General Chemistry M R NaimiJamal Faculty of Chemistry





- Slides: 57

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


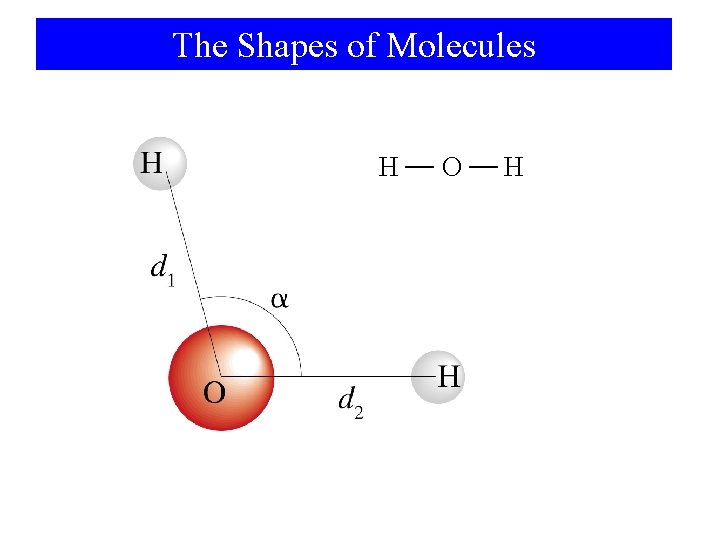
The Shapes of Molecules H O H

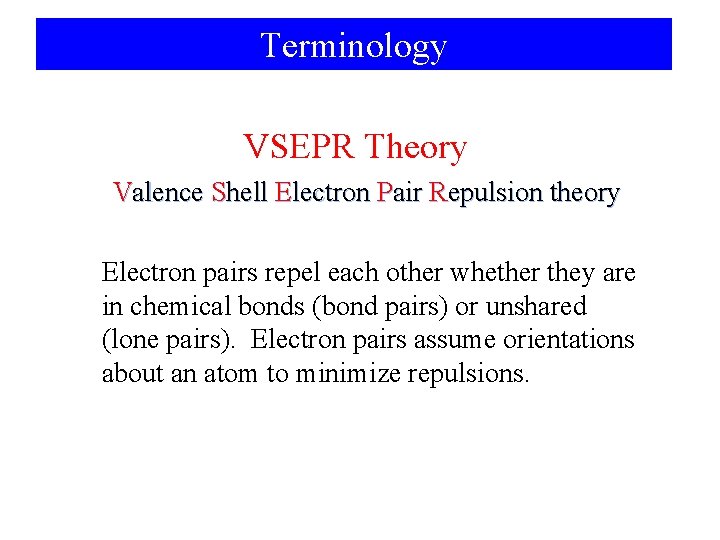
Terminology VSEPR Theory Valence Shell Electron Pair Repulsion theory Electron pairs repel each other whether they are in chemical bonds (bond pairs) or unshared (lone pairs). Electron pairs assume orientations about an atom to minimize repulsions.

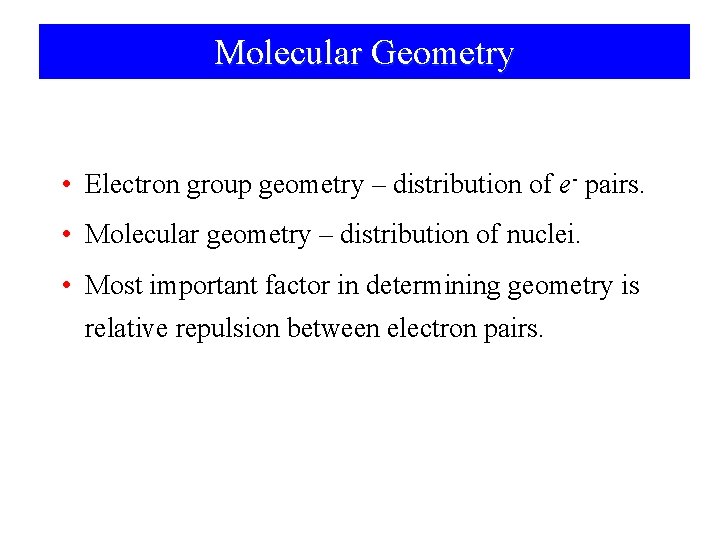
Molecular Geometry • Electron group geometry – distribution of e- pairs. • Molecular geometry – distribution of nuclei. • Most important factor in determining geometry is relative repulsion between electron pairs.

Balloon Analogy

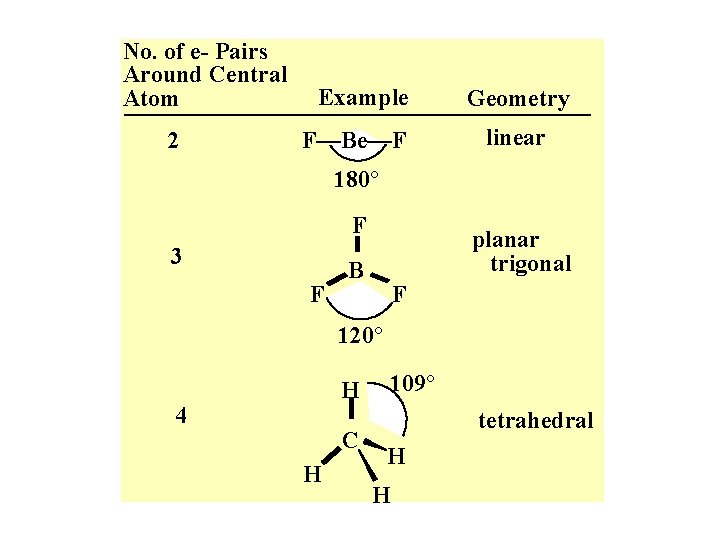
No. of e- Pairs Around Central Atom 2 Example F—Be—F Geometry linear 180° F 3 F planar trigonal B F 120° H 4 C H 109° tetrahedral H H

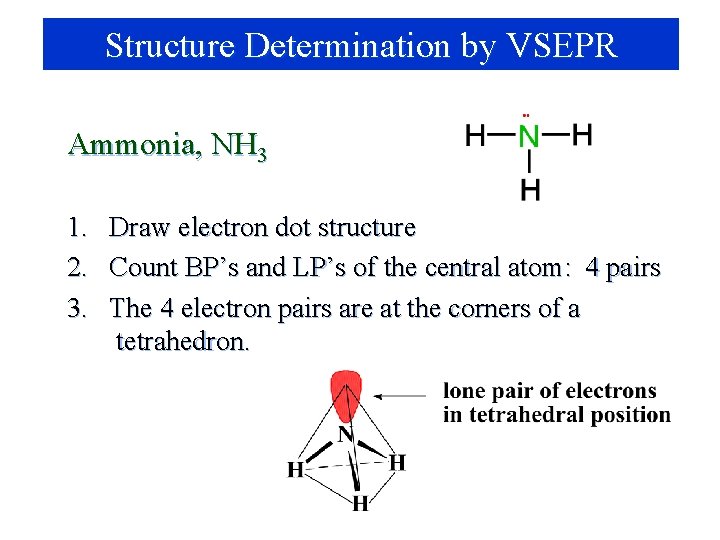
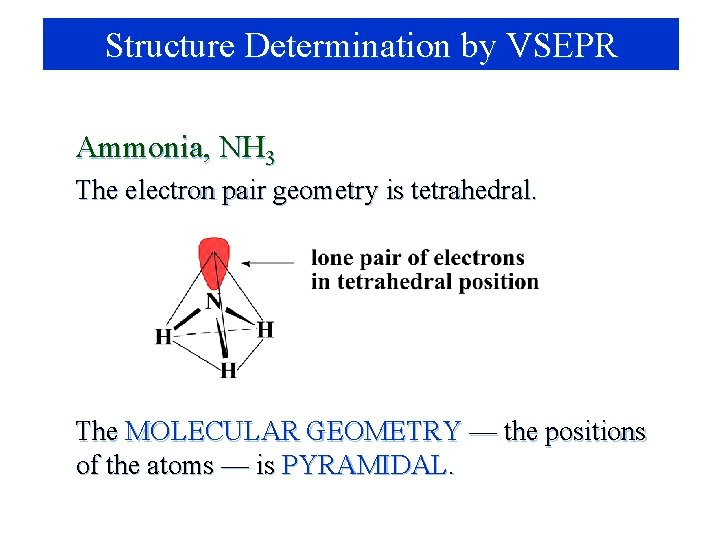
Structure Determination by VSEPR Ammonia, NH 3 1. 2. 3. Draw electron dot structure Count BP’s and LP’s of the central atom: 4 pairs The 4 electron pairs are at the corners of a tetrahedron.

