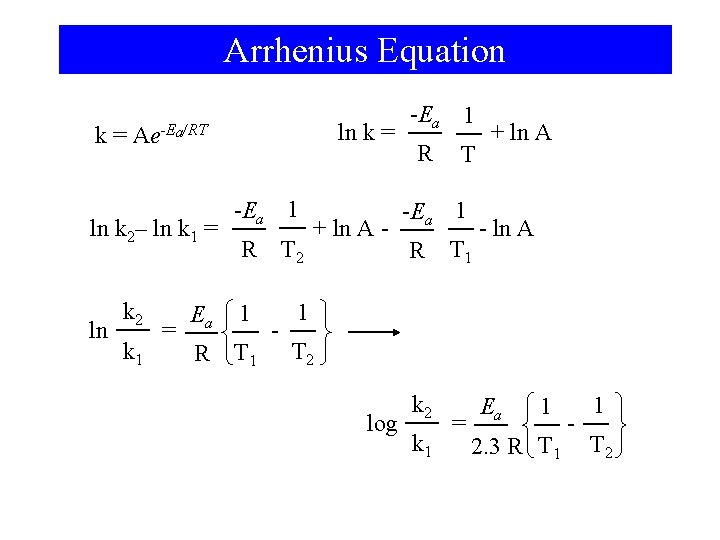
15 -9 Effect of Temperature on Reaction Rates • Svante Arrhenius demonstrated that many rate constants vary with temperature according to the equation: k = Ae-Ea/RT ln k = -Ea 1 R T + ln A

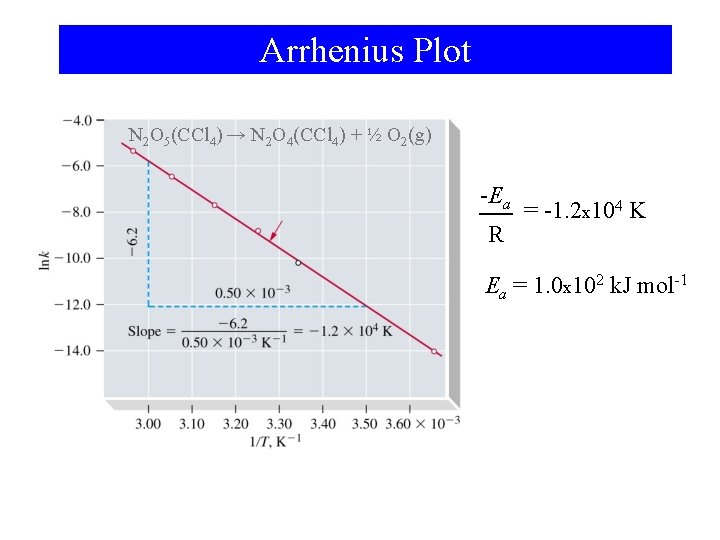
Arrhenius Plot N 2 O 5(CCl 4) → N 2 O 4(CCl 4) + ½ O 2(g) -Ea R = -1. 2 x 104 K Ea = 1. 0 x 102 k. J mol-1

Arrhenius Equation k= ln k 2– ln k 1 = ln ln k = Ae-Ea/RT k 2 k 1 = -Ea 1 R T 2 Ea 1 1 R T 1 - + ln A - -Ea 1 R T 1 + ln A - ln A T 2 log k 2 k 1 = Ea 1 2. 3 R T 1 - 1 T 2

A Rate Determining Step

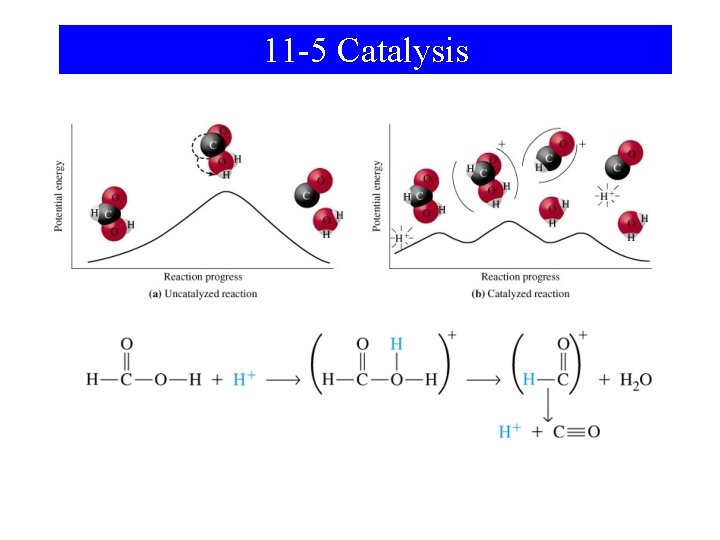
11 -5 Catalysis • Alternative reaction pathway of lower energy. • Homogeneous catalysis. – All species in the reaction are in solution. • Heterogeneous catalysis. – The catalyst is in the solid state. – Reactants from gas or solution phase are adsorbed. – Active sites on the catalytic surface are important.

11 -5 Catalysis
 Nit calicut chemistry faculty
Nit calicut chemistry faculty Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science faculty
University of bridgeport computer science faculty University of bridgeport computer engineering
University of bridgeport computer engineering Alamo colleges faculty salary schedule
Alamo colleges faculty salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine 002
002 Semmelweis
Semmelweis Penn state neurosurgery
Penn state neurosurgery Mercy college adjunct positions
Mercy college adjunct positions Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science Applied medical sciences
Applied medical sciences Carelli kutztown
Carelli kutztown Mch fsu
Mch fsu Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Electrical engineering umd
Electrical engineering umd Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Ecu faculty manual
Ecu faculty manual Faculty of engineering shoubra
Faculty of engineering shoubra Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Ucl computer science
Ucl computer science