General Chemistry M R NaimiJamal Faculty of Chemistry























- Slides: 45

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


Contents • • • Early chemical discoveries Electrons and the Nuclear Atom Chemical Elements Atomic Masses The Mole

Origins of Atomic Theory • Democritus (460 -370 BC) – Reasoned that if a piece of matter, such as gold, were divided into smaller and smaller pieces, one would ultimately arrive at a tiny particle of gold that could not be divided further, but that still retains the properties of gold – He called these particles “atoms” which means “uncuttable” – He taught that atoms are hard, move about spontaneously, and link to one another

Origins of Atomic Theory • Democritus used his concept to explain: –Different properties of metals High density and softness of lead versus iron which is less dense but much harder –Drying clothes –The appearance of moisture on the outside of a vessel containing cold water –How an odor moves through a room • To explain macroscopic events he saw, he imagined atoms scattering or collecting

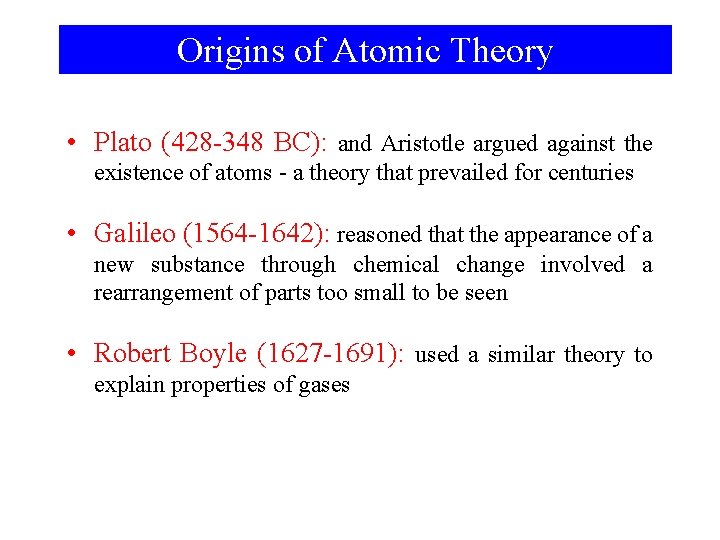
Origins of Atomic Theory • Plato (428 -348 BC): and Aristotle argued against the existence of atoms - a theory that prevailed for centuries • Galileo (1564 -1642): reasoned that the appearance of a new substance through chemical change involved a rearrangement of parts too small to be seen • Robert Boyle (1627 -1691): used a similar theory to explain properties of gases

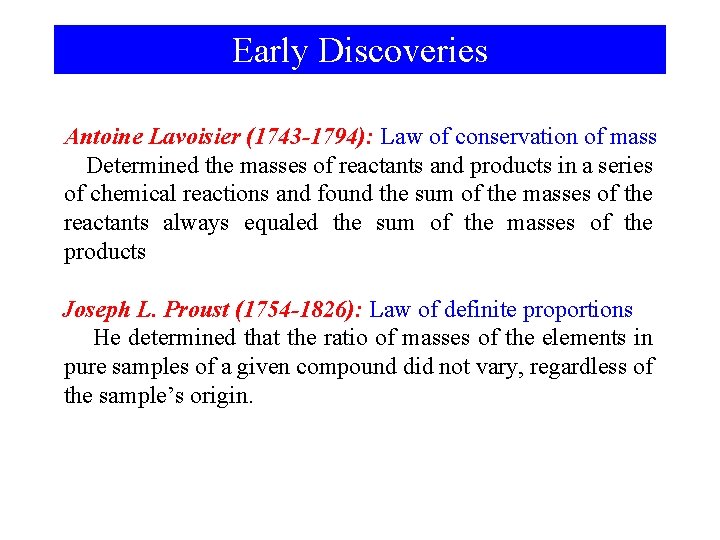
Early Discoveries Antoine Lavoisier (1743 -1794): Law of conservation of mass Determined the masses of reactants and products in a series of chemical reactions and found the sum of the masses of the reactants always equaled the sum of the masses of the products Joseph L. Proust (1754 -1826): Law of definite proportions He determined that the ratio of masses of the elements in pure samples of a given compound did not vary, regardless of the sample’s origin.