Structure Determination by VSEPR Ammonia, NH 3 The electron pair geometry is tetrahedral. The MOLECULAR GEOMETRY — the positions of the atoms — is PYRAMIDAL.

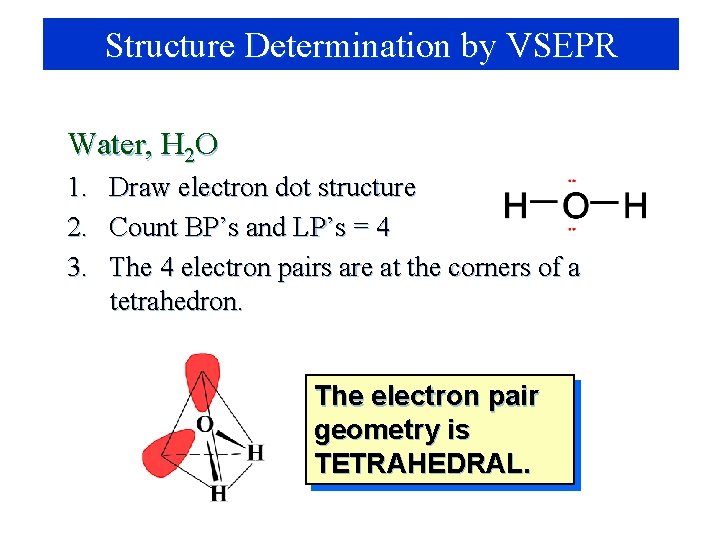
Structure Determination by VSEPR Water, H 2 O 1. 2. 3. Draw electron dot structure Count BP’s and LP’s = 4 The 4 electron pairs are at the corners of a tetrahedron. The electron pair geometry is TETRAHEDRAL.

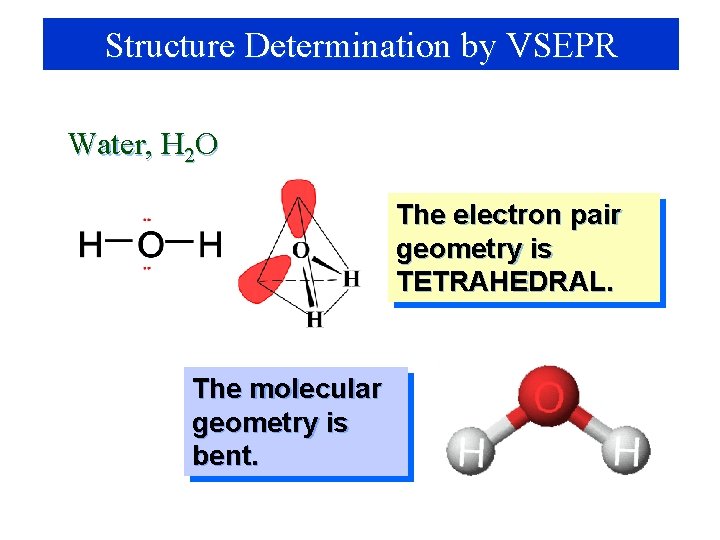
Structure Determination by VSEPR Water, H 2 O The electron pair geometry is TETRAHEDRAL. The molecular geometry is bent.

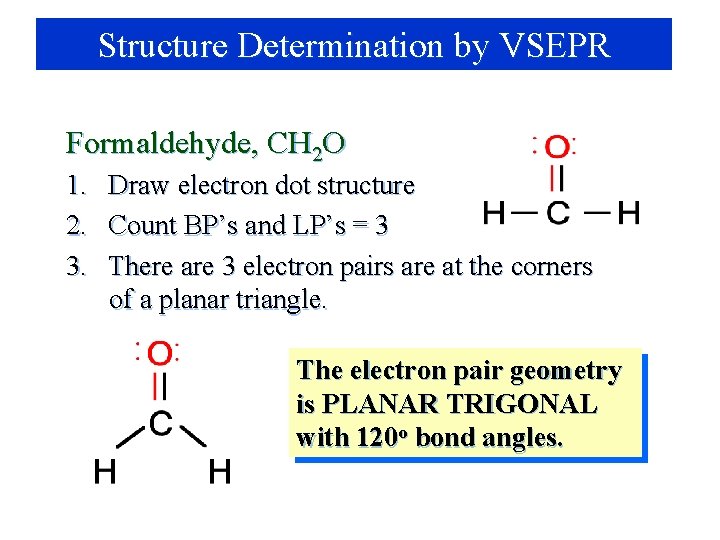
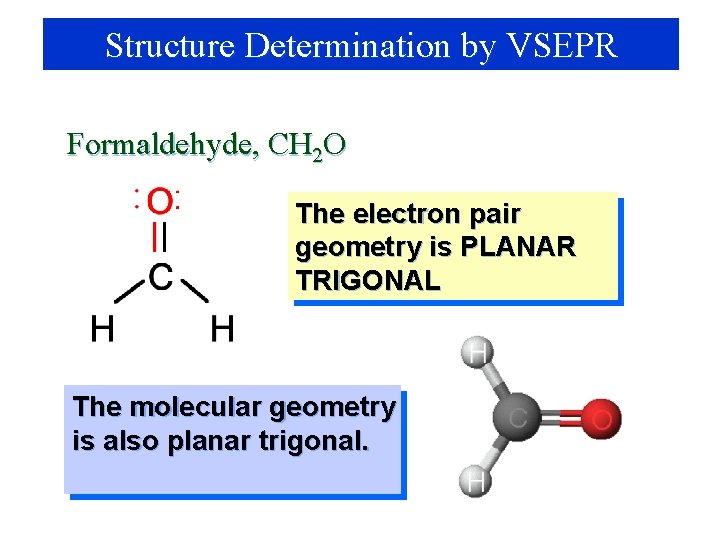
Structure Determination by VSEPR Formaldehyde, CH 2 O 1. Draw electron dot structure 2. Count BP’s and LP’s = 3 3. There are 3 electron pairs are at the corners of a planar triangle. The electron pair geometry is PLANAR TRIGONAL with 120 o bond angles.

Structure Determination by VSEPR Formaldehyde, CH 2 O The electron pair geometry is PLANAR TRIGONAL The molecular geometry is also planar trigonal.

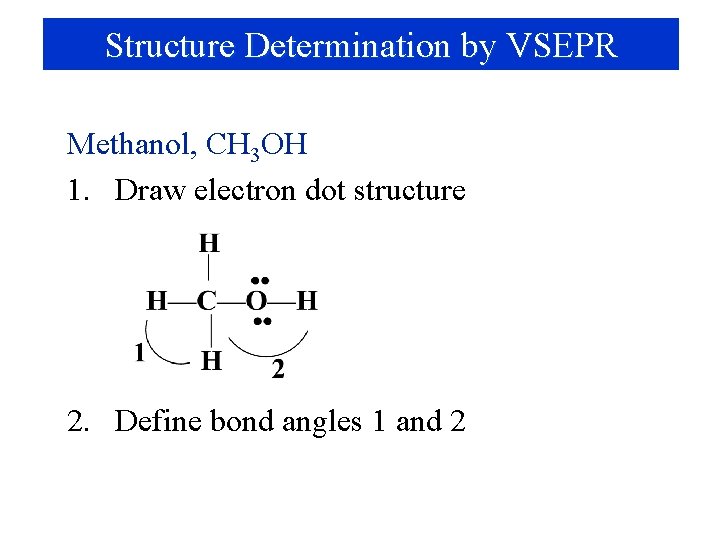
Structure Determination by VSEPR Methanol, CH 3 OH 1. Draw electron dot structure 2. Define bond angles 1 and 2

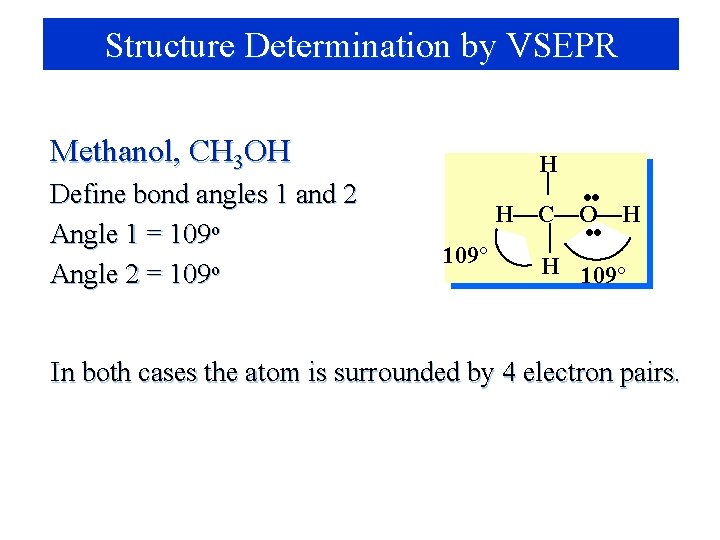
Structure Determination by VSEPR Methanol, CH 3 OH Define bond angles 1 and 2 Angle 1 = 109 o Angle 2 = 109 o H • • H—C—O—H • • 109° H 109° In both cases the atom is surrounded by 4 electron pairs.

