General Chemistry M R NaimiJamal Faculty of Chemistry


















- Slides: 43

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology





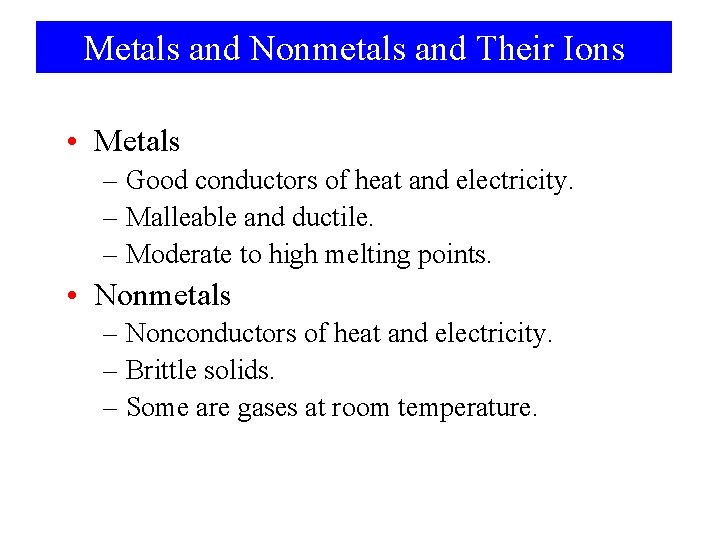
Metals and Nonmetals and Their Ions • Metals – Good conductors of heat and electricity. – Malleable and ductile. – Moderate to high melting points. • Nonmetals – Nonconductors of heat and electricity. – Brittle solids. – Some are gases at room temperature.