Dalton’s Atomic Theory Dalton (1803 -1888): Each element is composed of small particles called atoms. ‚ Atoms are neither created nor destroyed in chemical reactions. ƒ All atoms of a given element are identical. „ Compounds are formed when atoms of more than one element combine.

Dalton’s Atomic Theory • Dalton’s Law of Multiple Proportions – If two elements form two different compounds, the mass ratio of the elements making up one compound is a whole number multiple of the mass ratio of the elements in the second compound – Example: Oxides of Carbon

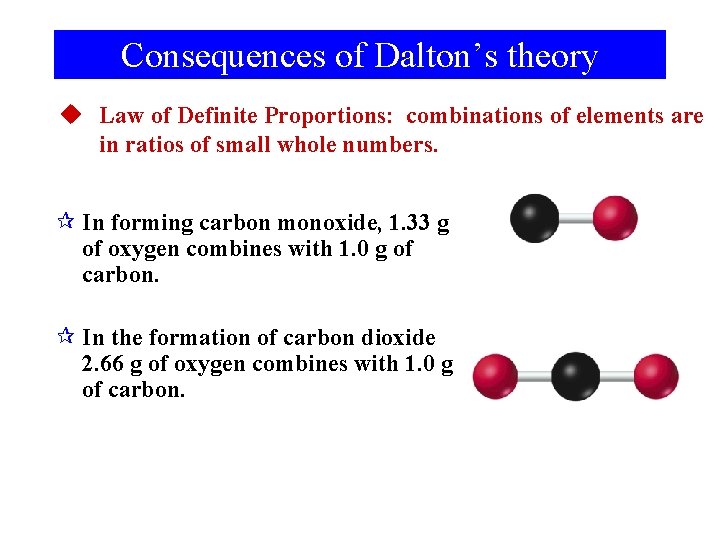
Consequences of Dalton’s theory u Law of Definite Proportions: combinations of elements are in ratios of small whole numbers. ¶ In forming carbon monoxide, 1. 33 g of oxygen combines with 1. 0 g of carbon. ¶ In the formation of carbon dioxide 2. 66 g of oxygen combines with 1. 0 g of carbon.

Protons, Neutrons, and Electrons • Dalton’s atomic theory said nothing about atoms having structure (Democritus’s idea) • Democritus’s idea that atoms stick together by a “hook and eye” mechanism is not accepted, but his idea that we better understand the behavior of elements by studying atomic structure was correct

Protons, Neutrons, and Electrons • Electricity – By the time of Benjamin Franklin (1706 -1790), two types of electric charge had been discovered – Franklin named them positive and negative because they appear as opposites and can repel one another • Like charges repel, and unlike charges attract – Franklin noted that charge is balanced: negative and positive present in same amount

Protons, Neutrons, and Electrons • Radioactivity: spontaneous emission of energy as atoms disintegrate – Henri Becquerel and Marie Curie • Marie Curie’s concept that atoms disintegrate contradicts Dalton’s idea that atoms are indivisible. If atoms can break apart, there must be something smaller than an atom: “subatomic particles”

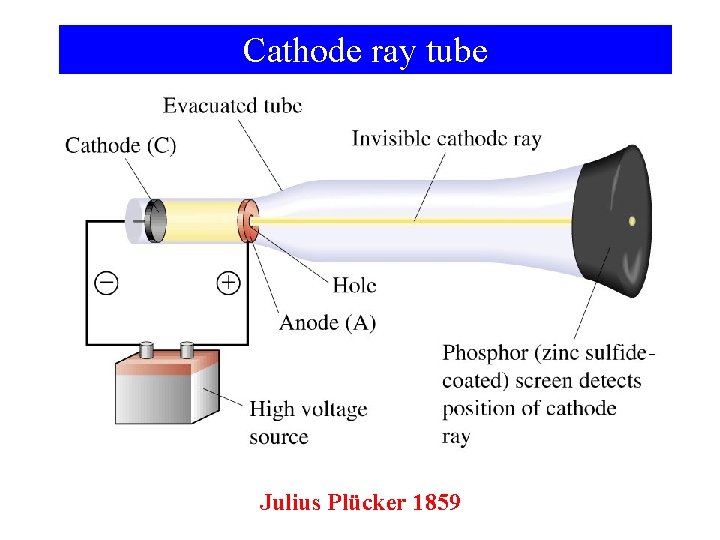
Protons, Neutrons, and Electrons • Michael Faraday (1791 -1867) experimented with electrolysis (passing a current through a solution) Found that different quantities of different metals “plated” at the same current (related to mass of the atoms of those elements) • As atoms are fundamental particles of elements, the fundamental particle of electricity was given the name “electron”

