General Chemistry M R NaimiJamal Faculty of Chemistry
























- Slides: 48

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


Contents 12 -1 12 -2 12 -3 12 -4 12 -5 12 -6 12 -7 Types of Solutions: Some Terminology Solution Concentration Intermolecular Forces and the Solution Process Solution Formation and Equilibrium Solubilities of Gases Vapor Pressure of Solutions Osmotic Pressure

Contents 12 -8 Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte solutions. 12 -9 Solutions of Electrolytes 12 -10 Colloidal Mixtures Focus on Chromatography

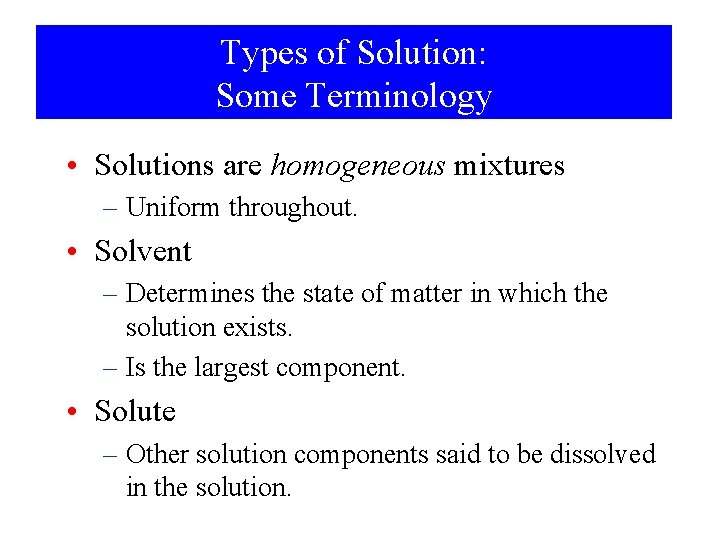
Types of Solution: Some Terminology • Solutions are homogeneous mixtures – Uniform throughout. • Solvent – Determines the state of matter in which the solution exists. – Is the largest component. • Solute – Other solution components said to be dissolved in the solution.

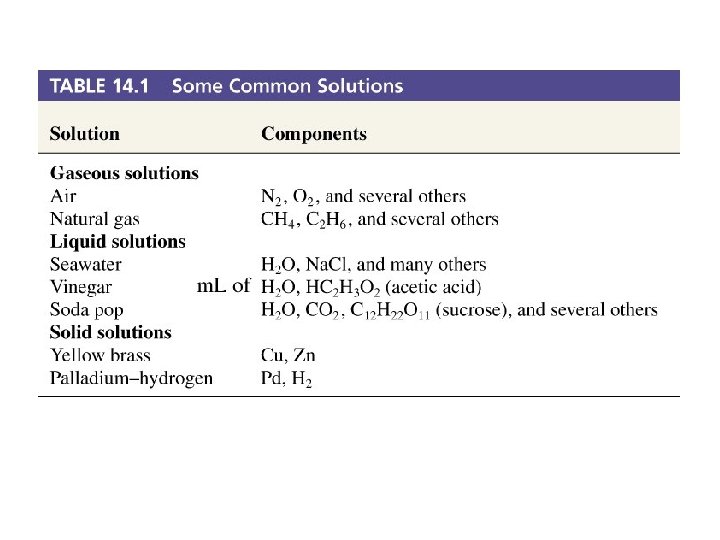
Table 14. 1 Some Common Solutions

Solution Concentration. • Mass percent. • Volume percent. • Mass/volume percent. (m/m) (v/v) (m/v) • Isotonic saline is prepared by dissolving 0. 9 g of Na. Cl in 100 m. L of water and is said to be: 0. 9% Na. Cl (mass/volume)

10% Ethanol Solution (v/v)

ppm, ppb and ppt • Very low solute concentrations are expressed as: ppm: parts per million ppb: parts per billion ppt: parts per trillion ( g/g, mg/L) (ng/g, g/L) (pg/g, ng/L) note that 1. 0 L x 1. 0 g/m. L = 1000 g ppm, ppb, and ppt are properly m/m or v/v.