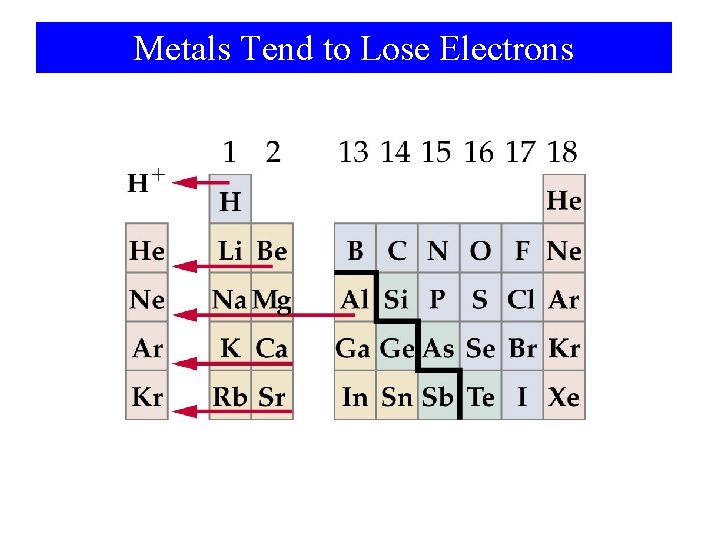
Metals Tend to Lose Electrons

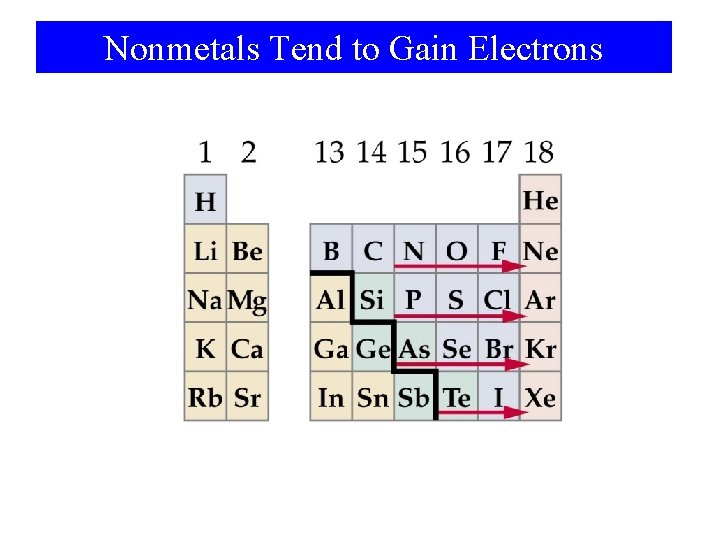
Nonmetals Tend to Gain Electrons

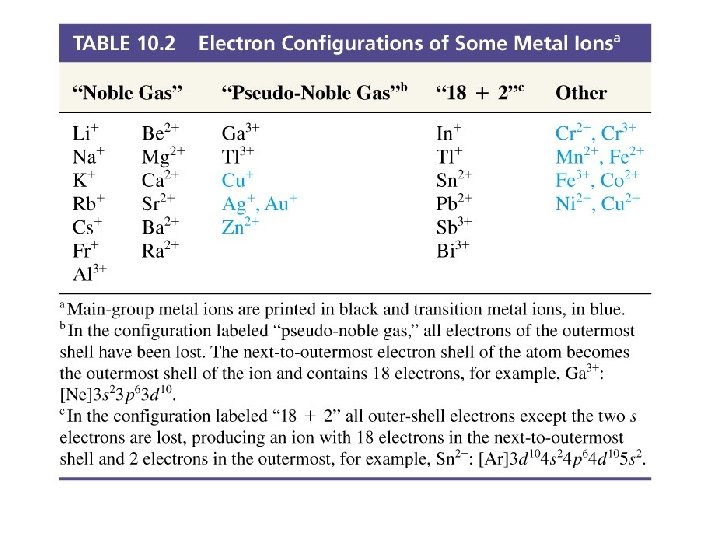
Electron Configuration of Some Ions



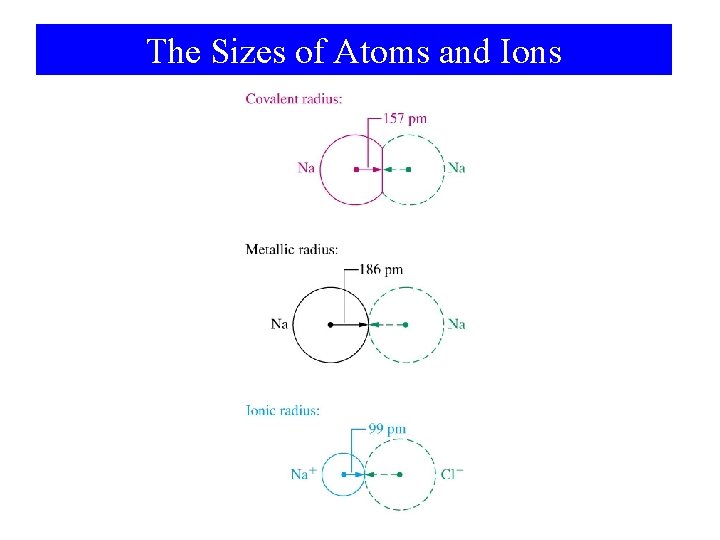
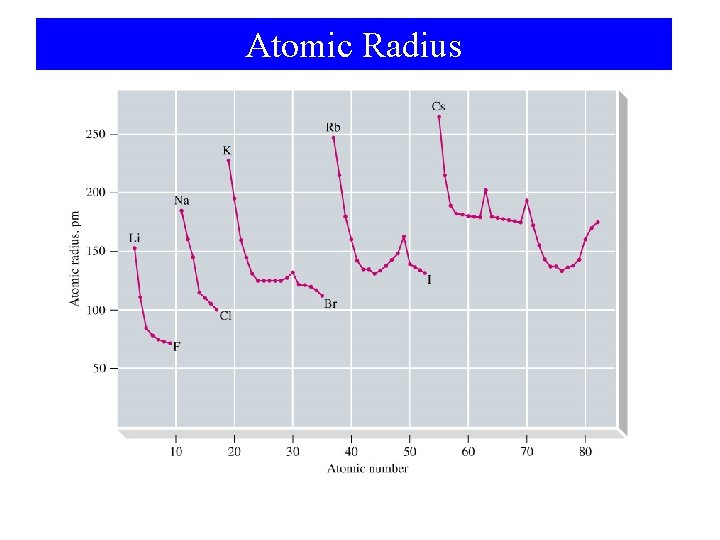
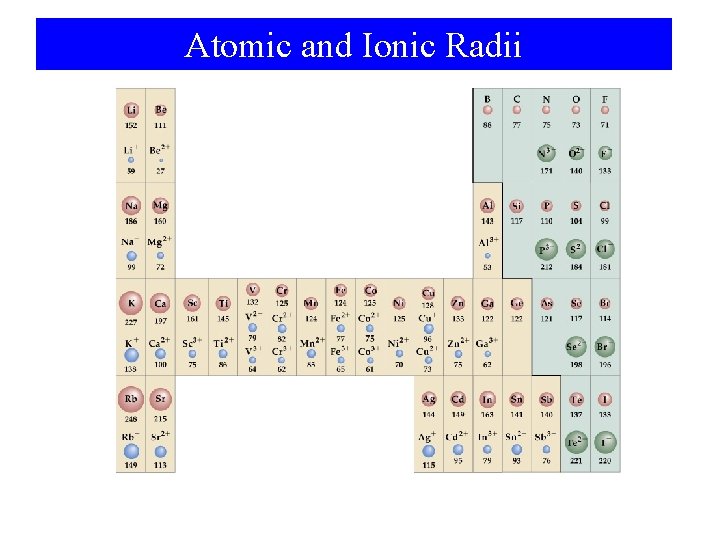
The Sizes of Atoms and Ions