Behavior of charges

Cathode ray tube Julius Plücker 1859

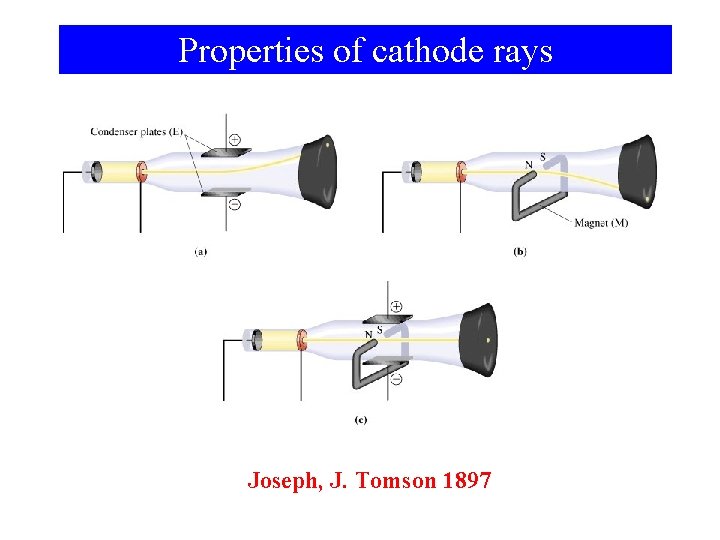
Properties of cathode rays Joseph, J. Tomson 1897


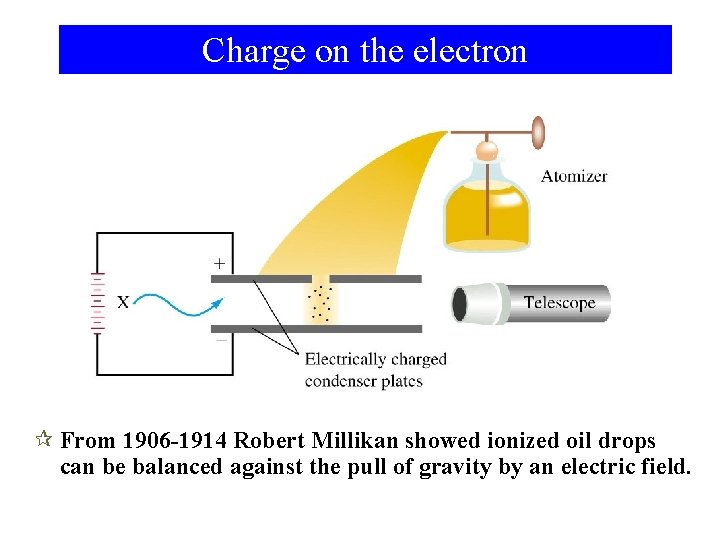
Charge on the electron ¶ From 1906 -1914 Robert Millikan showed ionized oil drops can be balanced against the pull of gravity by an electric field.