Mole Fraction and Mole Percent = Amount of component i (in moles) Total amount of all components (in moles) 1 + 2 + 3 + … n = 1 Mole % i = i x 100%

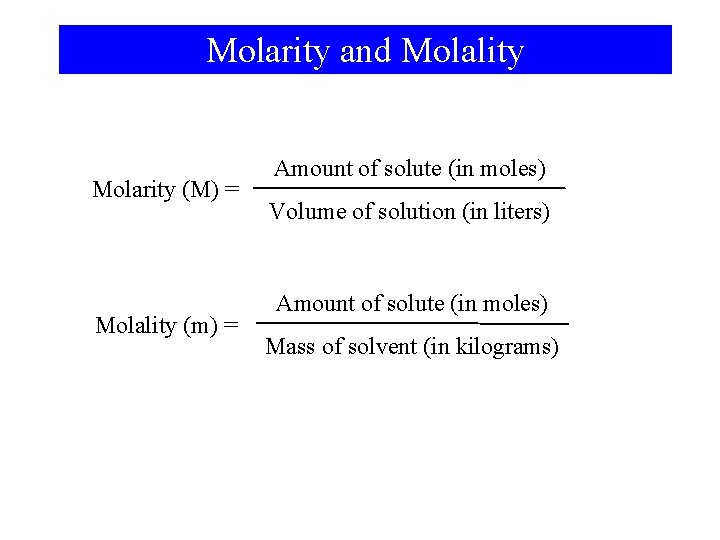
Molarity and Molality Molarity (M) = Molality (m) = Amount of solute (in moles) Volume of solution (in liters) Amount of solute (in moles) Mass of solvent (in kilograms)

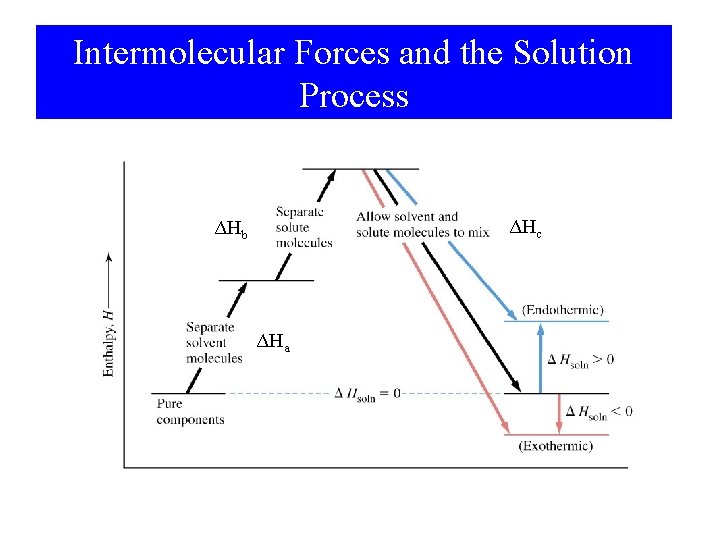
Intermolecular Forces and the Solution Process ΔHc ΔHb ΔHa

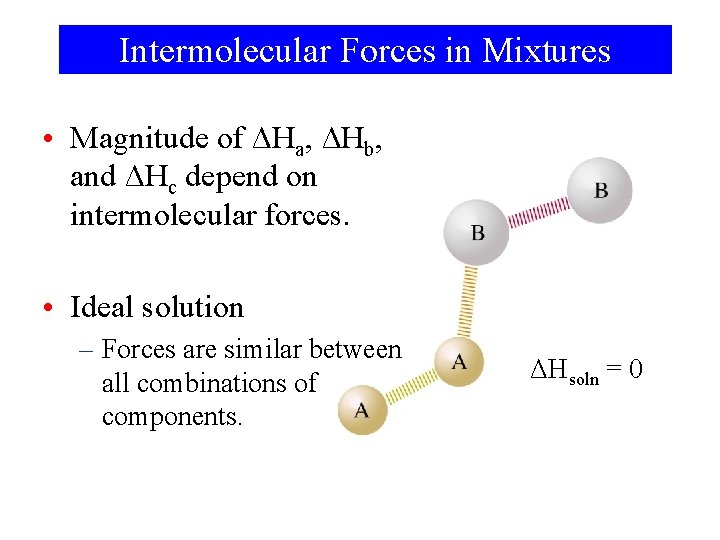
Intermolecular Forces in Mixtures • Magnitude of ΔHa, ΔHb, and ΔHc depend on intermolecular forces. • Ideal solution – Forces are similar between all combinations of components. ΔHsoln = 0