Atomic Radius

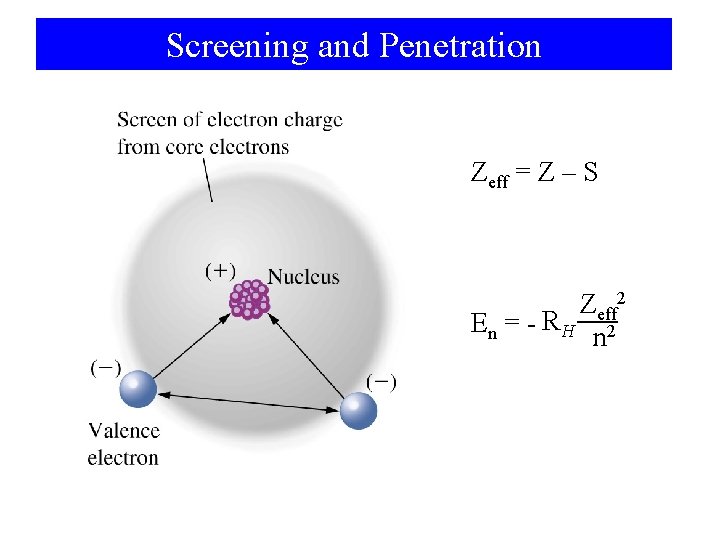
Screening and Penetration Zeff = Z – S Zeff 2 En = - RH 2 n