Milikan Experiment

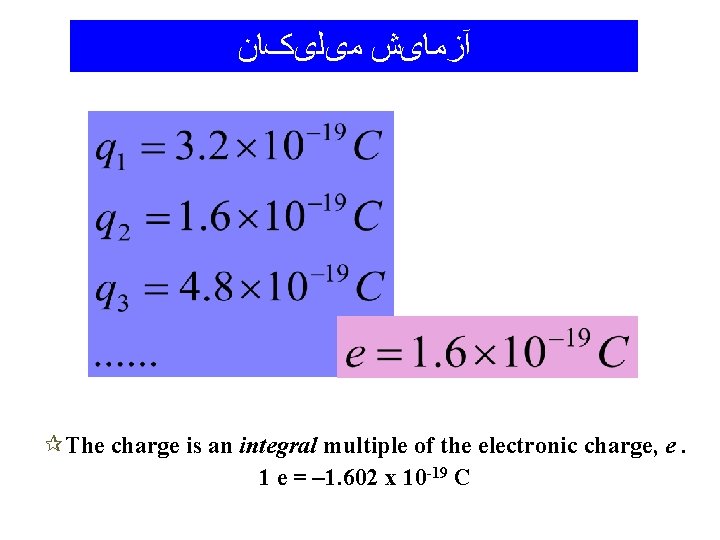
آﺰﻣﺎیﺶ ﻣیﻠیکﺎﻥ ¶The charge is an integral multiple of the electronic charge, e. 1 e = – 1. 602 x 10 -19 C


پﺮﻭﺗﻮﻥ q/m = + 9. 5791 x 104 C/g q = + e= +1. 06022 x 10 -19 C m= q/q/m= 1. 6726 x 10 -24



Radioactivity is the spontaneous emission of radiation from a substance. ¶ X-rays and g-rays are high-energy light. ¶ a-particles are a stream of helium nuclei, He 2+. ¶ b-particles are a stream of high speed electrons that originate in the nucleus.

a, b, and g Rays

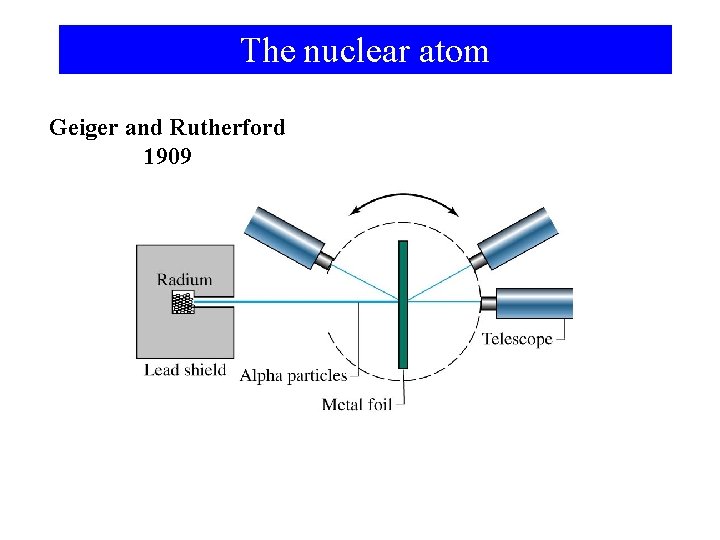
The nuclear atom Geiger and Rutherford 1909

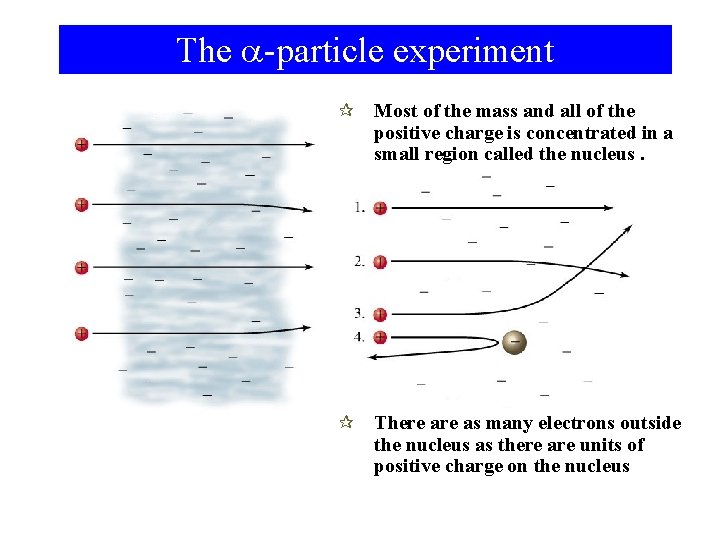
The a-particle experiment ¶ Most of the mass and all of the positive charge is concentrated in a small region called the nucleus. ¶ There as many electrons outside the nucleus as there are units of positive charge on the nucleus