Ideal Solution benzene toulene

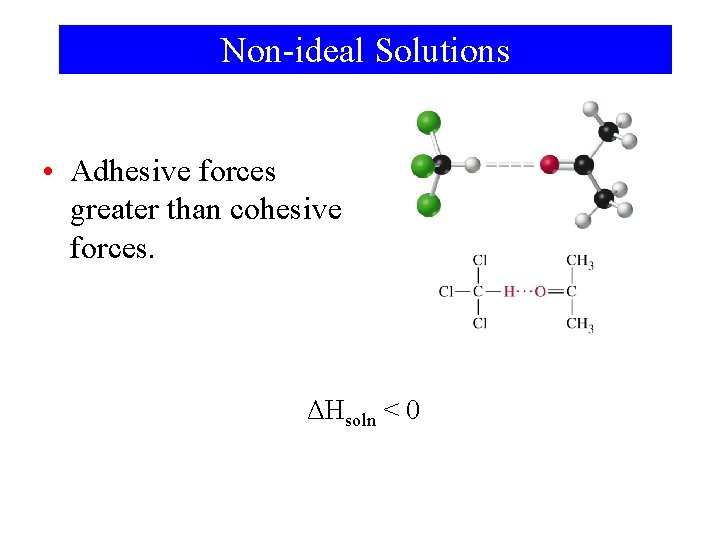
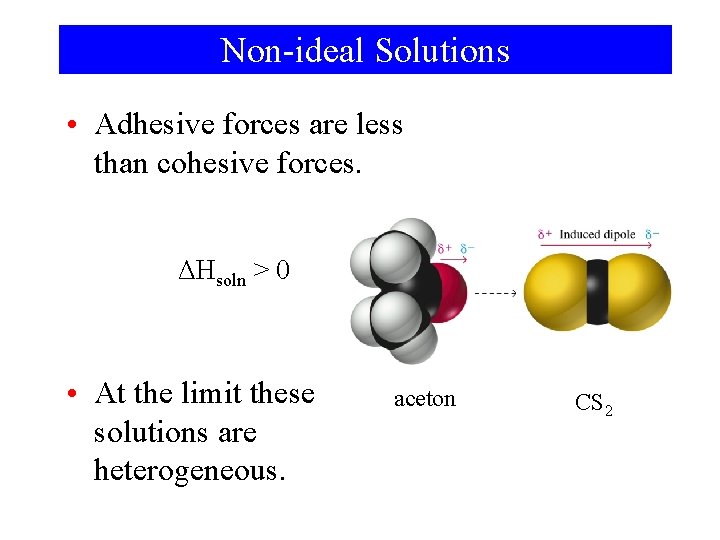
Non-ideal Solutions • Adhesive forces greater than cohesive forces. ΔHsoln < 0

Non-ideal Solutions • Adhesive forces are less than cohesive forces. ΔHsoln > 0 • At the limit these solutions are heterogeneous. aceton CS 2

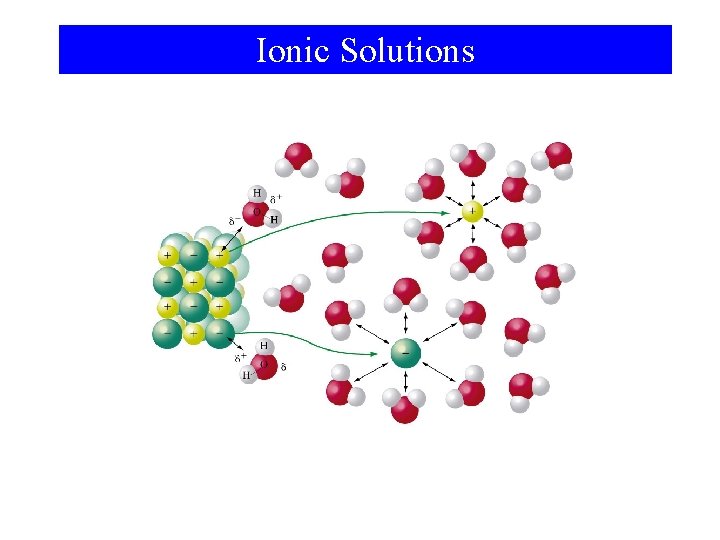
Ionic Solutions

Dissolution of Na. Cl

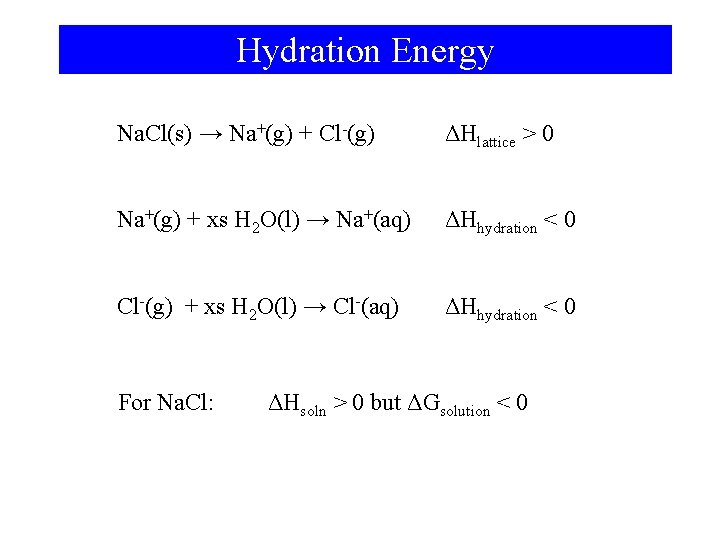
Hydration Energy Na. Cl(s) → Na+(g) + Cl-(g) ΔHlattice > 0 Na+(g) + xs H 2 O(l) → Na+(aq) ΔHhydration < 0 Cl-(g) + xs H 2 O(l) → Cl-(aq) ΔHhydration < 0 For Na. Cl: ΔHsoln > 0 but ΔGsolution < 0