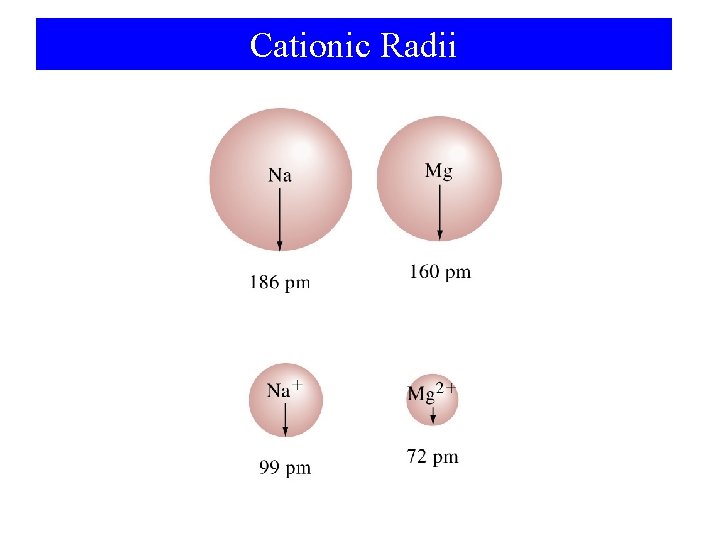
Cationic Radii

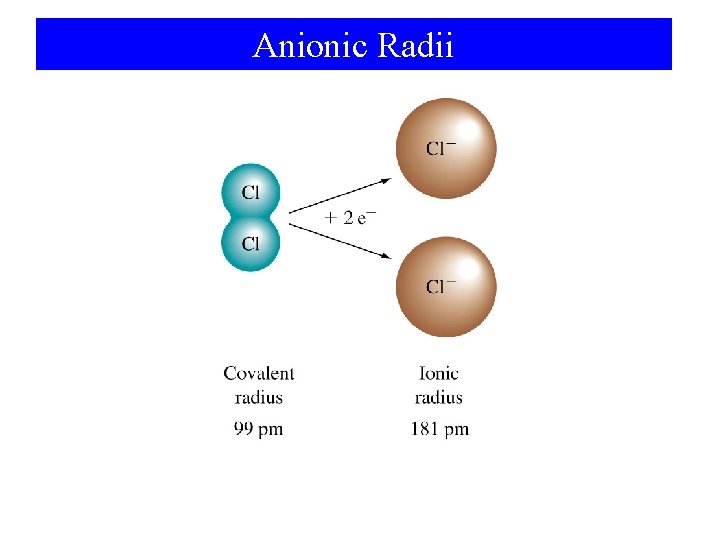
Anionic Radii

Atomic and Ionic Radii


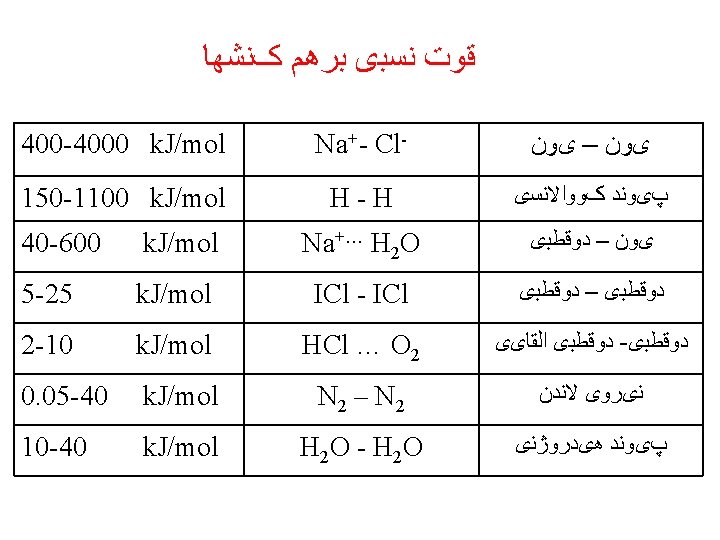
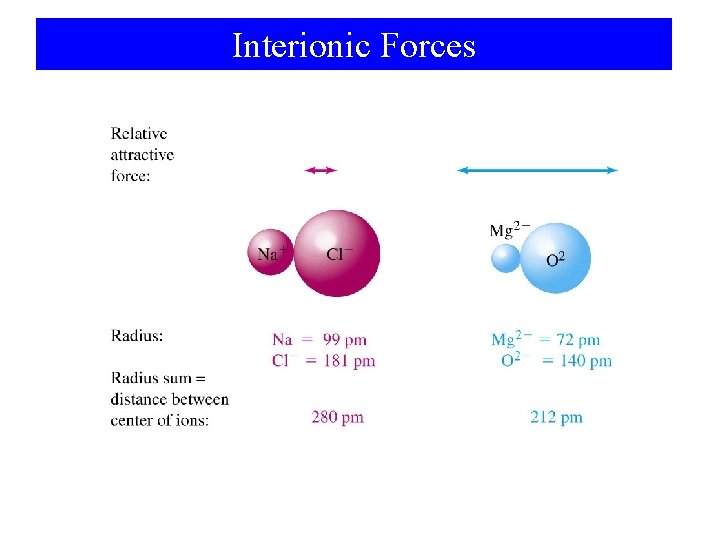
Interionic Forces


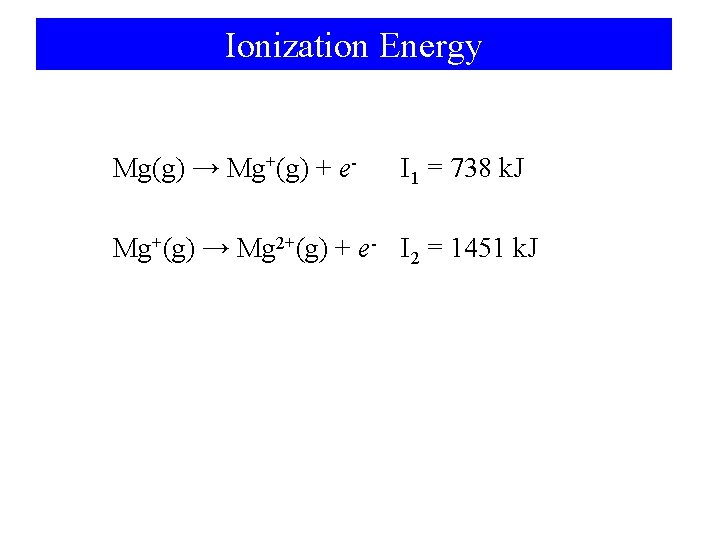
Ionization Energy Mg(g) → Mg+(g) + e- I 1 = 738 k. J Mg+(g) → Mg 2+(g) + e- I 2 = 1451 k. J