Structure Determination by VSEPR Acetonitrile, CH 3 CN Draw the electron dot structure

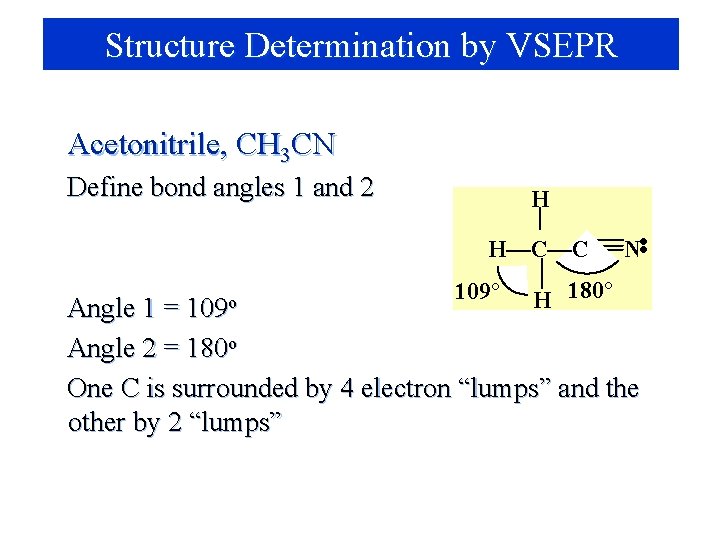
Structure Determination by VSEPR Acetonitrile, CH 3 CN Define bond angles 1 and 2 H 109 o 109° H 180° N • • H—C—C Angle 1 = Angle 2 = 180 o One C is surrounded by 4 electron “lumps” and the other by 2 “lumps”

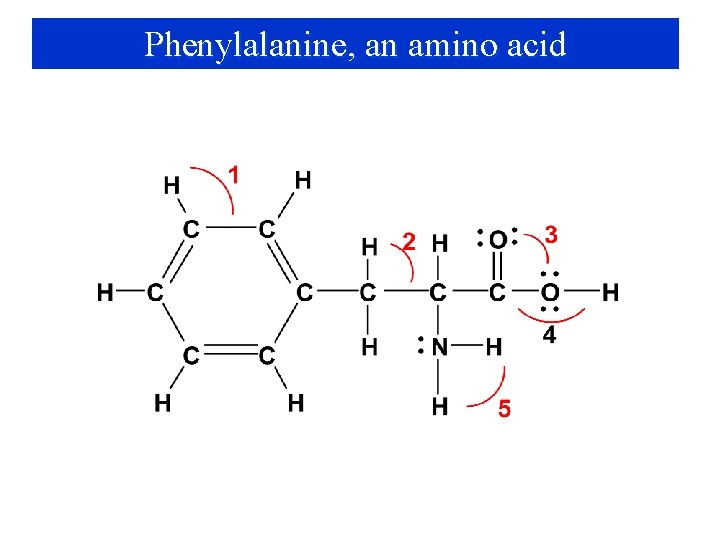
Phenylalanine, an amino acid

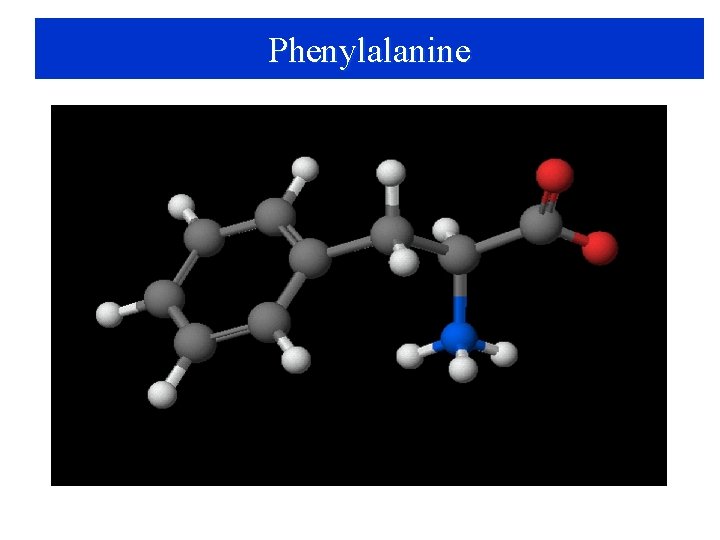
Phenylalanine

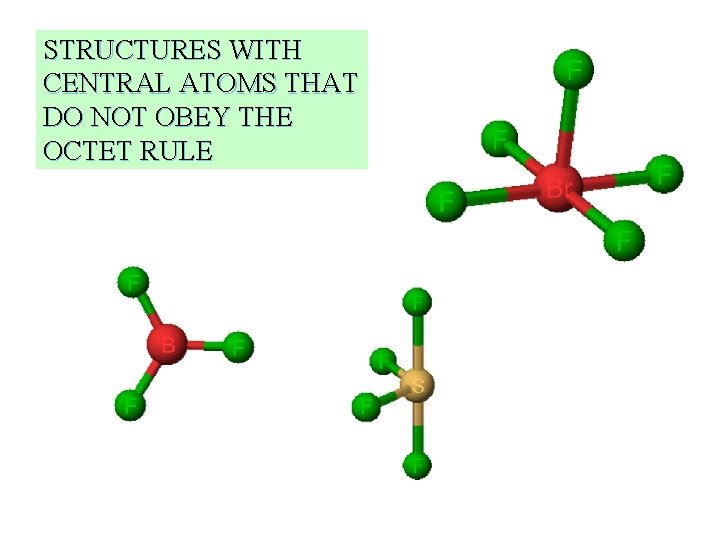
STRUCTURES WITH CENTRAL ATOMS THAT DO NOT OBEY THE OCTET RULE

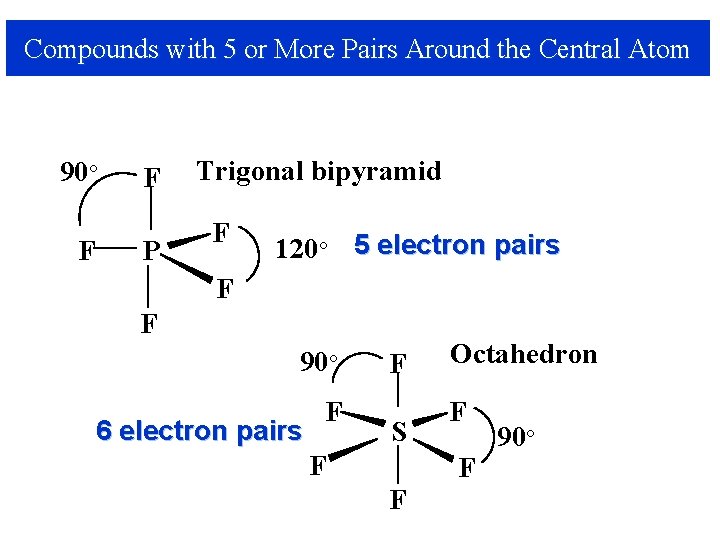
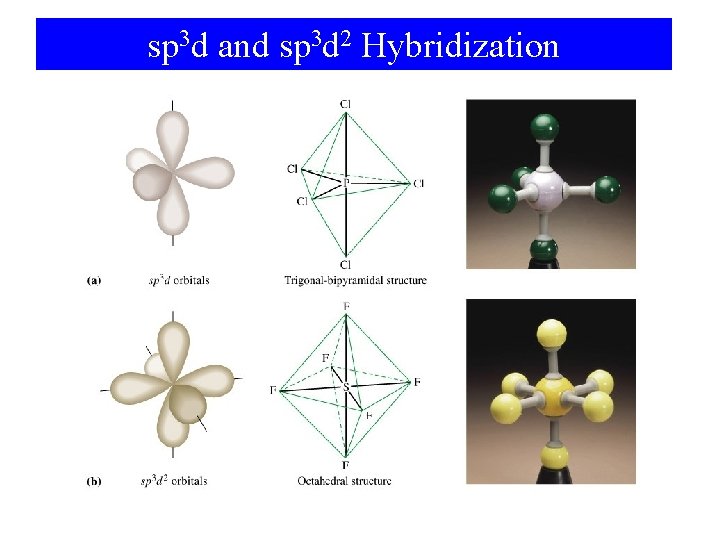
Compounds with 5 or More Pairs Around the Central Atom 90° F F P Trigonal bipyramid F 120° 5 electron pairs F F 90° 6 electron pairs F F S F Octahedron F F F 90°