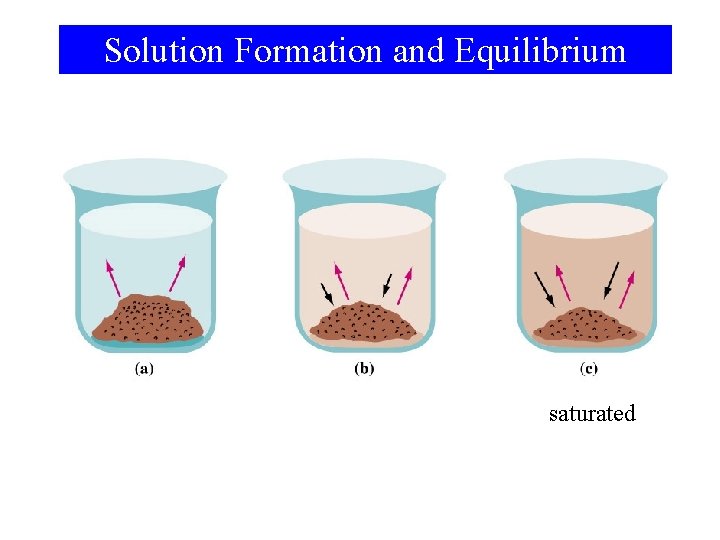
Solution Formation and Equilibrium saturated

Solubility Curves Supersaturated Unsaturated

Solubility of Gases • Most gases are less soluble in water as temperature increases. • In organic solvents the reverse is often true.

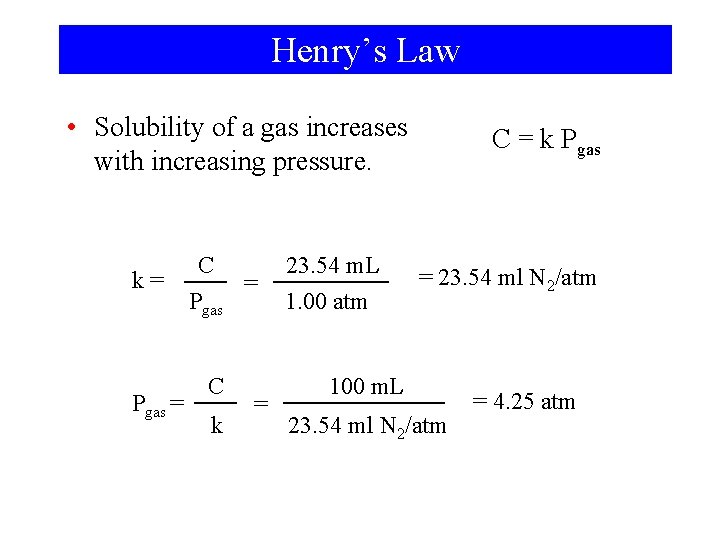
Henry’s Law

Henry’s Law • Solubility of a gas increases with increasing pressure. k= Pgas = C = Pgas C k = 23. 54 m. L 1. 00 atm C = k Pgas = 23. 54 ml N 2/atm 100 m. L 23. 54 ml N 2/atm = 4. 25 atm

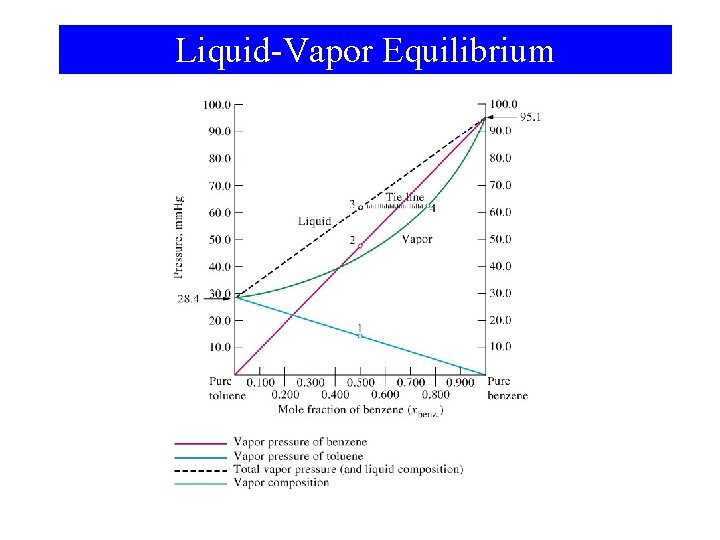
Vapor Pressures of Solutions • Roault, 1880 s. – Dissolved solute lowers vapor pressure of solvent. – The partial pressure exerted by solvent vapor above an ideal solution is the product of the mole fraction of solvent in the solution and the vapor pressure of the pure solvent at a given temperature. P A = A P A°