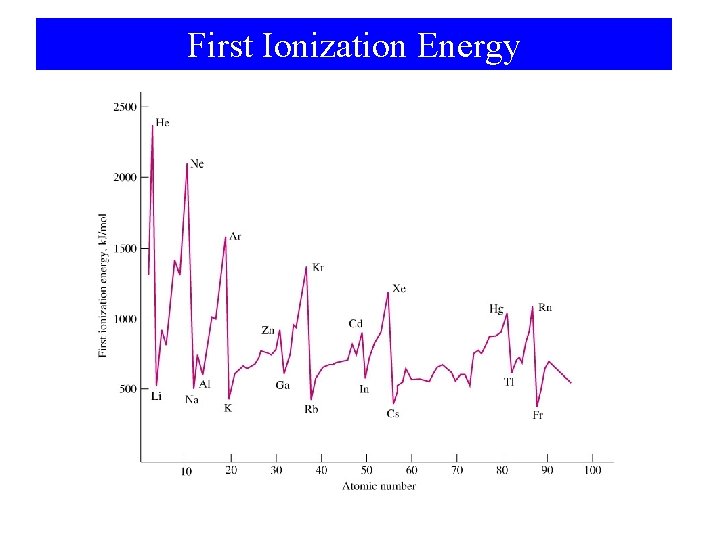
First Ionization Energy

Ionization Energy

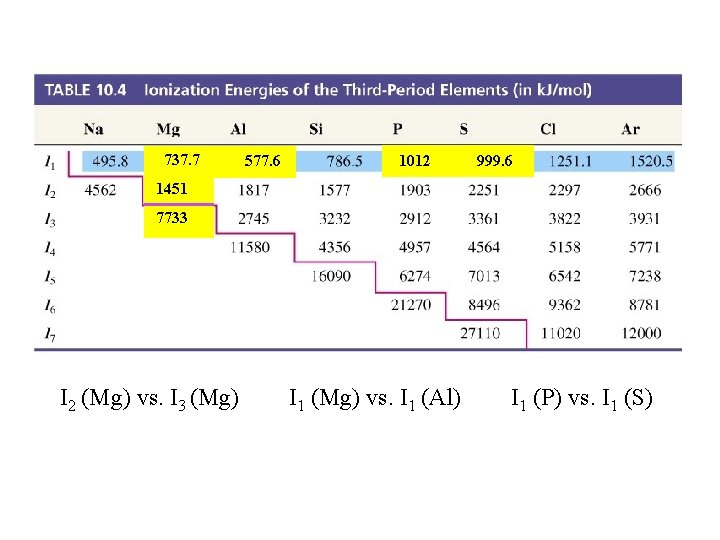
Table 10. 4 Ionization Energies of the Third-Period Elements (in k. J/mol) 737. 7 577. 6 1012 999. 6 1451 7733 I 2 (Mg) vs. I 3 (Mg) I 1 (Mg) vs. I 1 (Al) I 1 (P) vs. I 1 (S)


Electron Affinity F(g) + e- → F-(g) F (1 s 22 p 5) + EA = -328 k. J e- → Li(g) + e- → Li-(g) - F (1 s 22 p 6) EA = -59. 6 k. J