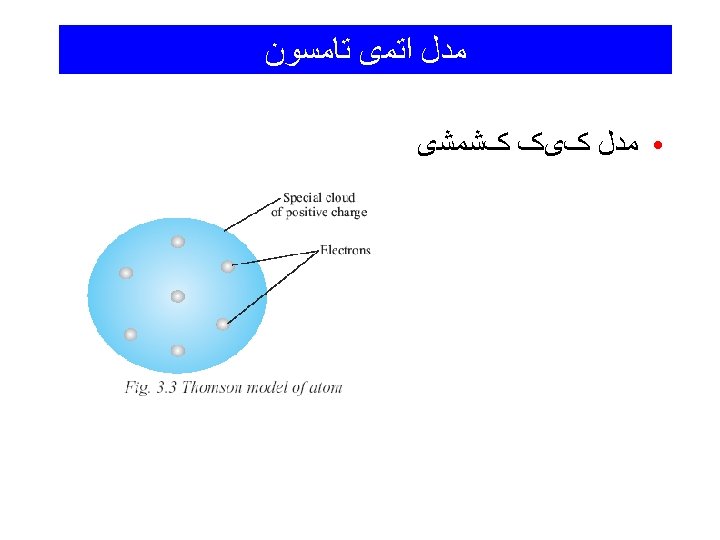
Atomic Structure • Ernest Rutherford called the tiny core of the atom (contains the protons and neutrons) the “nucleus” • The nucleus contains all of the + charge and almost all of the mass of the atom (protons and neutrons) • The electrons (-) surround the nucleus and occupy most of the volume of an atom • # of electrons = # of protons

Ratherford Experiment

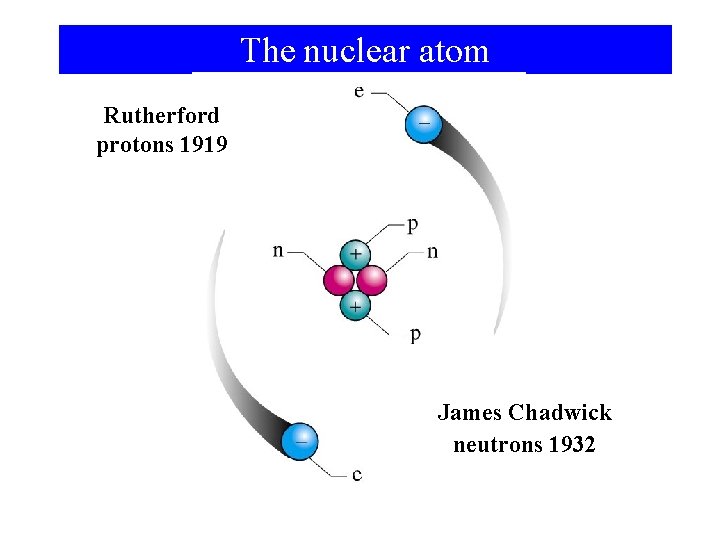
The nuclear atom Rutherford protons 1919 James Chadwick neutrons 1932

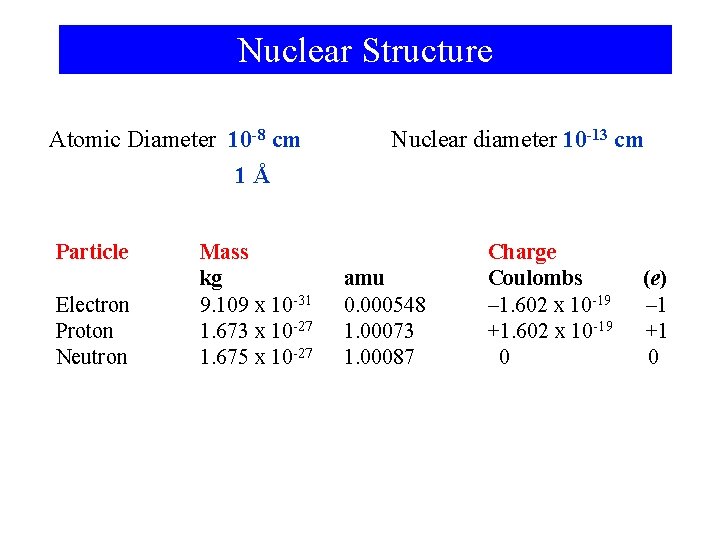
Nuclear Structure Atomic Diameter 10 -8 cm Nuclear diameter 10 -13 cm 1Å Particle Electron Proton Neutron Mass kg 9. 109 x 10 -31 1. 673 x 10 -27 1. 675 x 10 -27 amu 0. 000548 1. 00073 1. 00087 Charge Coulombs – 1. 602 x 10 -19 +1. 602 x 10 -19 0 (e) – 1 +1 0