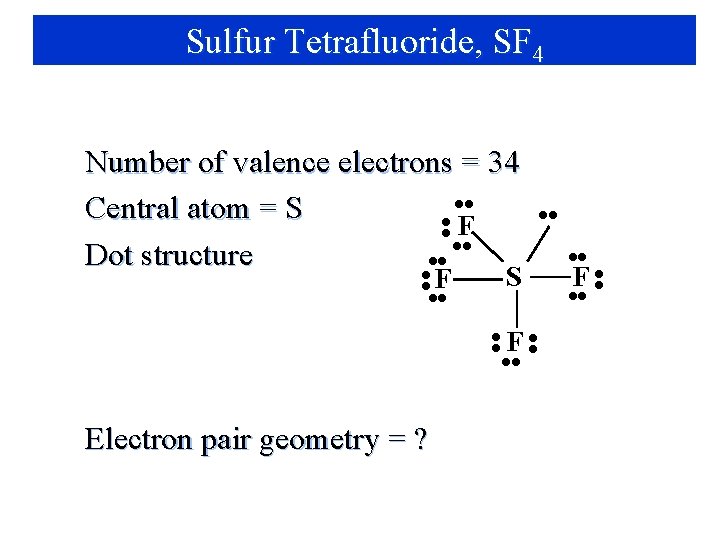
Sulfur Tetrafluoride, SF 4 Number of valence electrons = 34 • • Central atom = S • • F • • Dot structure • • F • • S • • F • • Electron pair geometry = ? F • •

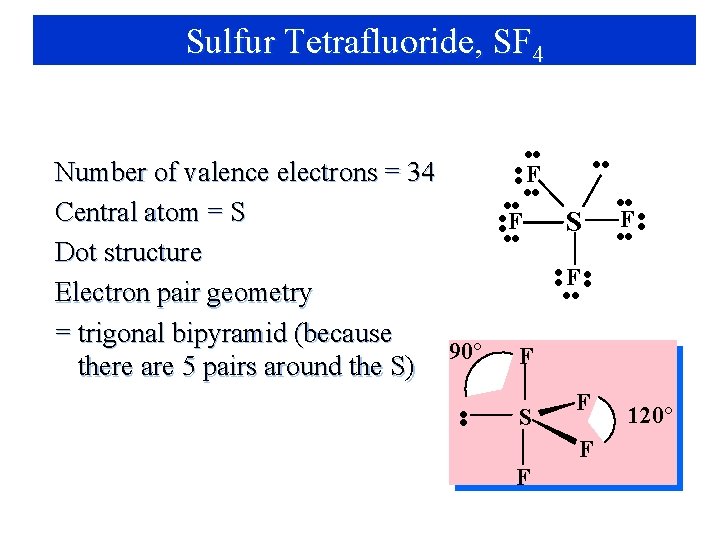
Sulfur Tetrafluoride, SF 4 Number of valence electrons = 34 Central atom = S Dot structure Electron pair geometry = trigonal bipyramid (because 90° there are 5 pairs around the S) • • • • F • • S • • F • • F S F F F 120°

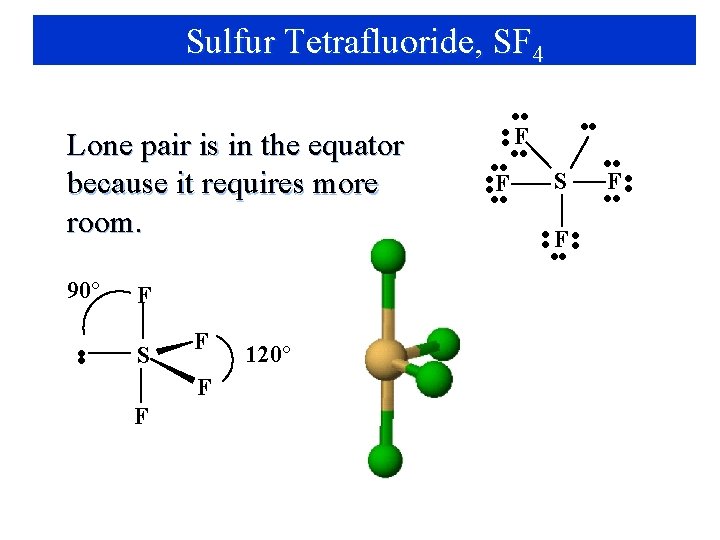
Sulfur Tetrafluoride, SF 4 Lone pair is in the equator because it requires more room. 90° F • • S F F F 120° • • F • • S • • F • •