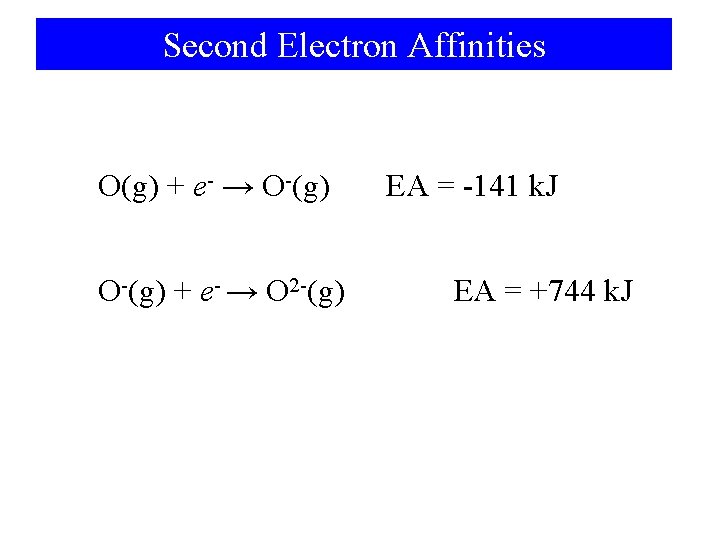
Second Electron Affinities O(g) + e- → O-(g) + e- → O 2 -(g) EA = -141 k. J EA = +744 k. J

First Electron Affinities

Electron Affinity

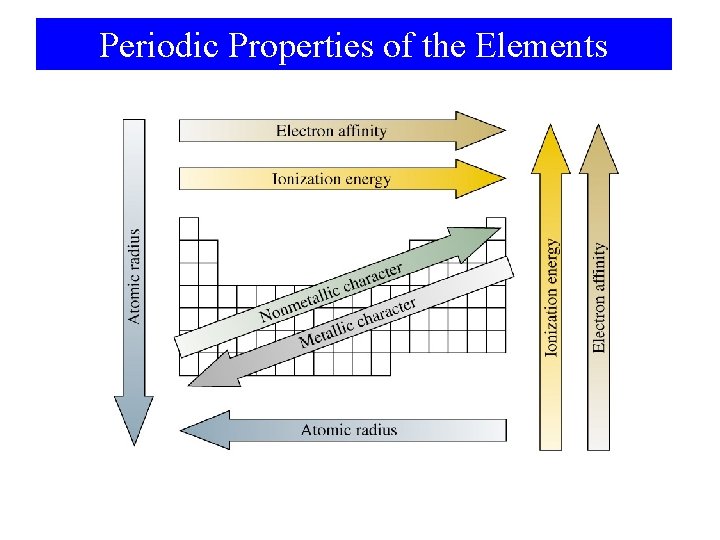
Periodic Properties of the Elements

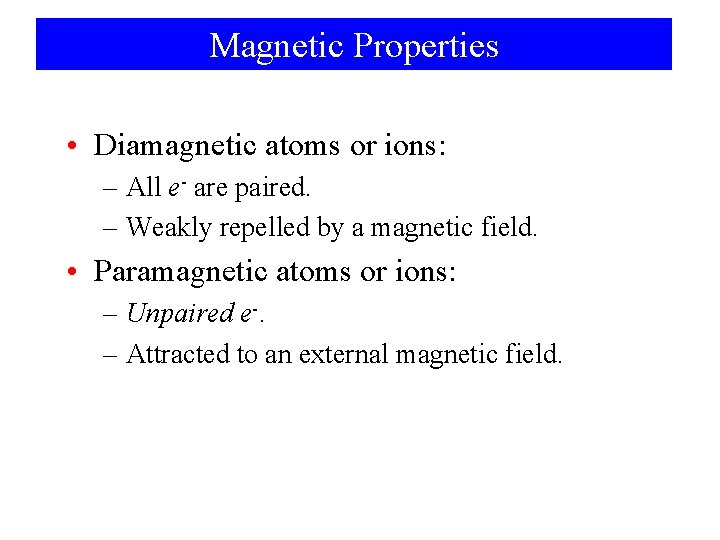
Magnetic Properties • Diamagnetic atoms or ions: – All e- are paired. – Weakly repelled by a magnetic field. • Paramagnetic atoms or ions: – Unpaired e-. – Attracted to an external magnetic field.