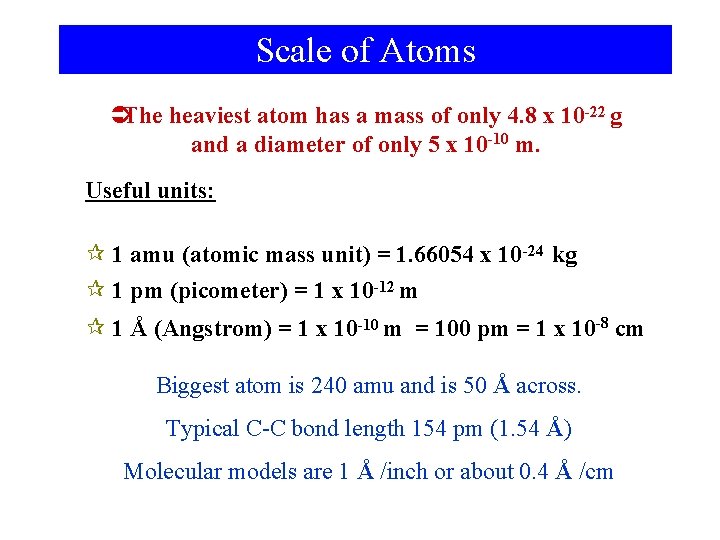
Scale of Atoms ÜThe heaviest atom has a mass of only 4. 8 x 10 -22 g and a diameter of only 5 x 10 -10 m. Useful units: ¶ 1 amu (atomic mass unit) = 1. 66054 x 10 -24 kg ¶ 1 pm (picometer) = 1 x 10 -12 m ¶ 1 Å (Angstrom) = 1 x 10 -10 m = 100 pm = 1 x 10 -8 cm Biggest atom is 240 amu and is 50 Å across. Typical C-C bond length 154 pm (1. 54 Å) Molecular models are 1 Å /inch or about 0. 4 Å /cm

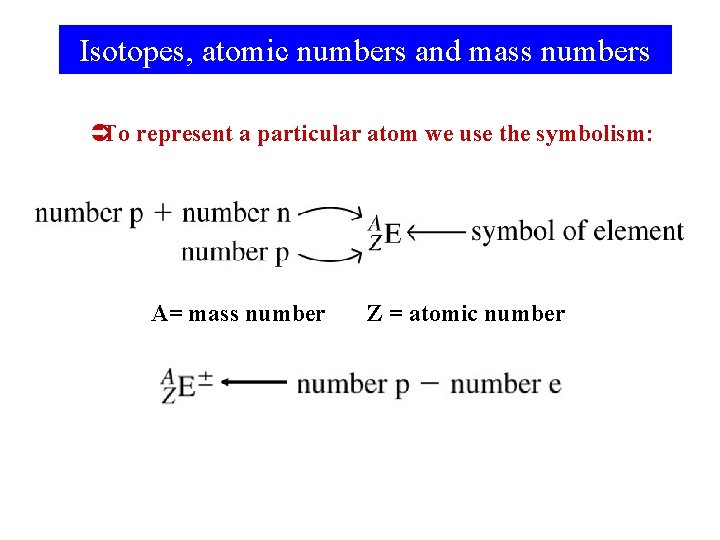
Isotopes, atomic numbers and mass numbers ÜTo represent a particular atom we use the symbolism: A= mass number Z = atomic number

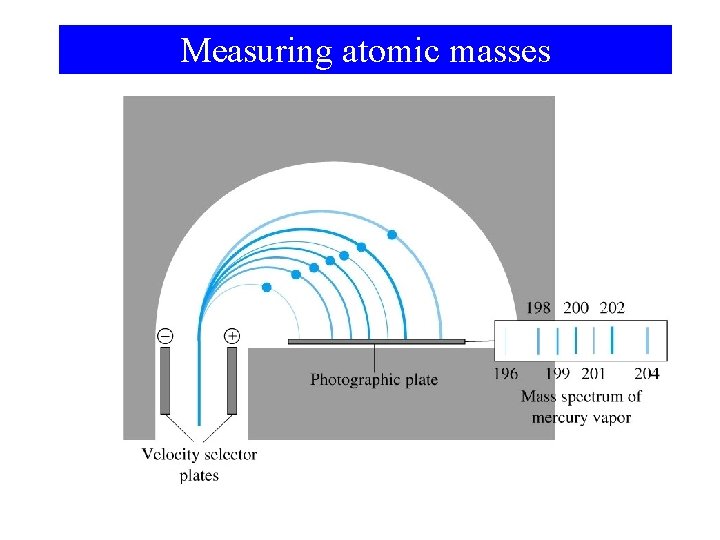
Measuring atomic masses

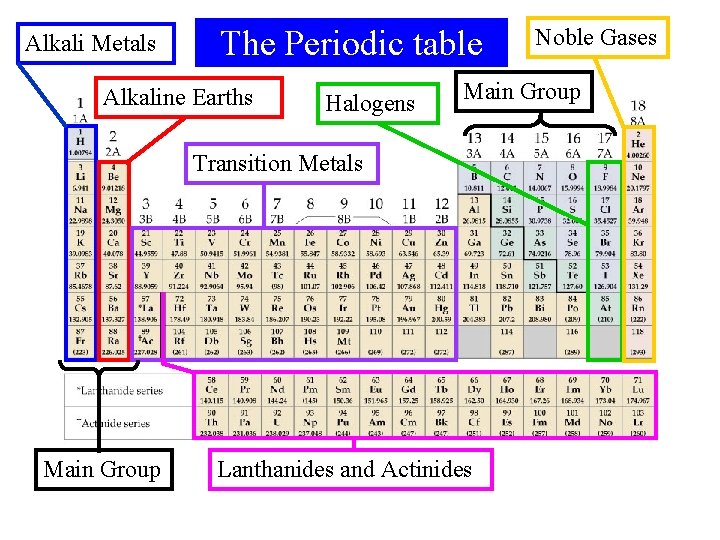
Alkali Metals The Periodic table Alkaline Earths Halogens Main Group Transition Metals Main Group Noble Gases Lanthanides and Actinides

Periodic Table

The Periodic Table • • Read atomic masses. Read the ions formed by main group elements. Read the electron configuration. Learn trends in physical and chemical properties. We will discuss these in detail later.

The Mole • Physically counting atoms is impossible. • We must be able to relate measured mass to numbers of atoms. – buying nails by the pound. – using atoms by the gram

Avogadro’s number v. The mole is an amount of substance that contains the same number of elementary entities as there are carbon-12 atoms in exactly 12 g of carbon-12. NA = 6. 02214199 x 1023 mol-1

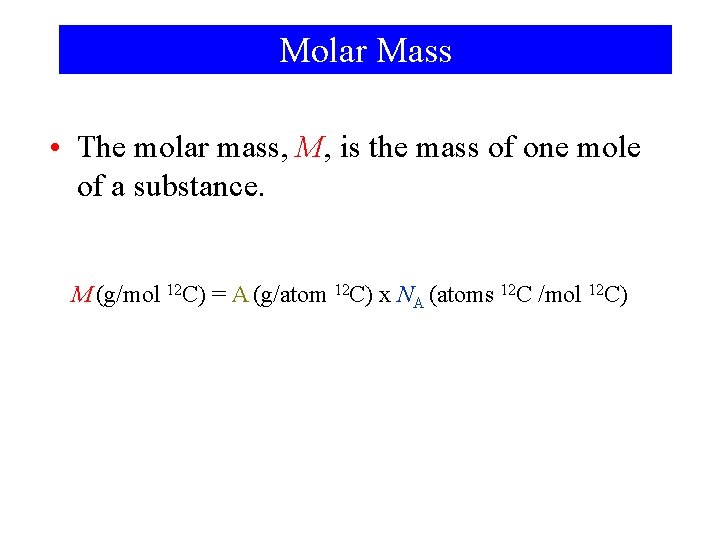
Molar Mass • The molar mass, M, is the mass of one mole of a substance. M (g/mol 12 C) = A (g/atom 12 C) x NA (atoms 12 C /mol 12 C)

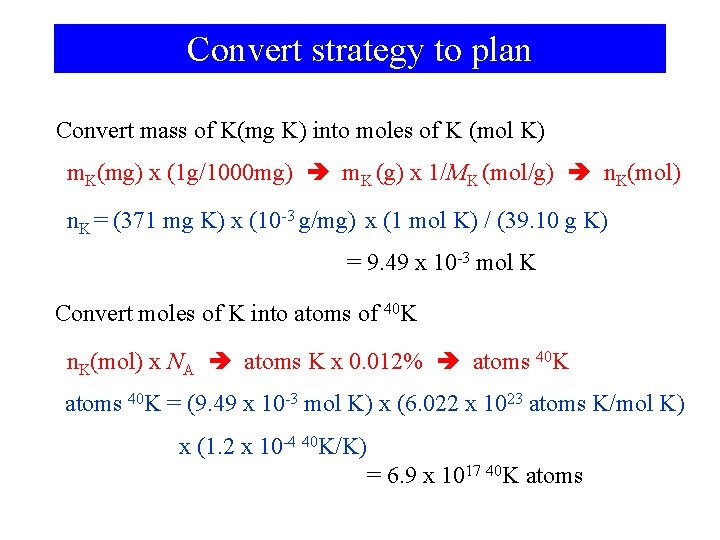
Example 2 -9 Combining Several Factors in a Calculation—Molar Mass, the Avogadro Constant, Percent Abundance. Potassium-40 is one of the few naturally occurring radioactive isotopes of elements of low atomic number. Its percent natural abundance among K isotopes is 0. 012%. How many 40 K atoms do you ingest by drinking one cup of whole milk containing 371 mg of K? Want atoms of 40 K, need atoms of K, Want atoms of K, need moles of K, Want moles of K, need mass and M(K).

Convert strategy to plan Convert mass of K(mg K) into moles of K (mol K) m. K(mg) x (1 g/1000 mg) m. K (g) x 1/MK (mol/g) n. K(mol) n. K = (371 mg K) x (10 -3 g/mg) x (1 mol K) / (39. 10 g K) = 9. 49 x 10 -3 mol K Convert moles of K into atoms of 40 K n. K(mol) x NA atoms K x 0. 012% atoms 40 K = (9. 49 x 10 -3 mol K) x (6. 022 x 1023 atoms K/mol K) x (1. 2 x 10 -4 40 K/K) = 6. 9 x 1017 40 K atoms

Chapter 2, Questions 4, 18, 20, 22, 24, 26, 29, 33
 Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry ders notları
General chemistry ders notları General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet Bridgeport engineering department
Bridgeport engineering department University of bridgeport computer engineering
University of bridgeport computer engineering Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school powerschool
Hahnville high school powerschool Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine 002
002 Solid thyroid nodule
Solid thyroid nodule Penn state neurosurgery faculty
Penn state neurosurgery faculty Mercy college adjunct positions
Mercy college adjunct positions Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Faculty kutztown carelli
Faculty kutztown carelli Florida state university ms in cs
Florida state university ms in cs Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd ece faculty
Umd ece faculty Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Czech technical university in prague civil engineering
Czech technical university in prague civil engineering Faculty 180 ecu
Faculty 180 ecu Faculty of engineering shoubra
Faculty of engineering shoubra Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv faculty senate
Unlv faculty senate Ulm nursing faculty
Ulm nursing faculty