Example Predicting vapor pressure of ideal solutions. The vapor pressures of pure benzene and toluene at 25°C are 95. 1 and 28. 4 mm Hg, respectively. A solution is prepared in which the mole fractions of benzene and toluene are both 0. 500. What are the partial pressures of the benzene and toluene above this solution? What is the total vapor pressure? Balanced Chemical Equation: Pbenzene = benzene P°benzene = (0. 500)(96. 1 mm Hg) = 47. 6 mm Hg Ptoluene = toluene P°toluene = (0. 500)(28. 4 mm Hg) = 14. 2 mm Hg Ptotal = Pbenzene + Ptoluene = 61. 8 mm Hg

Example Calculating the Composition of Vapor in Equilibrium with a Liquid Solution. What is the composition of the vapor in equilibrium with the benzene-toluene solution? Partial pressure and mole fraction: benzene = Pbenzene/Ptotal = 47. 6 mm Hg/61. 89 mm Hg = 0. 770 toluene = Ptoluene/Ptotal = 14. 2 mm Hg/61. 89 mm Hg = 0. 230

Liquid-Vapor Equilibrium

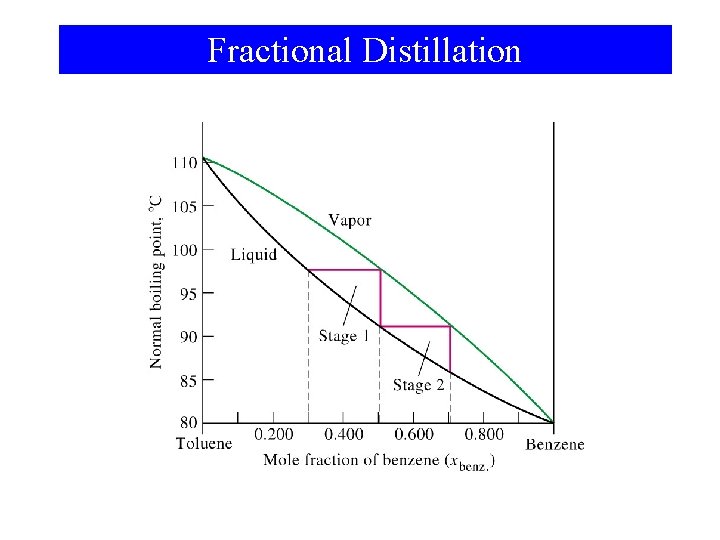
Fractional Distillation

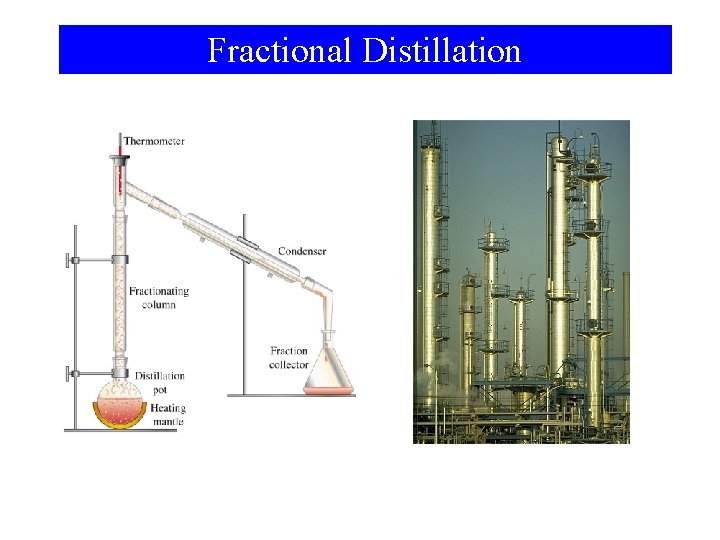
Fractional Distillation

Non-ideal behavior

Osmotic Pressure

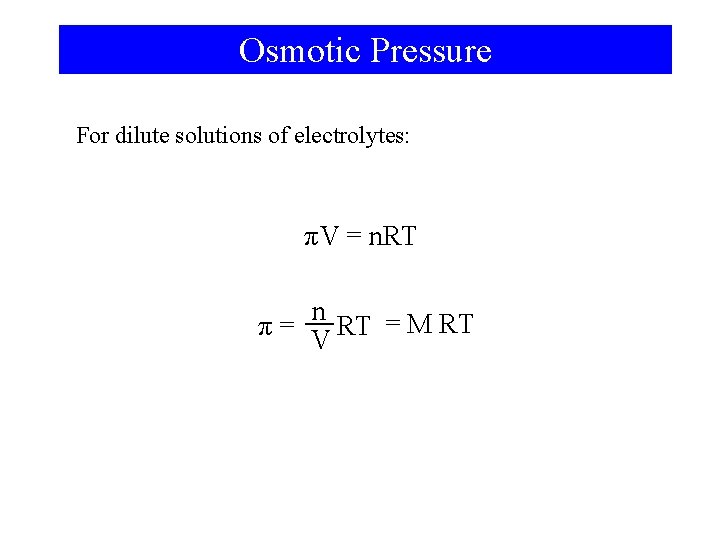
Osmotic Pressure For dilute solutions of electrolytes: πV = n. RT n π= RT = M RT V

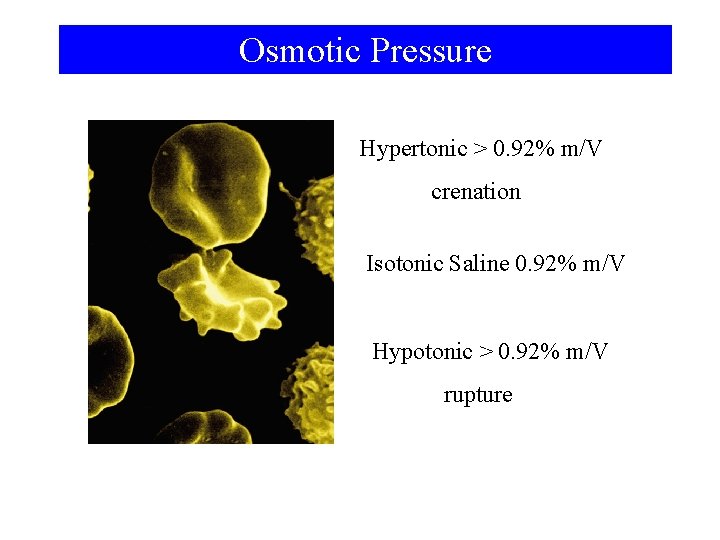
Osmotic Pressure Hypertonic > 0. 92% m/V crenation Isotonic Saline 0. 92% m/V Hypotonic > 0. 92% m/V rupture

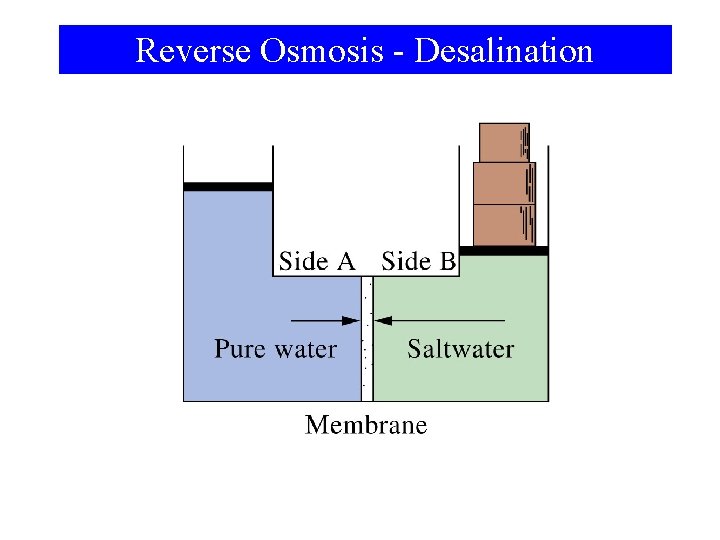
Reverse Osmosis - Desalination

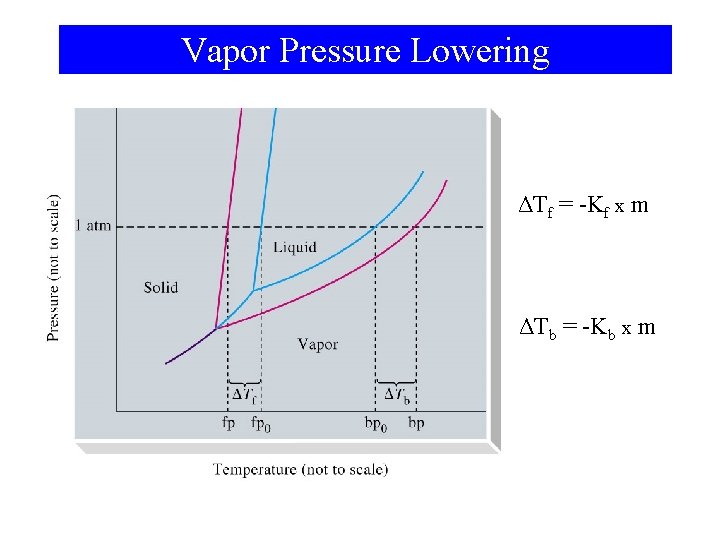
Freezing-Point Depression and Boiling Point Elevation of Nonelectrolyte Solutions • Vapor pressure is lowered when a solute is present. – This results in boiling point elevation. – Freezing point is also effected and is lowered. • Colligative properties. – Depends on the number of particles present.