Paramagnetism

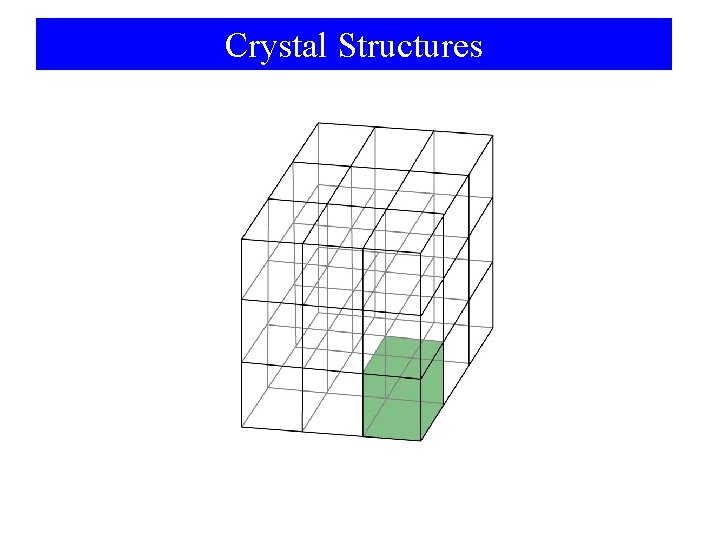
Crystal Structures

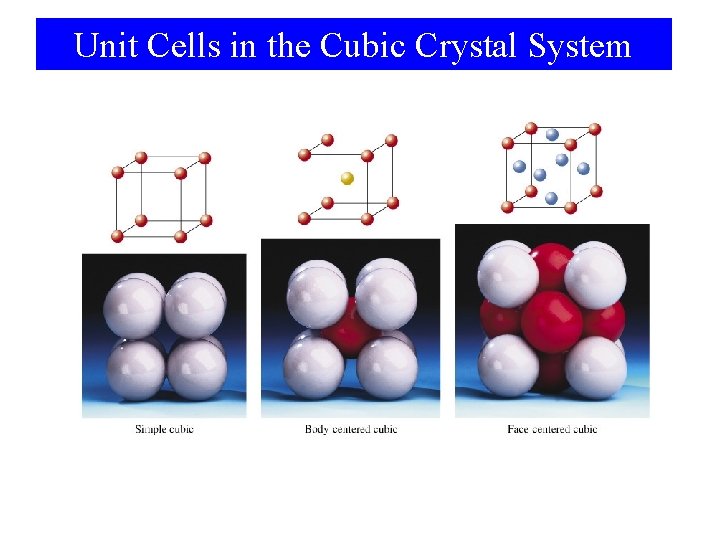
Unit Cells in the Cubic Crystal System

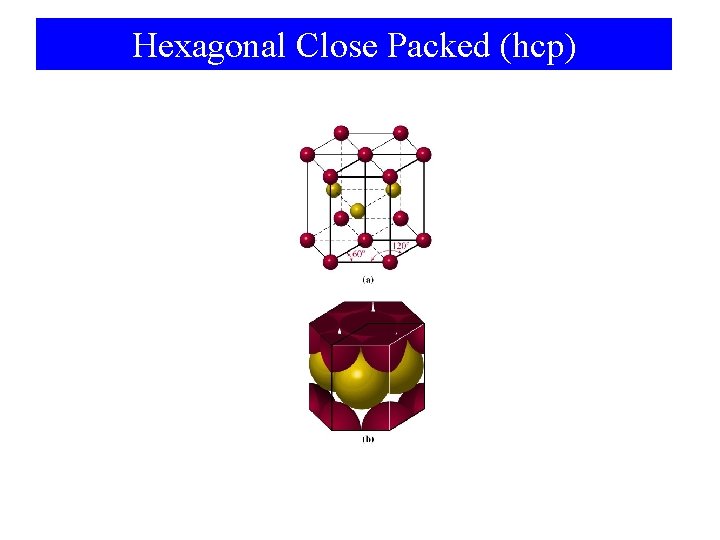
Hexagonal Close Packed (hcp)