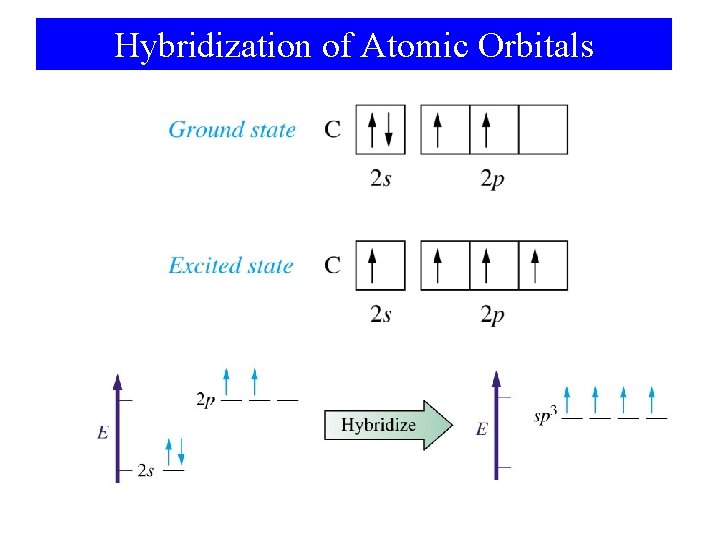
Hybridization of Atomic Orbitals

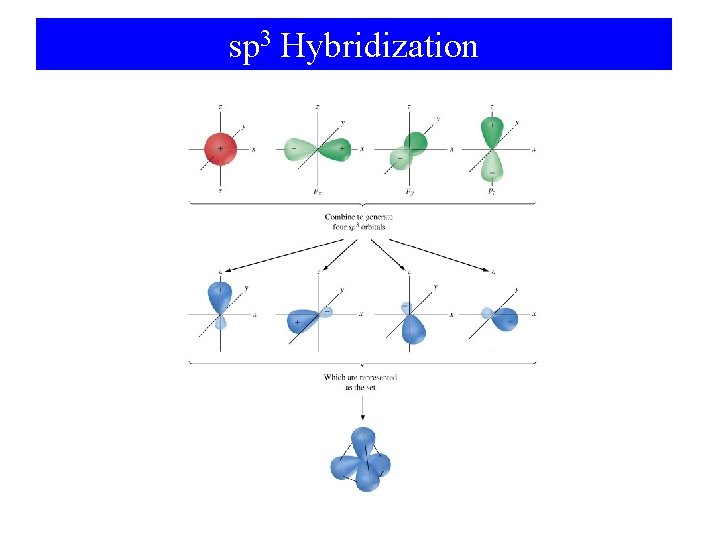
sp 3 Hybridization

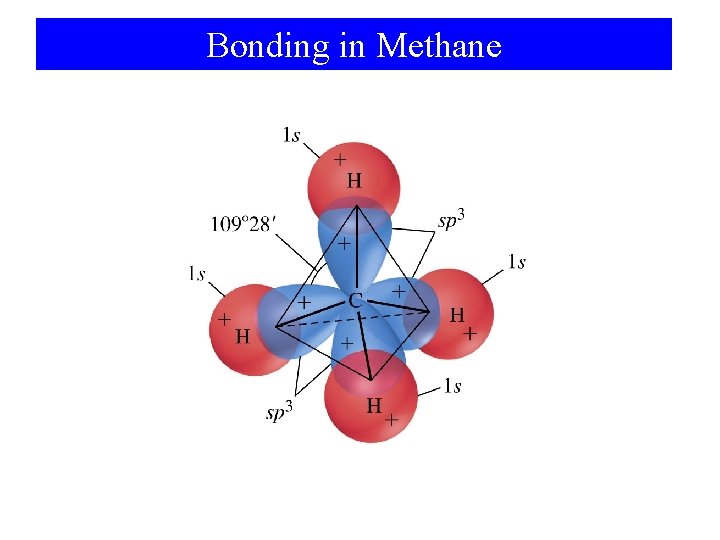
Bonding in Methane

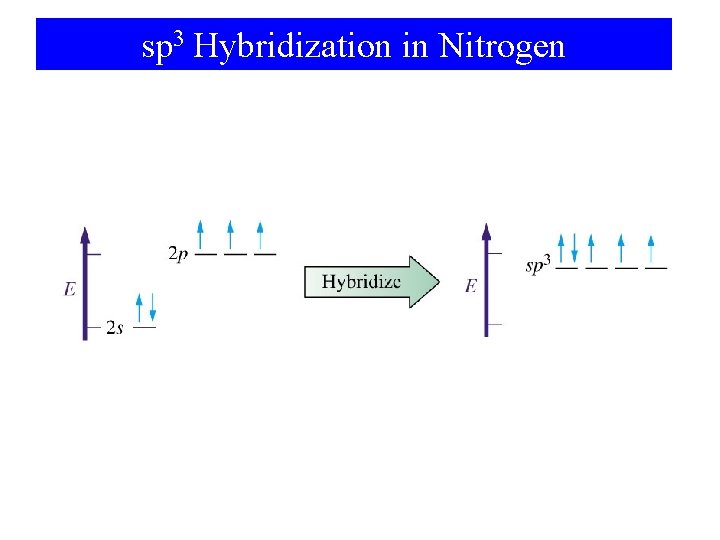
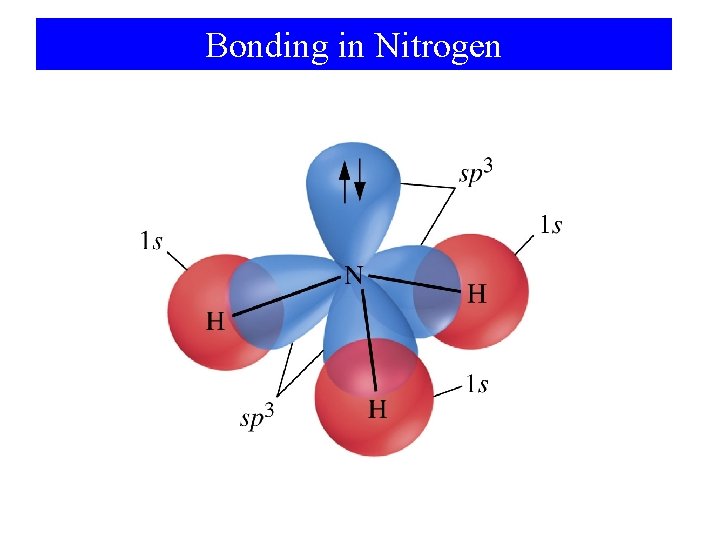
sp 3 Hybridization in Nitrogen

Bonding in Nitrogen

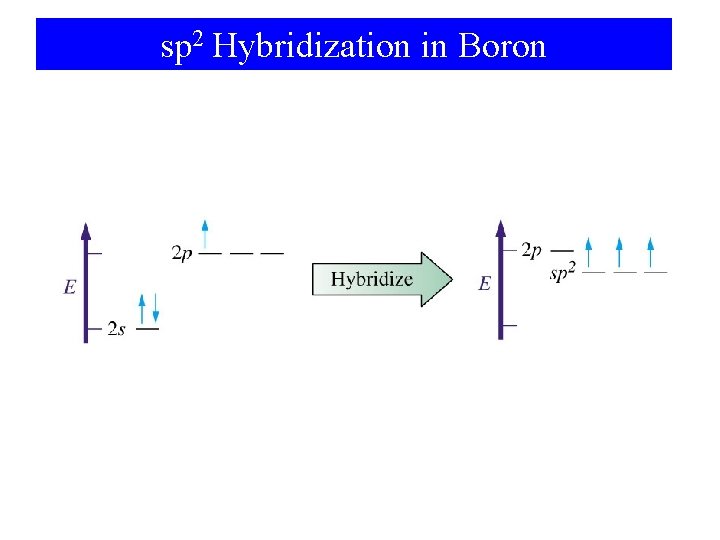
sp 2 Hybridization in Boron

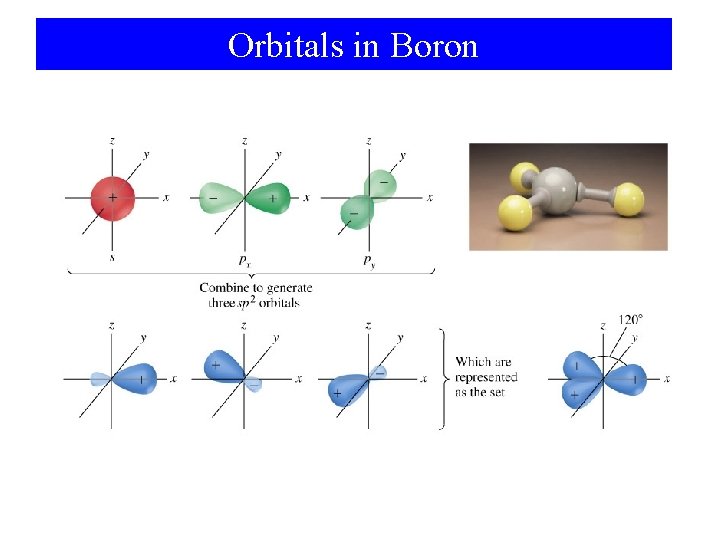
Orbitals in Boron

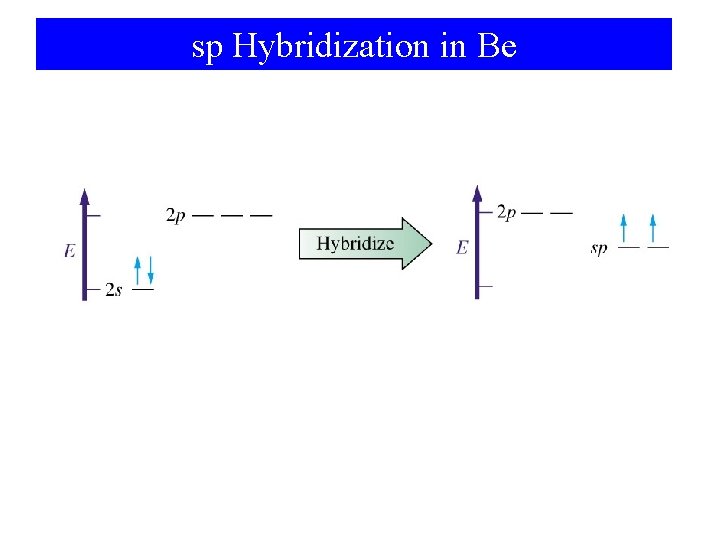
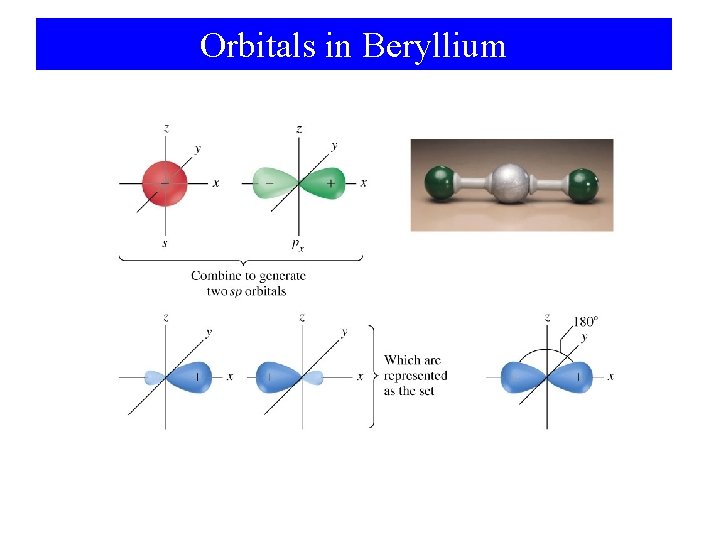
sp Hybridization in Be

Orbitals in Beryllium

sp 3 d and sp 3 d 2 Hybridization

Hybrid Orbitals and VSEPR • Write a plausible Lewis structure. • Use VSEPR to predict electron geometry. • Select the appropriate hybridization.

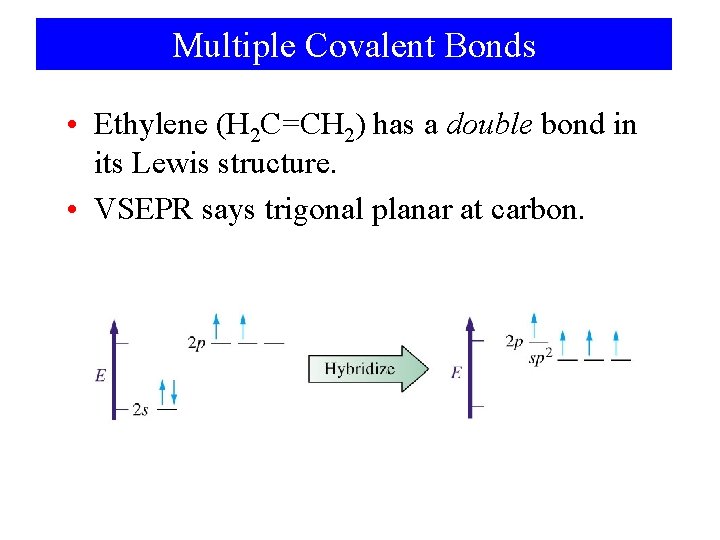
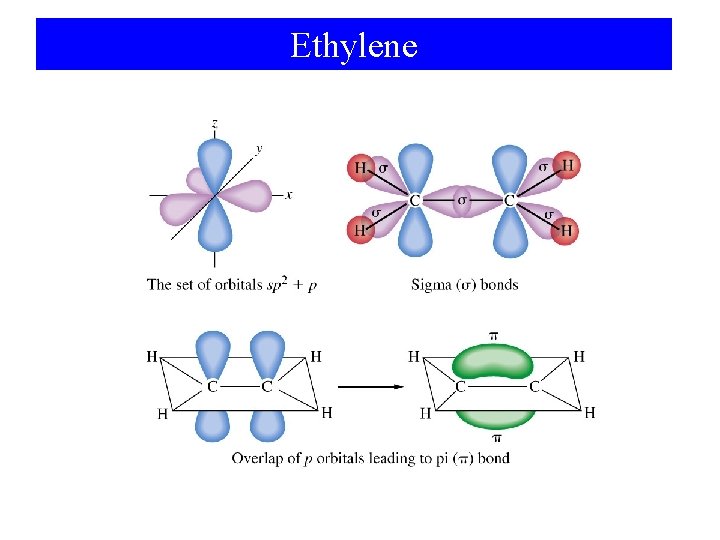
Multiple Covalent Bonds • Ethylene (H 2 C=CH 2) has a double bond in its Lewis structure. • VSEPR says trigonal planar at carbon.

Ethylene

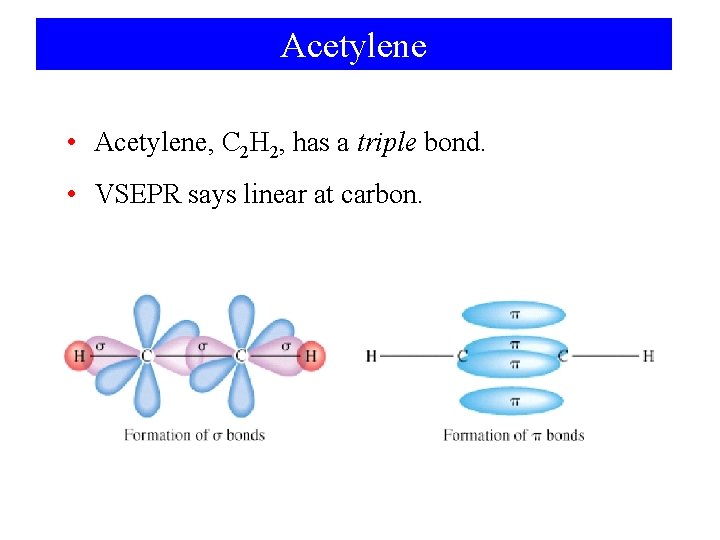
Acetylene • Acetylene, C 2 H 2, has a triple bond. • VSEPR says linear at carbon.

Applying VSEPR Theory • Draw a plausible Lewis structure. • Determine the number of e- groups and identify them as bond or lone pairs. • Establish the e- group geometry. • Determine the molecular geometry. • Multiple bonds count as one group of electrons. • More than one central atom can be handled individually.

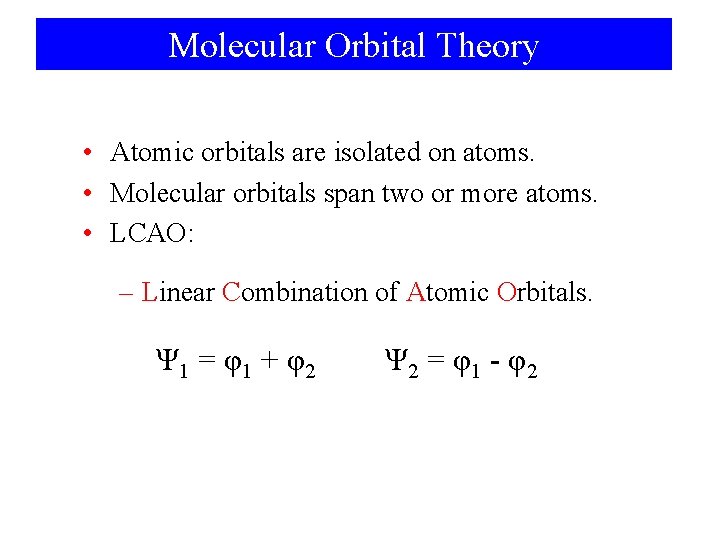
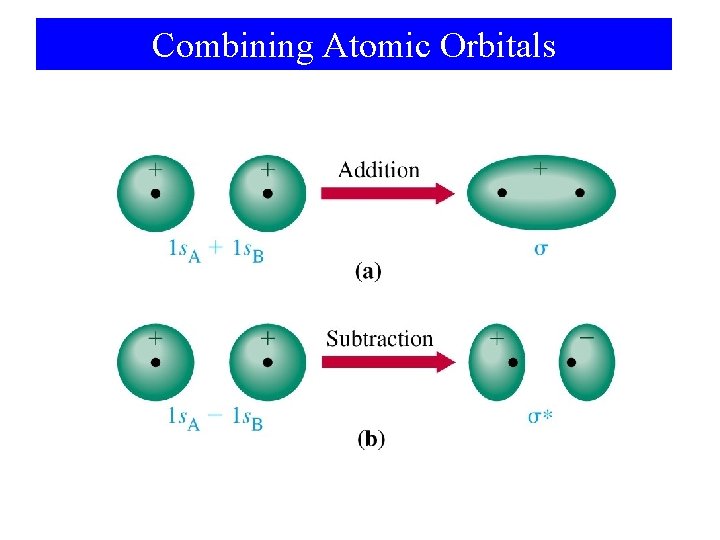
Molecular Orbital Theory • Atomic orbitals are isolated on atoms. • Molecular orbitals span two or more atoms. • LCAO: – Linear Combination of Atomic Orbitals. Ψ 1 = φ1 + φ2 Ψ 2 = φ1 - φ2

Combining Atomic Orbitals

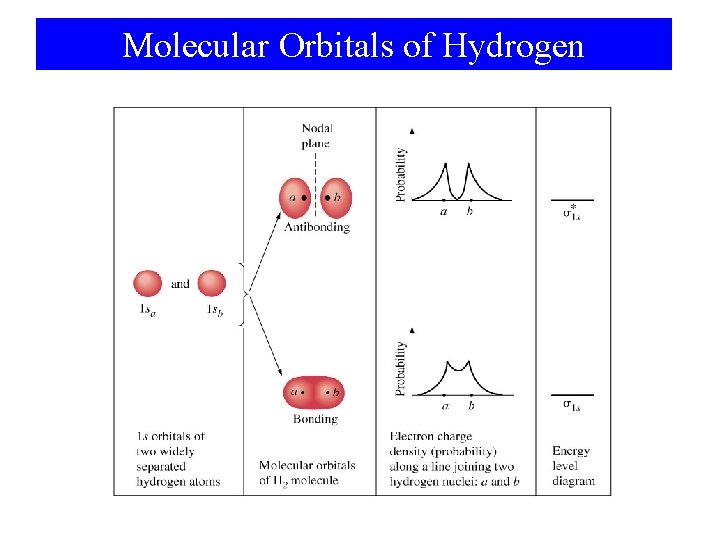
Molecular Orbitals of Hydrogen

Basic Ideas Concerning MOs • Number of MOs = Number of AOs. • Bonding and antibonding MOs formed from AOs. • e- fill the lowest energy MO first. • Pauli exclusion principle is followed. • Hund’s rule is followed

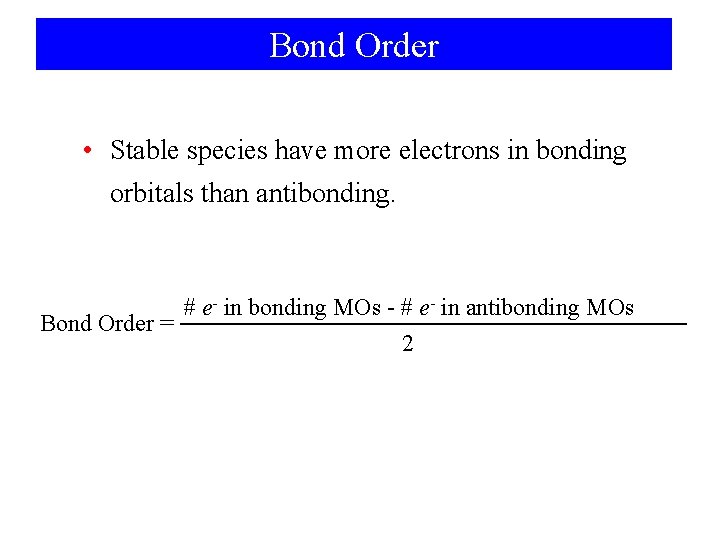
Bond Order • Stable species have more electrons in bonding orbitals than antibonding. # e- in bonding MOs - # e- in antibonding MOs Bond Order = 2

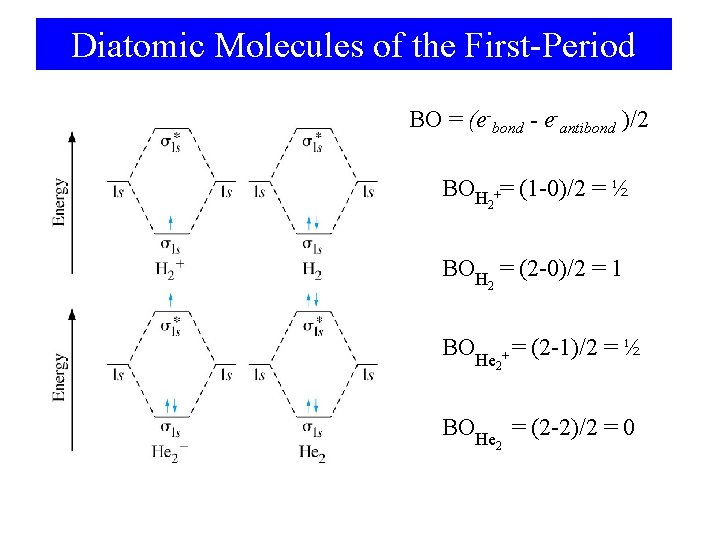
Diatomic Molecules of the First-Period BO = (e-bond - e-antibond )/2 BOH += (1 -0)/2 = ½ 2 BOH = (2 -0)/2 = 1 2 BOHe + = (2 -1)/2 = ½ 2 BOHe = (2 -2)/2 = 0 2

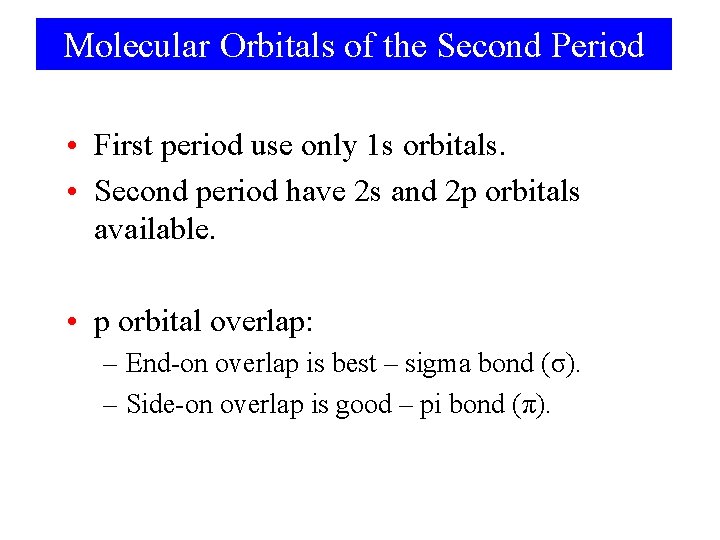
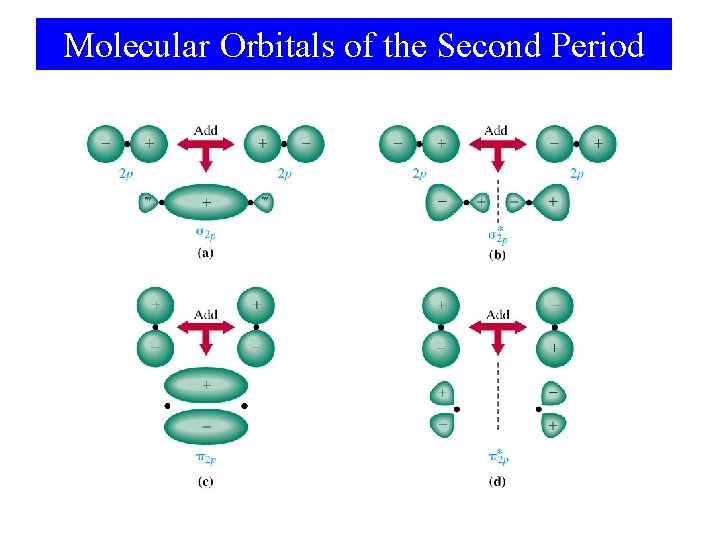
Molecular Orbitals of the Second Period • First period use only 1 s orbitals. • Second period have 2 s and 2 p orbitals available. • p orbital overlap: – End-on overlap is best – sigma bond (σ). – Side-on overlap is good – pi bond (π).

Molecular Orbitals of the Second Period

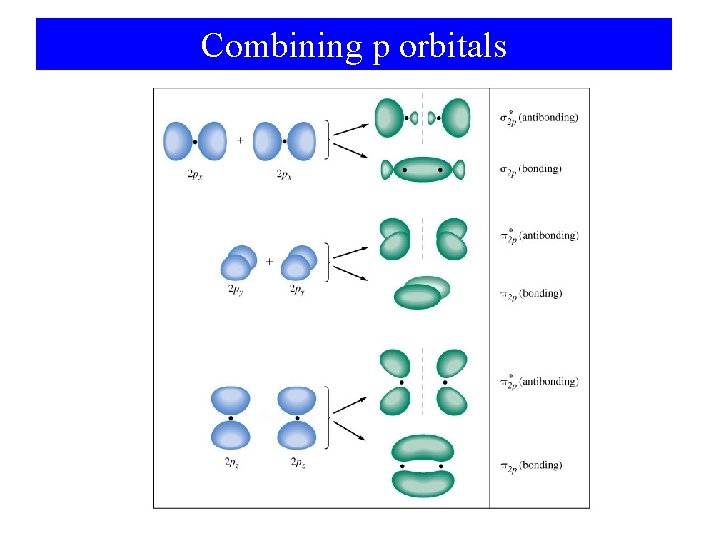
Combining p orbitals

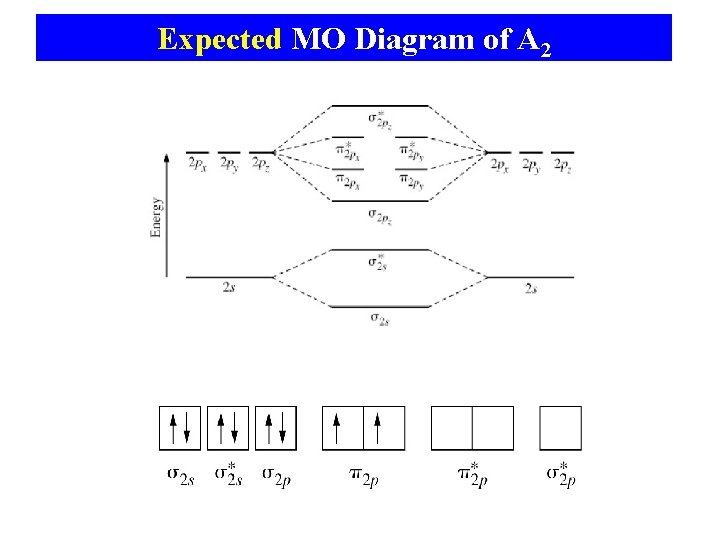
Expected MO Diagram of A 2

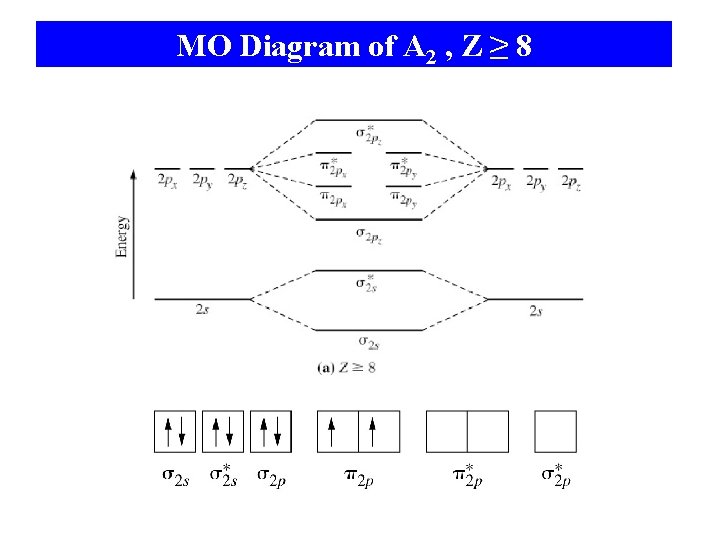
MO Diagram of A 2 , Z ≥ 8

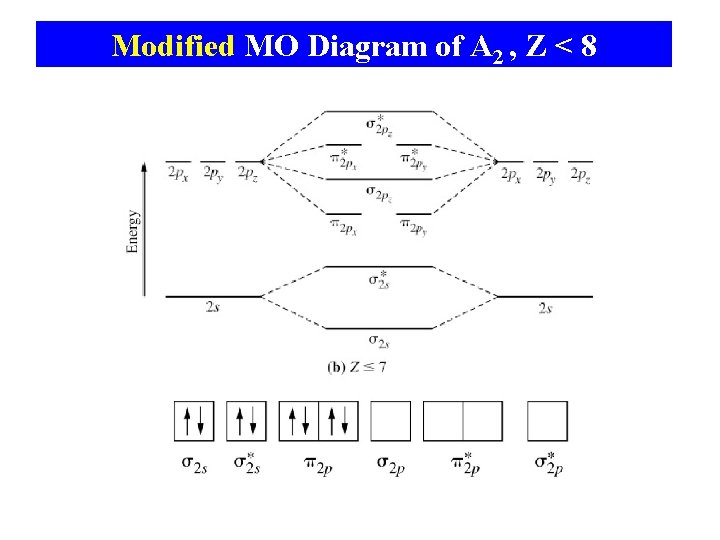
Modified MO Diagram of A 2 , Z < 8

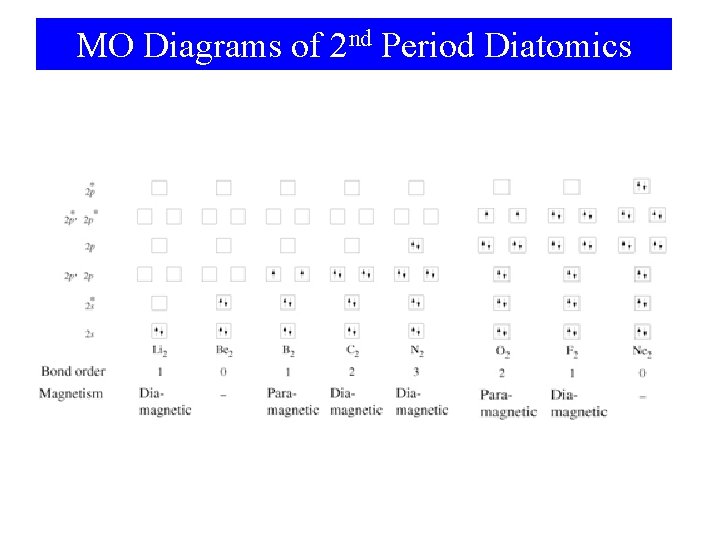
MO Diagrams of 2 nd Period Diatomics


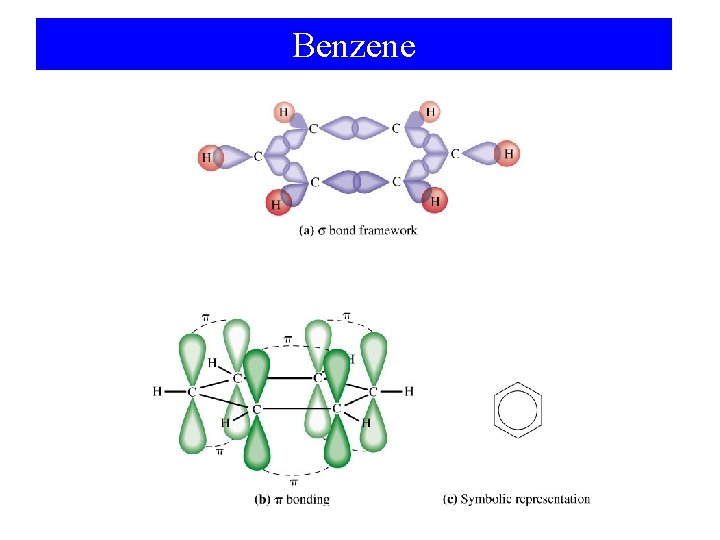
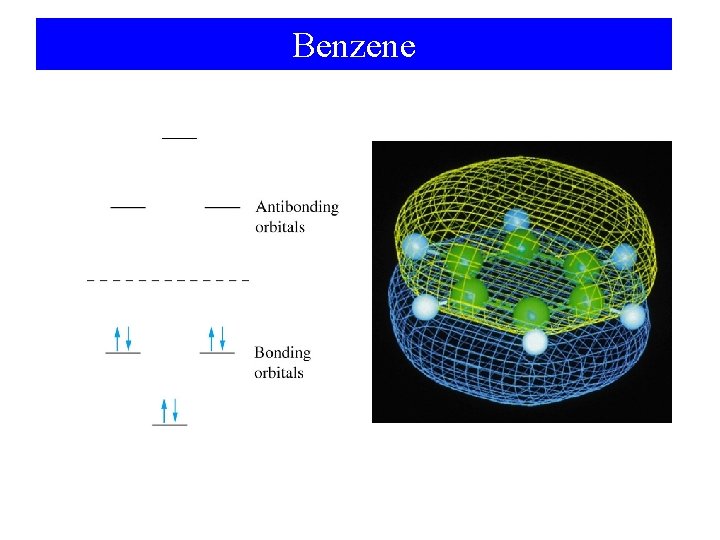
Benzene

Benzene

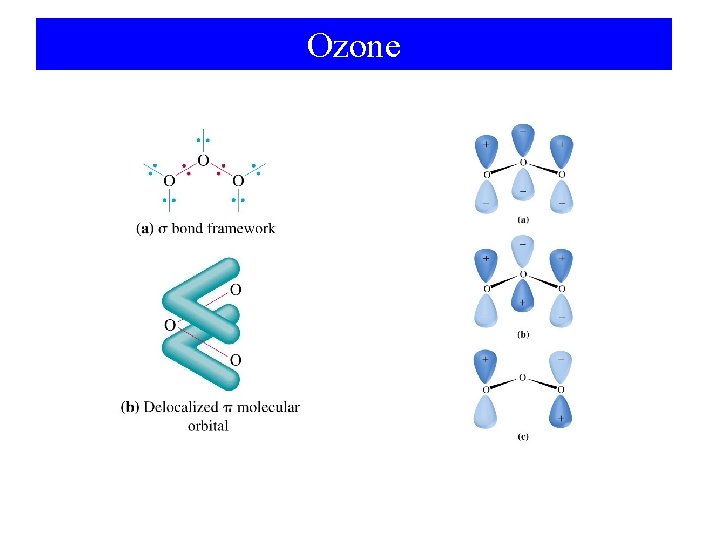
Ozone

Chapter 9 Questions 7, 16, 22, 25, 33 34, 35, 43
 Nit calicut chemistry faculty
Nit calicut chemistry faculty Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 Organik kimya adlandırma öncelik sırası
Organik kimya adlandırma öncelik sırası General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science faculty
University of bridgeport computer science faculty Bridgeport university computer science
Bridgeport university computer science Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school powerschool
Hahnville high school powerschool Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine 002
002 Semmelweis
Semmelweis Penn state neurosurgery
Penn state neurosurgery Mercy college adjunct positions
Mercy college adjunct positions Faculty of medicine nursing and health sciences
Faculty of medicine nursing and health sciences Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Carelli
Carelli Fsu computer science department
Fsu computer science department Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Electrical engineering umd
Electrical engineering umd Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Czech technical university in prague civil engineering
Czech technical university in prague civil engineering Faculty 180 ecu
Faculty 180 ecu Faculty of engineering shoubra
Faculty of engineering shoubra Singularity university faculty
Singularity university faculty Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Mathematical computation ucl
Mathematical computation ucl Elibrary symbiosis
Elibrary symbiosis Training and development metrics
Training and development metrics Short story for memory test
Short story for memory test Agnes csaki semmelweis
Agnes csaki semmelweis Parson kutztown
Parson kutztown Student faculty ratio for nba
Student faculty ratio for nba Kfupm faculty housing pictures
Kfupm faculty housing pictures Ascaris lumbricoides ova
Ascaris lumbricoides ova Faculty model of training
Faculty model of training Utd cs1 server
Utd cs1 server Fulbright faculty development program
Fulbright faculty development program