Vapor Pressure Lowering ΔTf = -Kf x m ΔTb = -Kb x m

Practical Applications

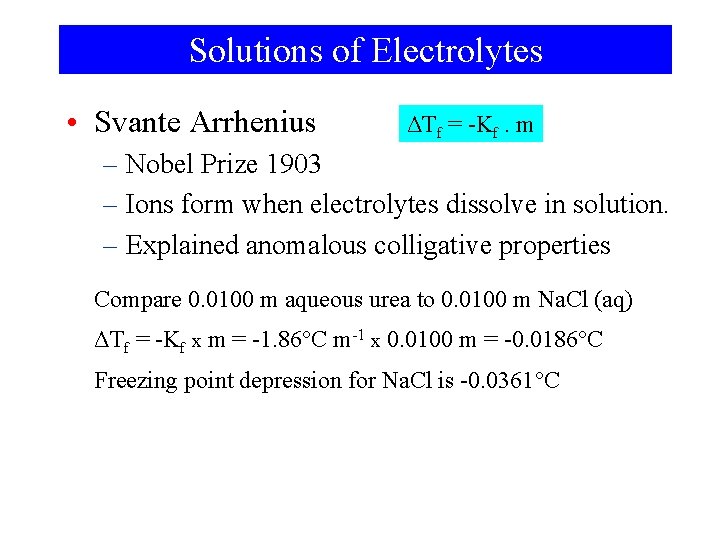
Solutions of Electrolytes • Svante Arrhenius ΔTf = -Kf. m – Nobel Prize 1903 – Ions form when electrolytes dissolve in solution. – Explained anomalous colligative properties Compare 0. 0100 m aqueous urea to 0. 0100 m Na. Cl (aq) ΔTf = -Kf x m = -1. 86°C m-1 x 0. 0100 m = -0. 0186°C Freezing point depression for Na. Cl is -0. 0361°C

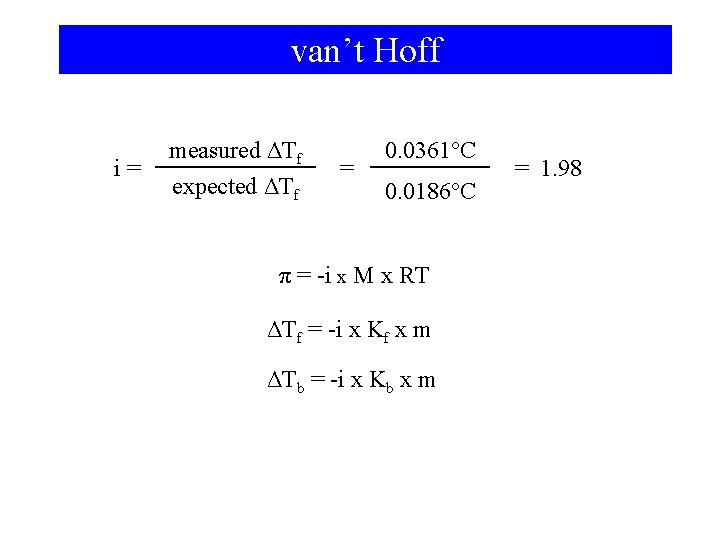
van’t Hoff i= measured ΔTf expected ΔTf = 0. 0361°C 0. 0186°C π = -i x M x RT ΔTf = -i x Kf x m ΔTb = -i x Kb x m = 1. 98

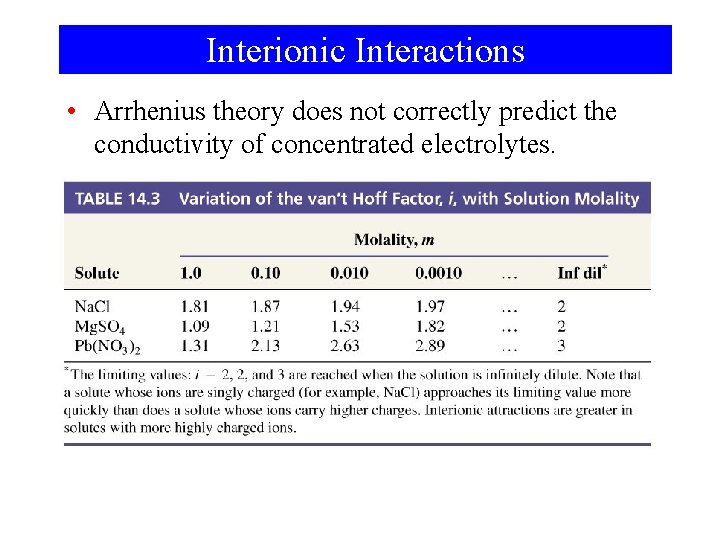
Interionic Interactions • Arrhenius theory does not correctly predict the conductivity of concentrated electrolytes.

Debye and Hückel • 1923 – Ions in solution do not behave independently. – Each ion is surrounded by others of opposite charge. – Ion mobility is reduced by the drag of the ionic atmosphere.

Colloidal Mixtures

Colloids • Particles of 1 -1000 nm size – Nanoparticles of various shapes: rods, discs, spheres – Particles can remain suspended indefinitly • Milk is colloidal • Increasing ionic strength can cause precipitation

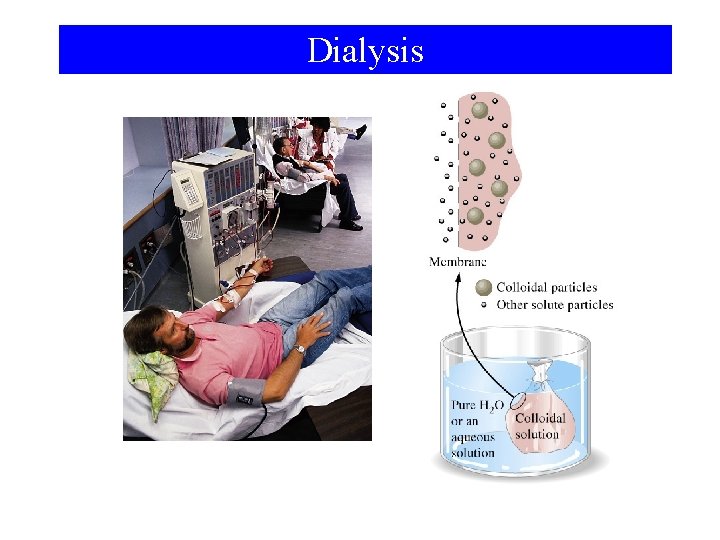
Dialysis

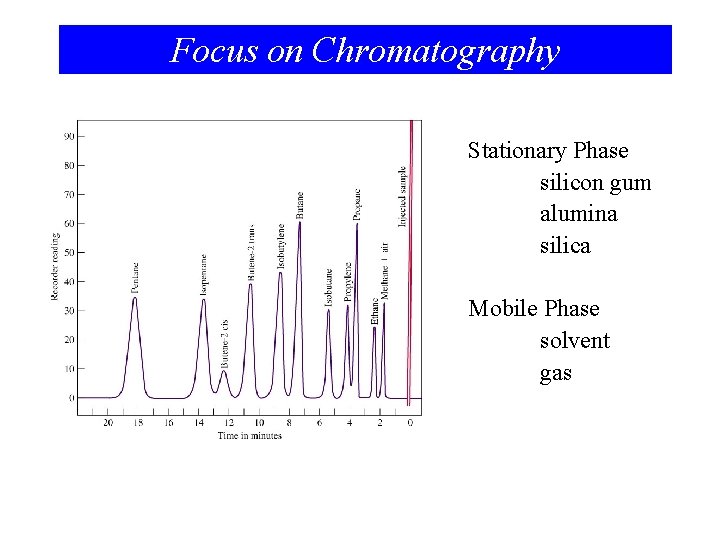
Focus on Chromatography Stationary Phase silicon gum alumina silica Mobile Phase solvent gas

Chromatography

Chapter 12 Questions 8, 14, 15, 22, 23, 29, 36, 49, 66, 73, 81, 85
 Nit calicut chemistry faculty
Nit calicut chemistry faculty Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet University of bridgeport computer science
University of bridgeport computer science Bridgeport university computer science
Bridgeport university computer science Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine 002
002 Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Penn state neurosurgery faculty
Penn state neurosurgery faculty Mercy college adjunct positions
Mercy college adjunct positions Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Carelli kutztown
Carelli kutztown Fsu cybersecurity major
Fsu cybersecurity major Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd electrical engineering
Umd electrical engineering Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Ecu faculty 180
Ecu faculty 180 Benha faculty of engineering
Benha faculty of engineering Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Graham roberts ucl
Graham roberts ucl Symbiosis centre for distance learning (scdl)
Symbiosis centre for distance learning (scdl) Faculty model of training
Faculty model of training