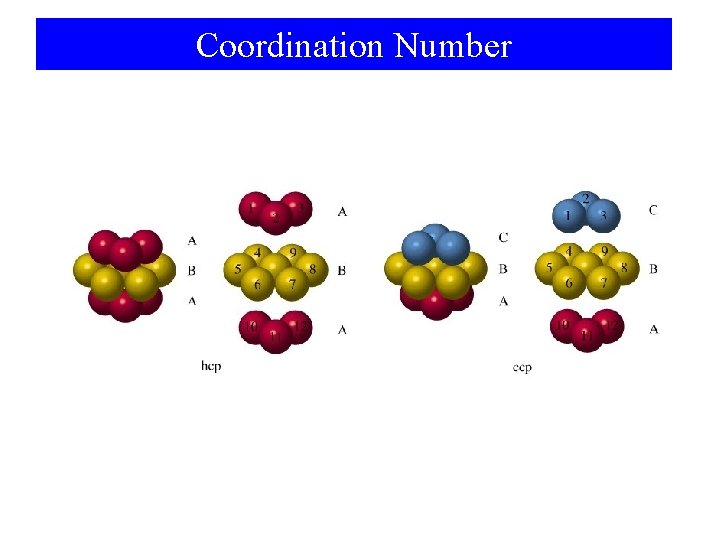
Coordination Number

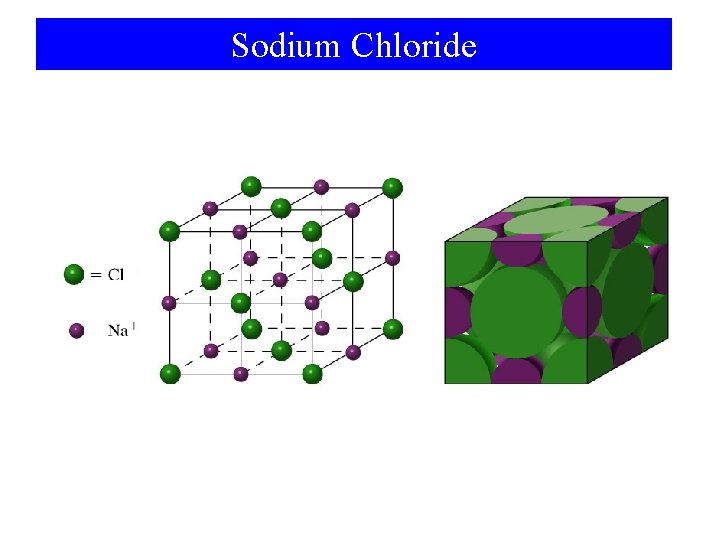
Sodium Chloride

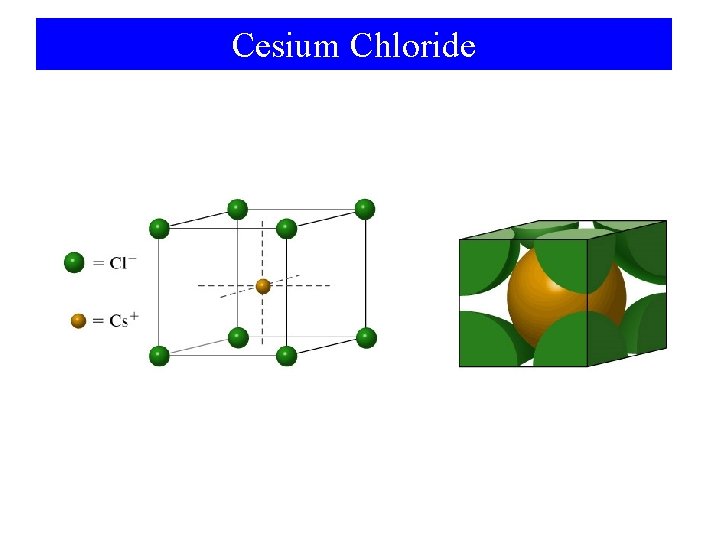
Cesium Chloride

Lattice Energy • Ionic compounds are solids under normal conditions and their structures contain positive and negative ions arranged in a 3 -D lattice • Lattice energy, ∆Elattice, is the energy of formation of one mole of solid crystalline ionic compound when ions in the gas phase combine • Tells us the strength of ionic bonding in solids on ion sizes and ion charges

Energy Changes in the Formation of Ionic Crystals

Chapter 7 Questions 5, 8, 14, 17, 20 24, 31, 38, 47
 Nit calicut chemistry faculty
Nit calicut chemistry faculty Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet University of bridgeport computer science
University of bridgeport computer science University of bridgeport engineering
University of bridgeport engineering Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Http://www-bcf.usc.edu/~gareth/isl/advertising.csv
Http://www-bcf.usc.edu/~gareth/isl/advertising.csv Solid thyroid nodule
Solid thyroid nodule Penn state neurosurgery
Penn state neurosurgery Mercy faculty forward
Mercy faculty forward Faculty of medicine nursing and health sciences
Faculty of medicine nursing and health sciences Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Carelli kutztown
Carelli kutztown Fsu cs faculty
Fsu cs faculty Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd ee
Umd ee Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Faculty 180 ecu
Faculty 180 ecu Faculty of engineering shoubra
Faculty of engineering shoubra Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs