Organic Chemistry M R NaimiJamal Faculty of Chemistry





















- Slides: 71

Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology

Chapter 2. Alkanes and Cycloalkanes Based on: Mc. Murry’s Fundamental of Organic Chemistry, 4 th edition, Chapter 2

Families of Organic Compounds n Organic compounds can be grouped into families by their common structural features n We shall survey the nature of the compounds in a tour of the families in this course n This chapter deals with alkanes, compounds that contain only carbons and hydrogens, all connected exclusively by single bonds 3

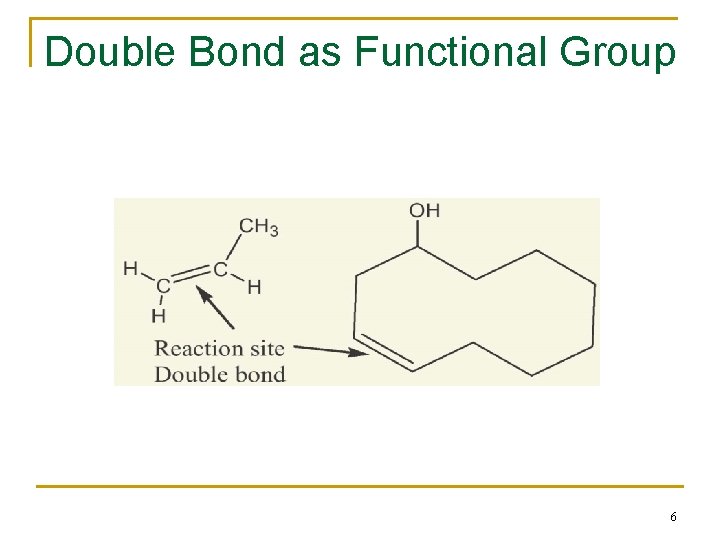
2. 1 Functional Groups n Functional group - collection of atoms at a site within a molecule with a common bonding pattern n The group reacts in a typical way, generally independent of the rest of the molecule n For example, the double bonds in simple and complex alkenes react with bromine in the same way 4

5

Double Bond as Functional Group 6

Survey of Functional Groups n n n Table 3. 1 lists a wide variety of functional groups that you should recognize As you learn about them in each chapter it will be easier to recognize them The functional groups affect the reactions, structure, and physical properties of every compound in which they occur 7

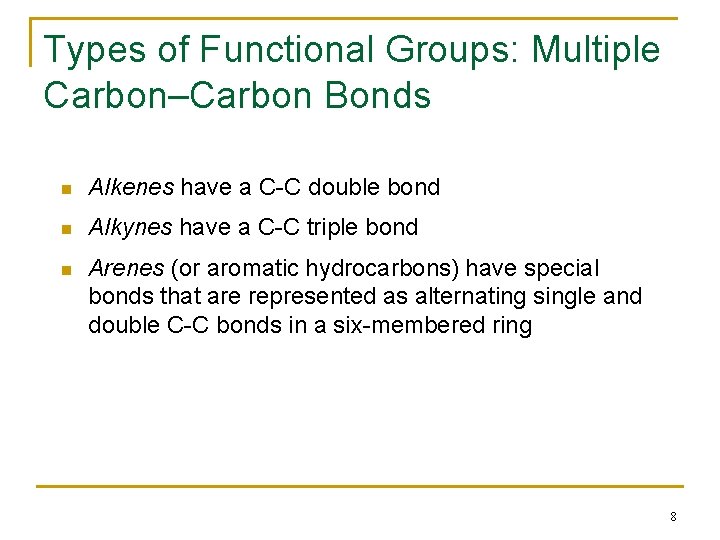
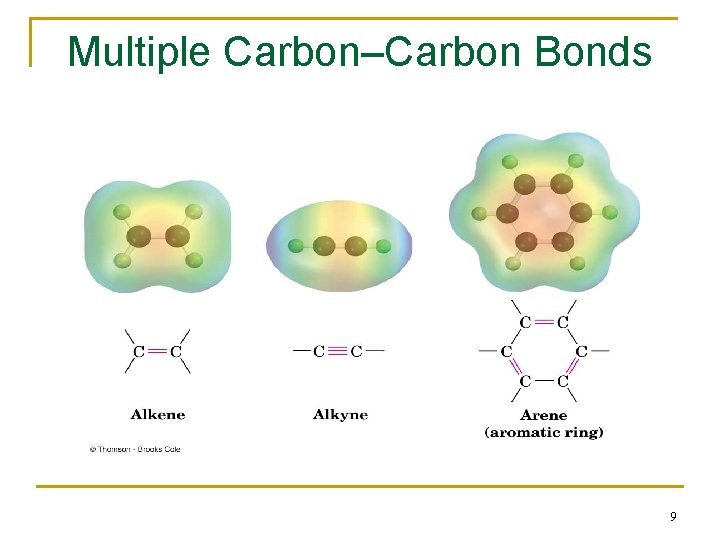
Types of Functional Groups: Multiple Carbon–Carbon Bonds n Alkenes have a C-C double bond n Alkynes have a C-C triple bond n Arenes (or aromatic hydrocarbons) have special bonds that are represented as alternating single and double C-C bonds in a six-membered ring 8

Multiple Carbon–Carbon Bonds 9

Functional Groups with Carbon Singly Bonded to an Electronegative Atom n n n n Alkyl halide: C bonded to halogen (C-X) Alcohol: C bonded O of a hydroxyl group (C-OH) Ether: Two C’s bonded to the same O (C-O-C) Amine: C bonded to N (C-N) Thiol: C bonded to SH group (C-SH) Sulfide: Two C’s bonded to same S (C-S-C) Bonds are polar, with partial positive charge on C ( +) and partial negative charge ( ) on electronegative atom 10

11

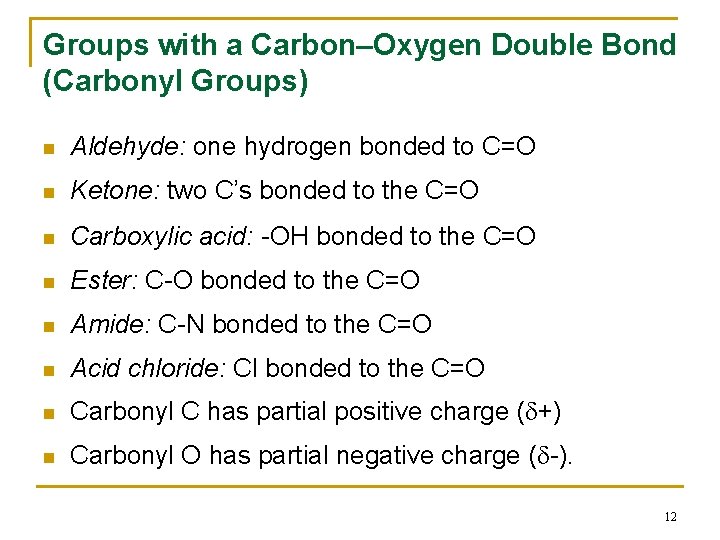
Groups with a Carbon–Oxygen Double Bond (Carbonyl Groups) n Aldehyde: one hydrogen bonded to C=O n Ketone: two C’s bonded to the C=O n Carboxylic acid: -OH bonded to the C=O n Ester: C-O bonded to the C=O n Amide: C-N bonded to the C=O n Acid chloride: Cl bonded to the C=O n Carbonyl C has partial positive charge ( +) n Carbonyl O has partial negative charge ( -). 12

13

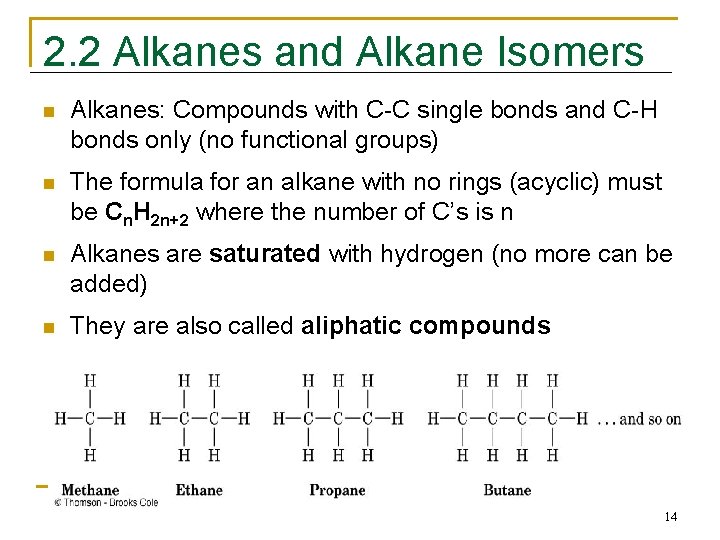
2. 2 Alkanes and Alkane Isomers n Alkanes: Compounds with C-C single bonds and C-H bonds only (no functional groups) n The formula for an alkane with no rings (acyclic) must be Cn. H 2 n+2 where the number of C’s is n n Alkanes are saturated with hydrogen (no more can be added) n They are also called aliphatic compounds 14

A “saturated” fat (glyceryl stearate): 15

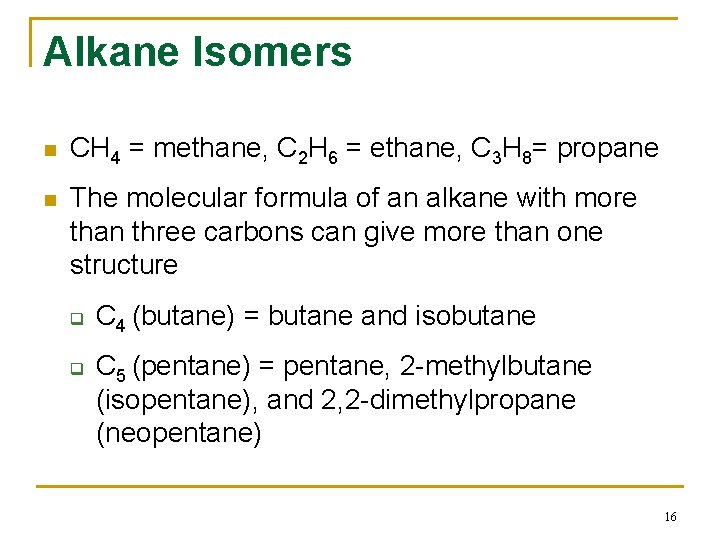
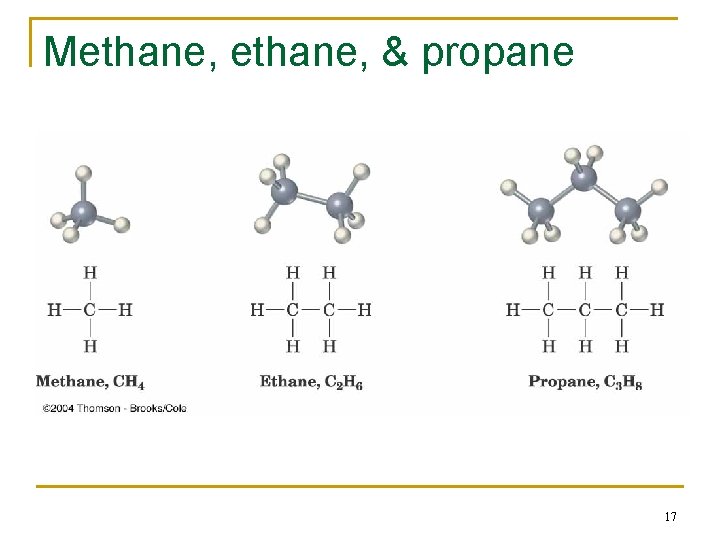
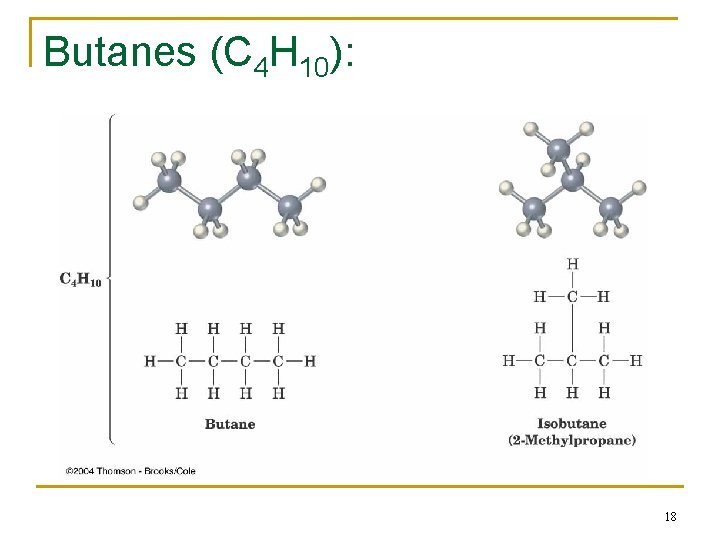
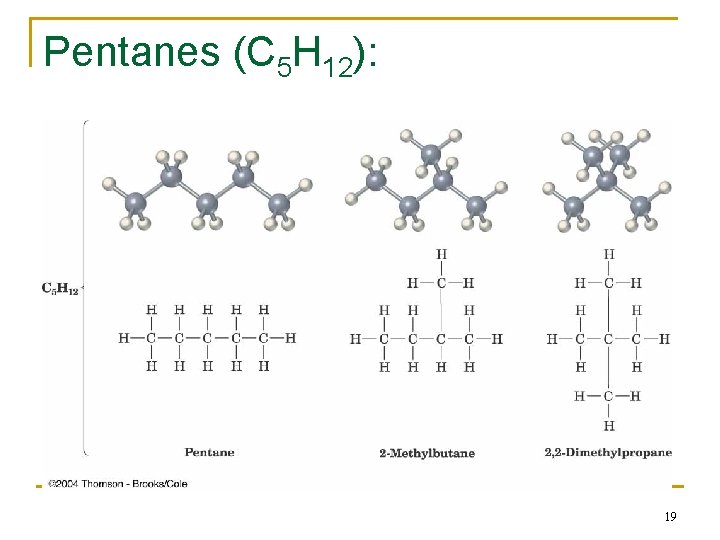
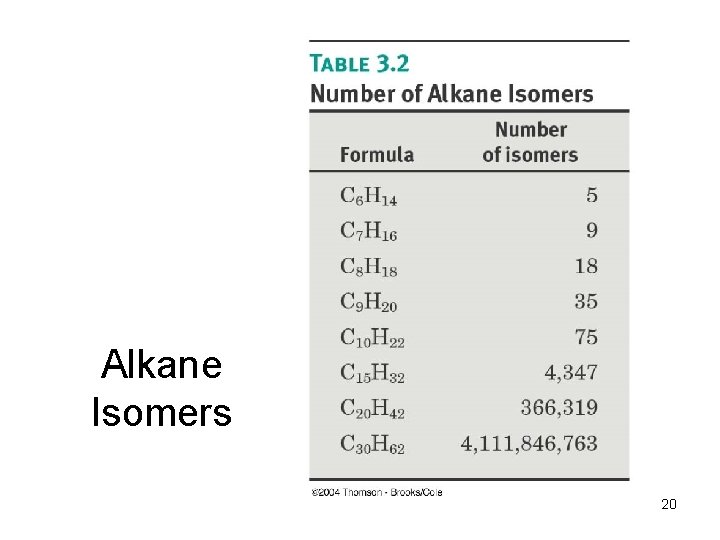
Alkane Isomers n CH 4 = methane, C 2 H 6 = ethane, C 3 H 8= propane n The molecular formula of an alkane with more than three carbons can give more than one structure q q C 4 (butane) = butane and isobutane C 5 (pentane) = pentane, 2 -methylbutane (isopentane), and 2, 2 -dimethylpropane (neopentane) 16

Methane, & propane 17

Butanes (C 4 H 10): 18

Pentanes (C 5 H 12): 19

Alkane Isomers 20

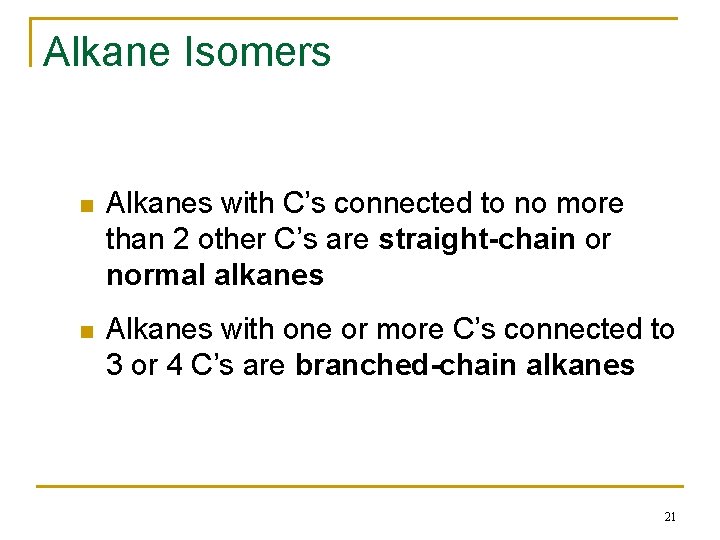
Alkane Isomers n Alkanes with C’s connected to no more than 2 other C’s are straight-chain or normal alkanes n Alkanes with one or more C’s connected to 3 or 4 C’s are branched-chain alkanes 21

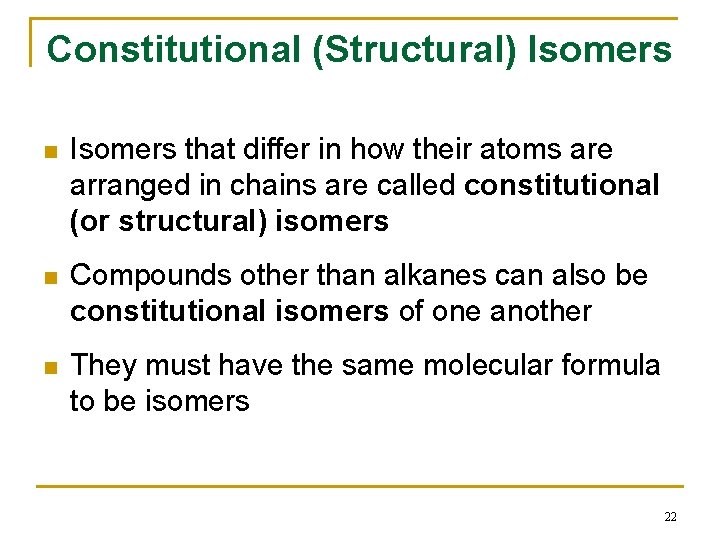
Constitutional (Structural) Isomers n Isomers that differ in how their atoms are arranged in chains are called constitutional (or structural) isomers n Compounds other than alkanes can also be constitutional isomers of one another n They must have the same molecular formula to be isomers 22

Constitutional Isomers 23

Condensed Structures of Alkanes n n We can represent an alkane in a brief form or in many types of extended form A condensed structure does not show bonds but lists atoms, such as q CH 3 CH 2 CH 3 (propane) q CH 3(CH 2)2 CH 3 (2, 2 -dimethylpropane) 24

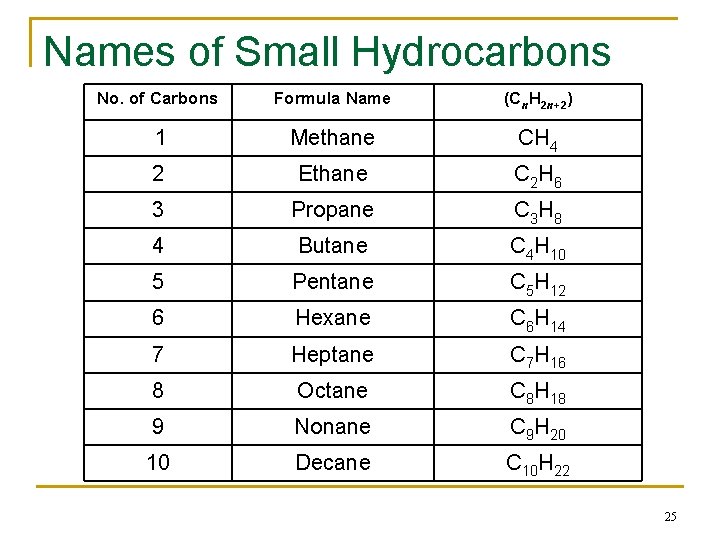
Names of Small Hydrocarbons No. of Carbons Formula Name (Cn. H 2 n+2) 1 Methane CH 4 2 Ethane C 2 H 6 3 Propane C 3 H 8 4 Butane C 4 H 10 5 Pentane C 5 H 12 6 Hexane C 6 H 14 7 Heptane C 7 H 16 8 Octane C 8 H 18 9 Nonane C 9 H 20 10 Decane C 10 H 22 25

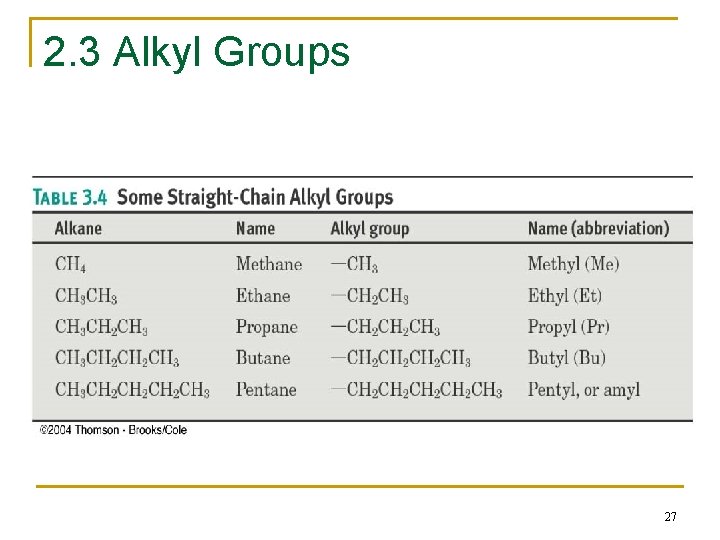
2. 3 Alkyl Groups n Alkyl group – remove one H from an alkane (a part of a structure) n General abbreviation “R” (for Radical, an incomplete species or the “rest” of the molecule) n Name: replace -ane ending of alkane with -yl ending q q -CH 3 is “methyl” (from methane) -CH 2 CH 3 is “ethyl” from ethane 26

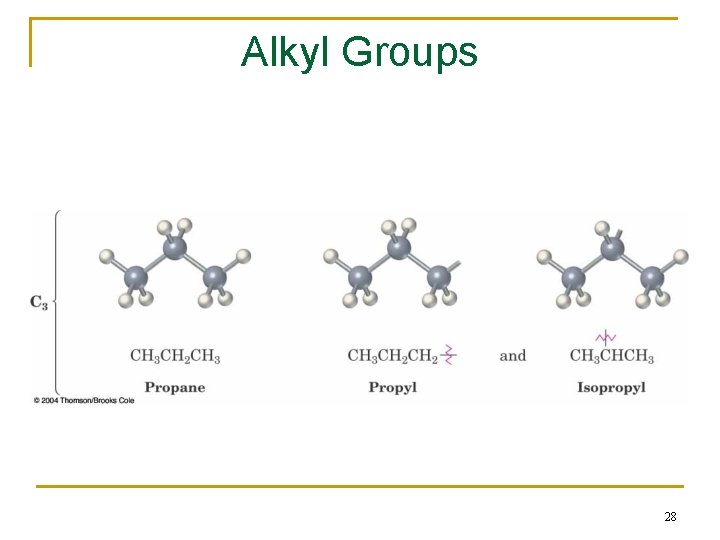
2. 3 Alkyl Groups 27

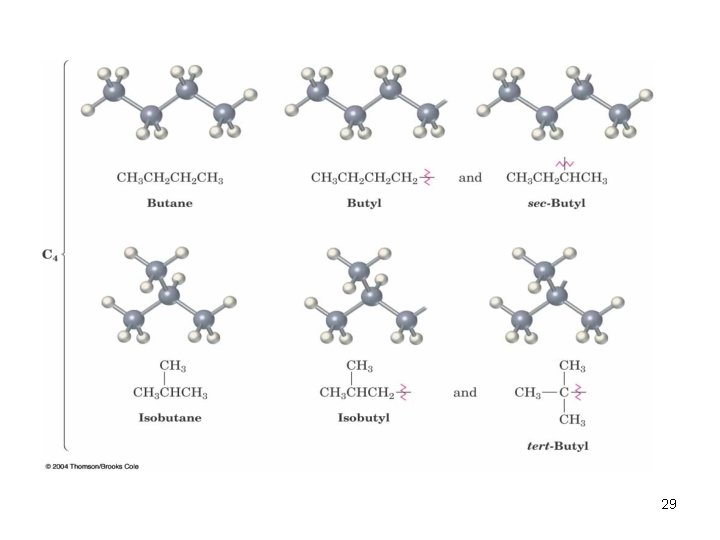
Alkyl Groups 28

29

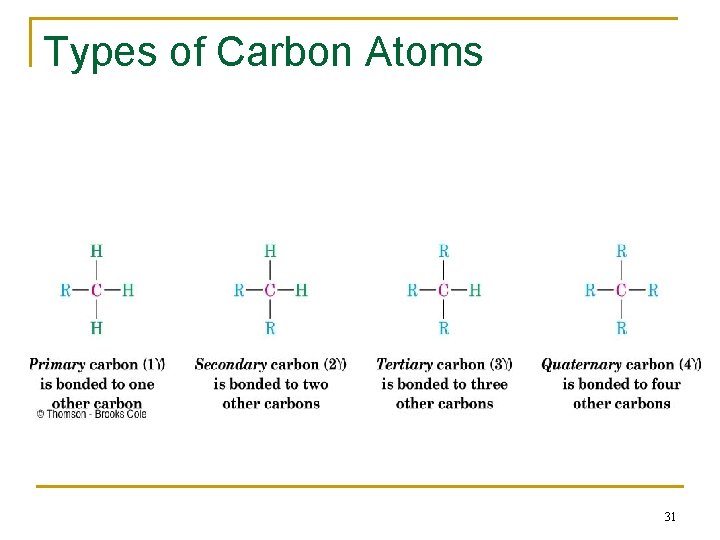
Types of Alkyl groups n n n a carbon at the end of a chain (primary alkyl group) a carbon in the middle of a chain (secondary alkyl group) a carbon with three carbons attached to it (tertiary alkyl group) 30

Types of Carbon Atoms 31

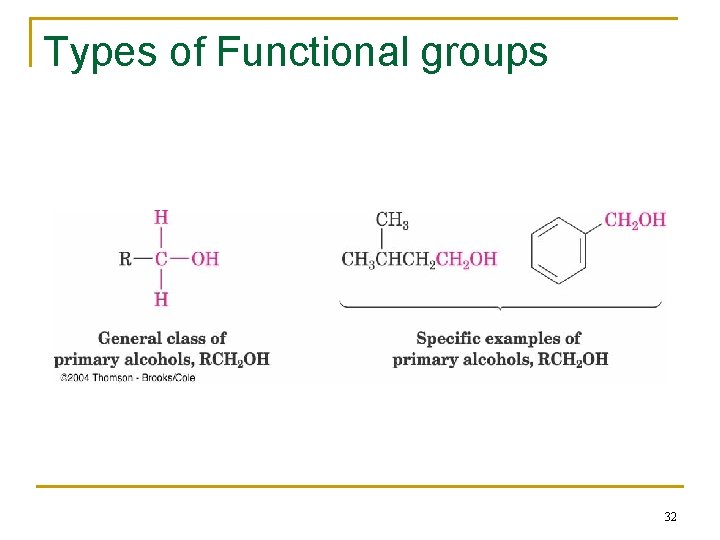
Types of Functional groups 32

Types of Hydrogens: 33

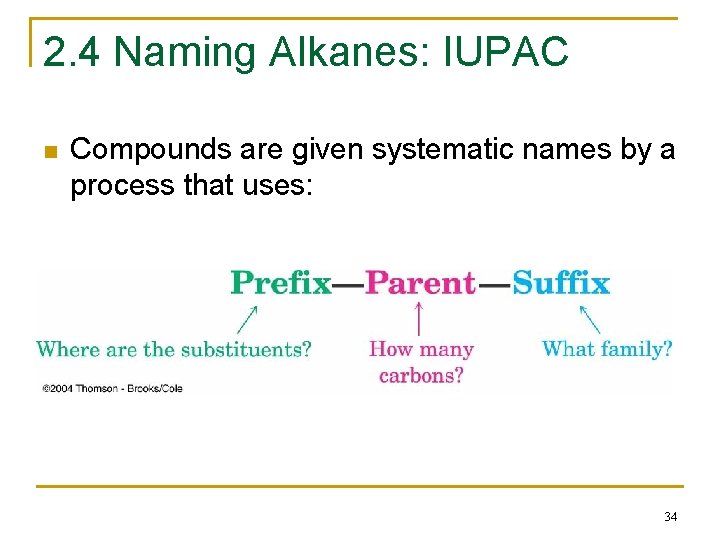
2. 4 Naming Alkanes: IUPAC n Compounds are given systematic names by a process that uses: 34

2. 4 Naming Alkanes: IUPAC n Follows specific rules q q q Named as longest contiunous chain of C’s Carbons in that chain are numbered in sequence Substituents are numbered at their point of attachment Compound name is one word (German style) Complex substituents are named similarly 35

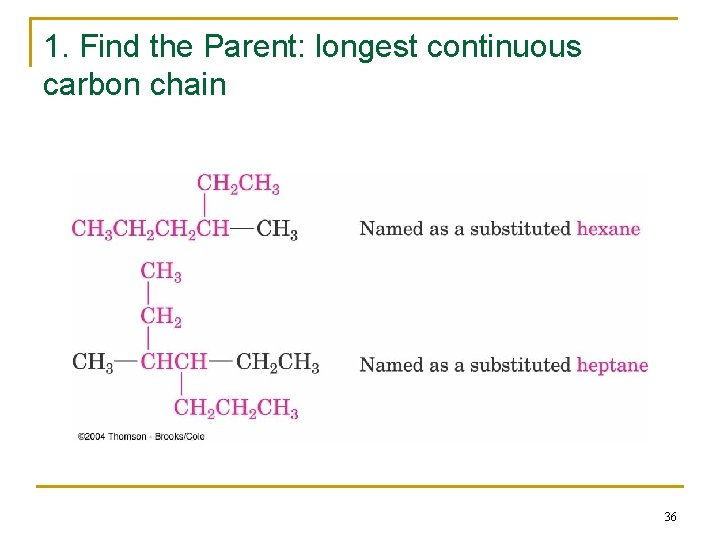
1. Find the Parent: longest continuous carbon chain 36

2. Number the atoms in the chain with the end of the chain nearer the first substituent 37

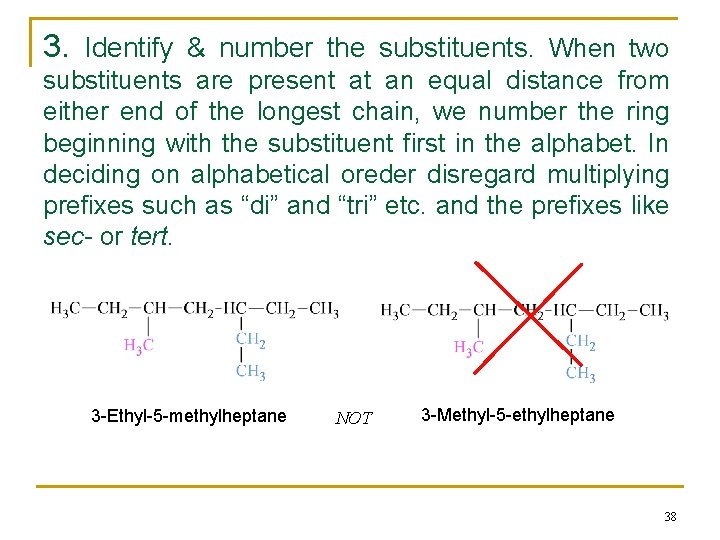
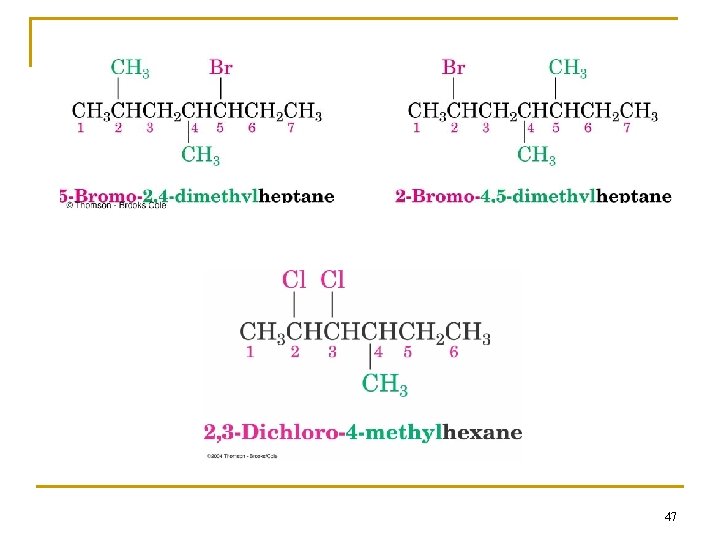
3. Identify & number the substituents. When two substituents are present at an equal distance from either end of the longest chain, we number the ring beginning with the substituent first in the alphabet. In deciding on alphabetical oreder disregard multiplying prefixes such as “di” and “tri” etc. and the prefixes like sec- or tert. 3 -Ethyl-5 -methylheptane NOT 3 -Methyl-5 -ethylheptane 38

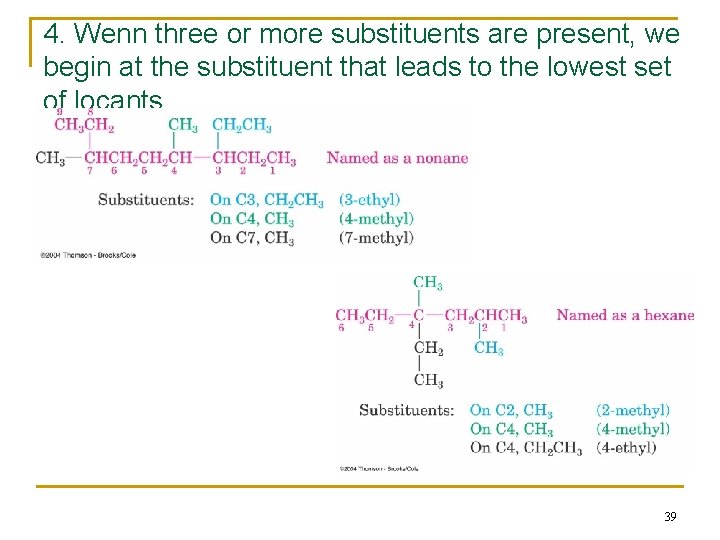
4. Wenn three or more substituents are present, we begin at the substituent that leads to the lowest set of locants. 39

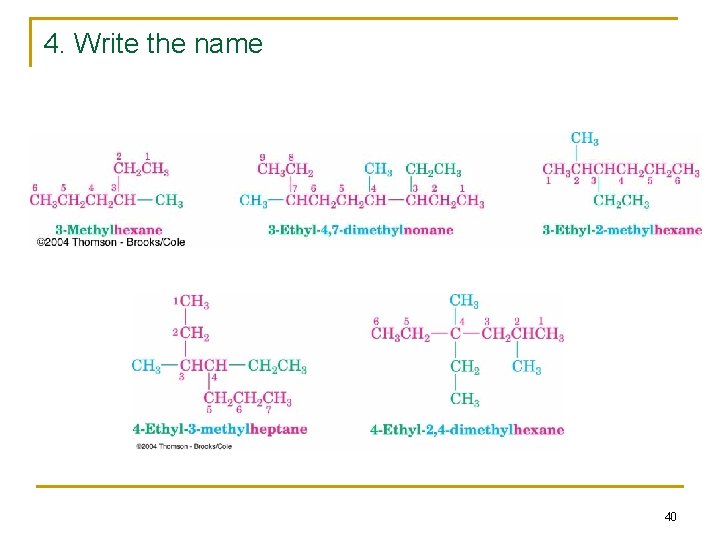
4. Write the name 40

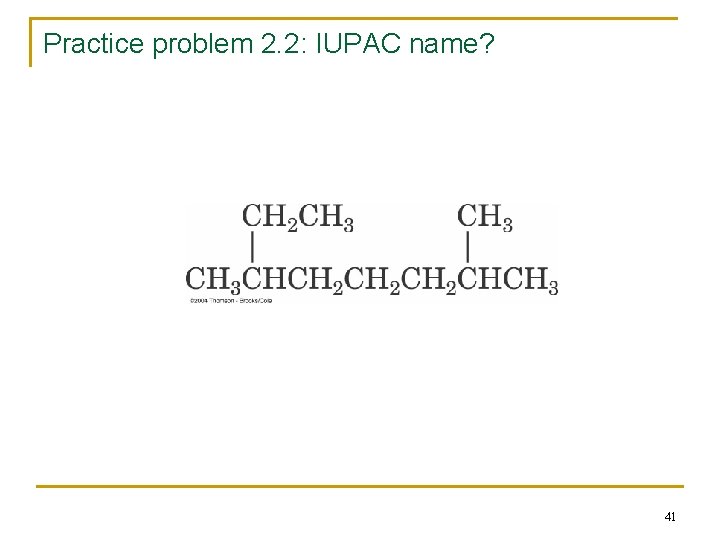
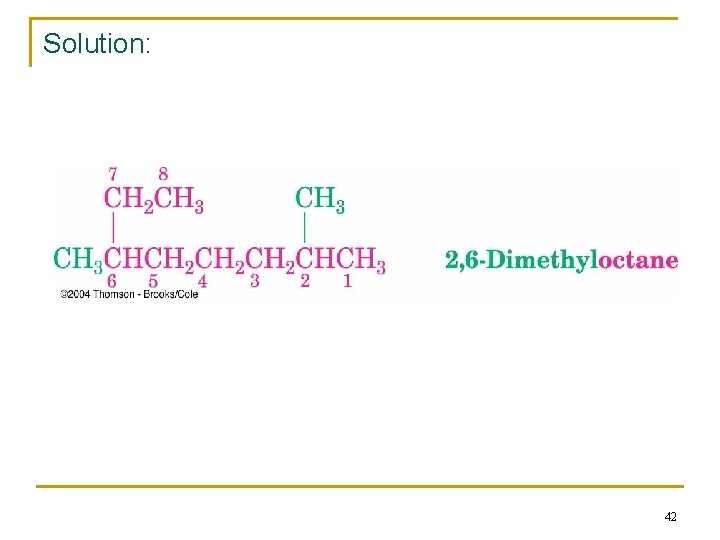
Practice problem 2. 2: IUPAC name? 41

Solution: 42

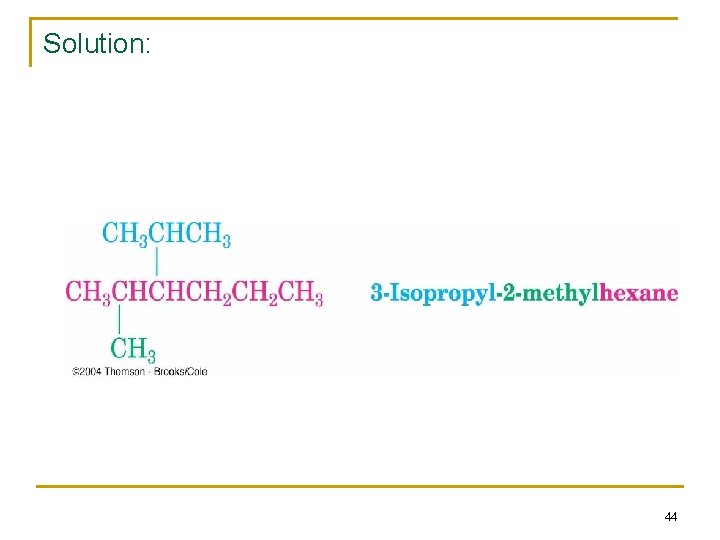
Practice prob. 2. 3: structure? 3 -isopropyl-2 -methylhexane n n n C-C-C-C Two substituents: isopropyl & methyl Add hydrogens to complete the structure 43

Solution: 44

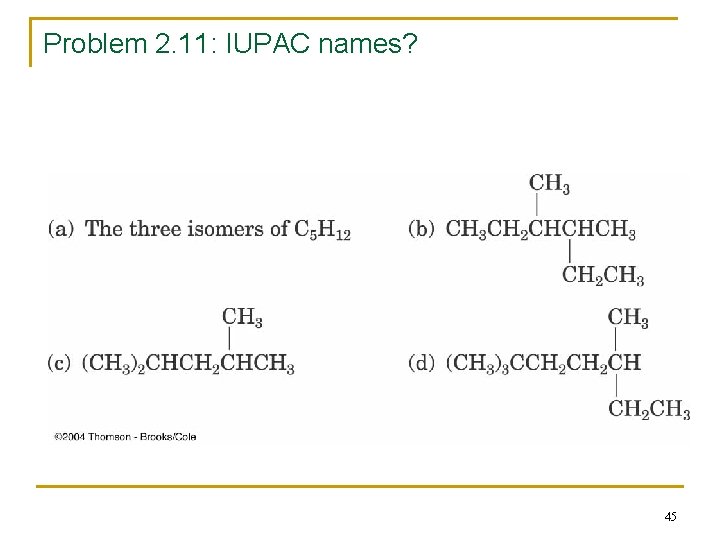
Problem 2. 11: IUPAC names? 45

10. 1 Naming Alkyl Halides n Name is based on longest carbon chain q (Contains double or triple bond if present) q Number from end nearest any substituent (alkyl or halogen) q Halogens have same priority as alkyl groups 46

47

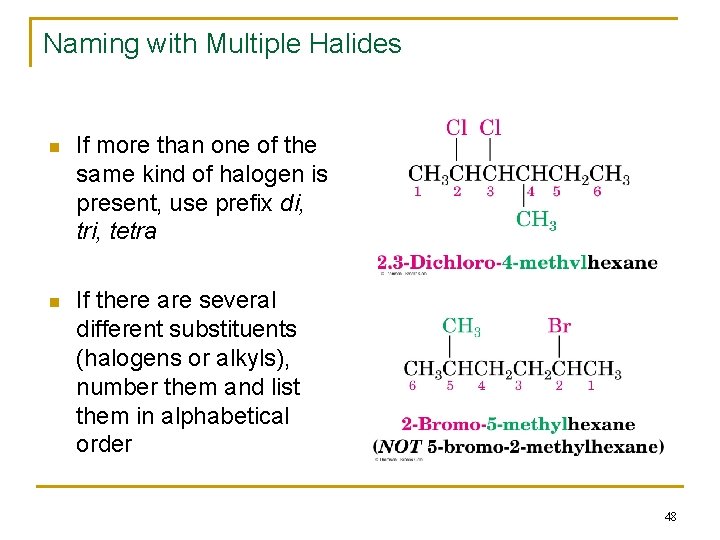
Naming with Multiple Halides n If more than one of the same kind of halogen is present, use prefix di, tri, tetra n If there are several different substituents (halogens or alkyls), number them and list them in alphabetical order 48

Naming if Two Halides or Alkyl Are Equally Distant from Ends of Chain n Begin at the end nearer the substituent whose name comes first in the alphabet 49

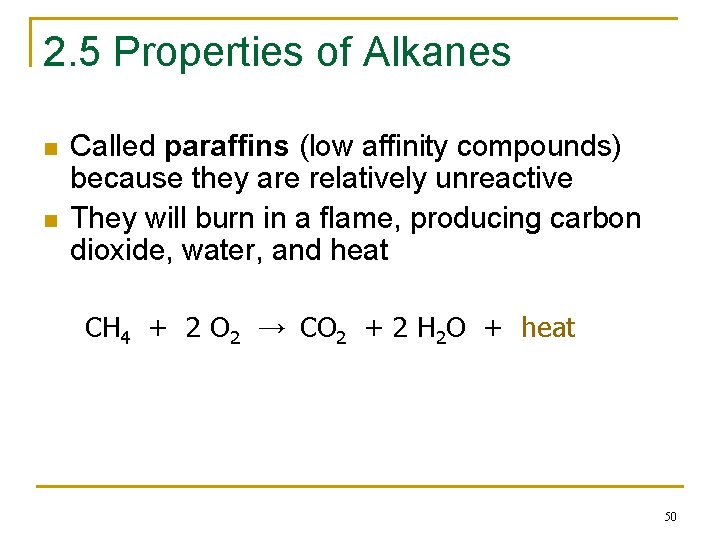
2. 5 Properties of Alkanes n n Called paraffins (low affinity compounds) because they are relatively unreactive They will burn in a flame, producing carbon dioxide, water, and heat CH 4 + 2 O 2 → CO 2 + 2 H 2 O + heat 50

2. 5 Properties of Alkanes n They react with Cl 2 in the presence of light to replace H’s with Cl’s (not easily controlled) 51

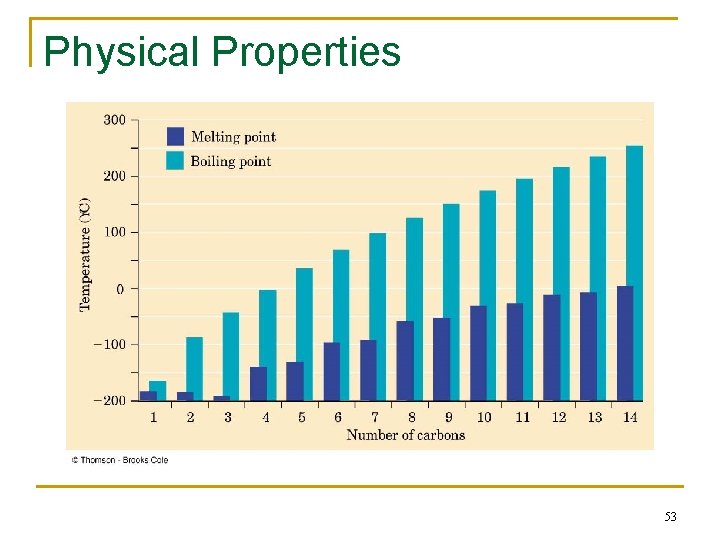
Physical Properties n n Boiling points and melting points increase as size of alkane increases Forces between molecules (temporary dipoles, dispersion) are weak 52

Physical Properties 53

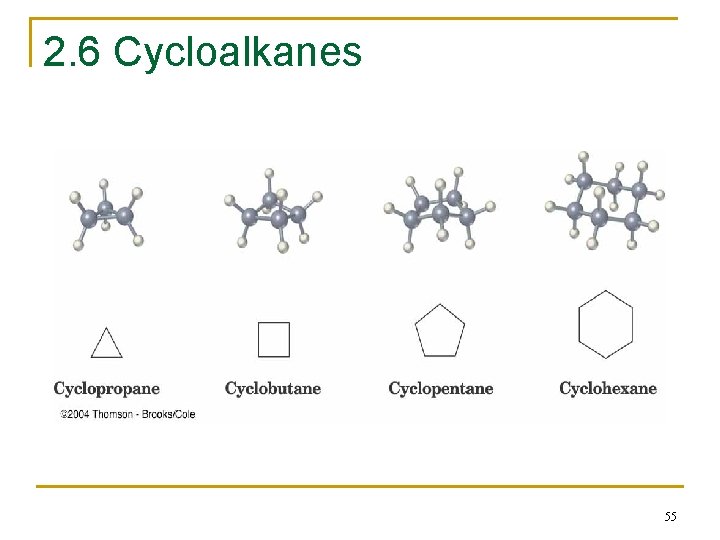
2. 6 Cycloalkanes n n n Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) Simple cycloalkanes are rings of CH 2 units, (CH 2)n, or Cn. H 2 n Structure is shown as a regular polygon with the number of vertices equal to the number of C’s (a projection of the actual structure) 54

2. 6 Cycloalkanes 55

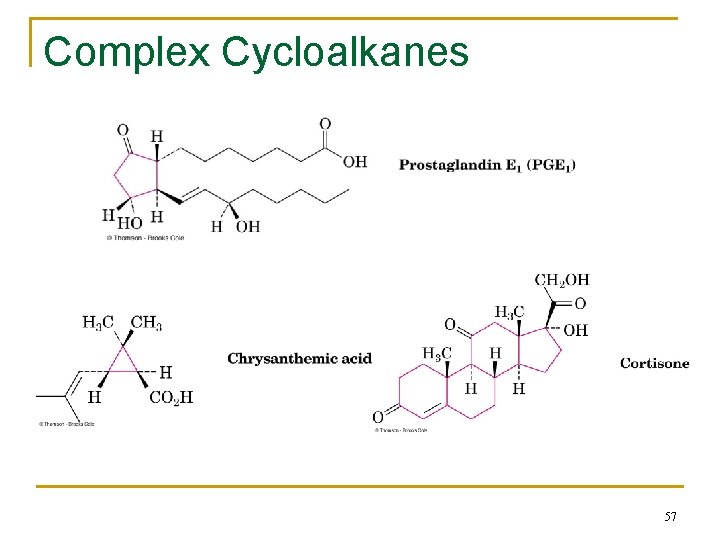
Complex Cycloalkanes n Naturally occurring materials contain cycloalkane structures n Examples: q chrysanthemic acid (cyclopropane), q prostaglandins (cyclopentane), q steroids (cyclohexanes and cyclopentane) 56

Complex Cycloalkanes 57

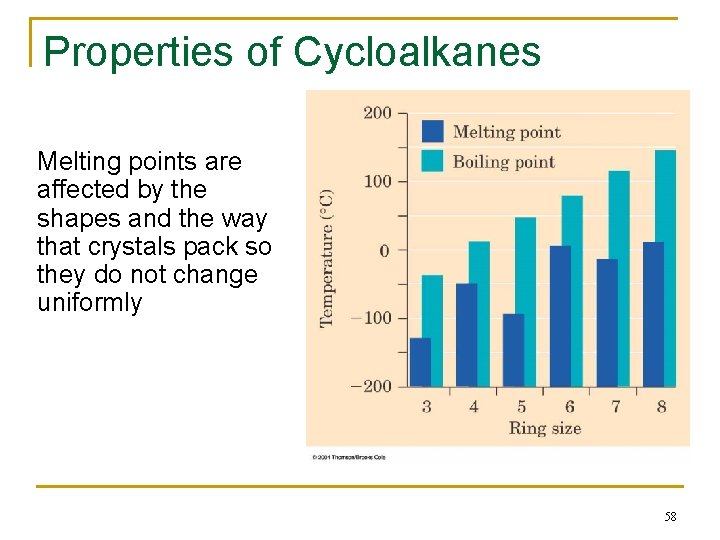
Properties of Cycloalkanes Melting points are affected by the shapes and the way that crystals pack so they do not change uniformly 58

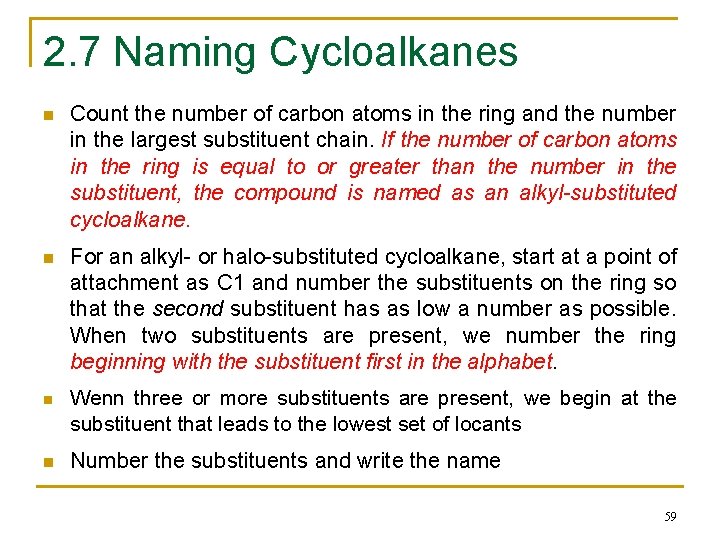
2. 7 Naming Cycloalkanes n Count the number of carbon atoms in the ring and the number in the largest substituent chain. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane. n For an alkyl- or halo-substituted cycloalkane, start at a point of attachment as C 1 and number the substituents on the ring so that the second substituent has as low a number as possible. When two substituents are present, we number the ring beginning with the substituent first in the alphabet. n Wenn three or more substituents are present, we begin at the substituent that leads to the lowest set of locants n Number the substituents and write the name 59

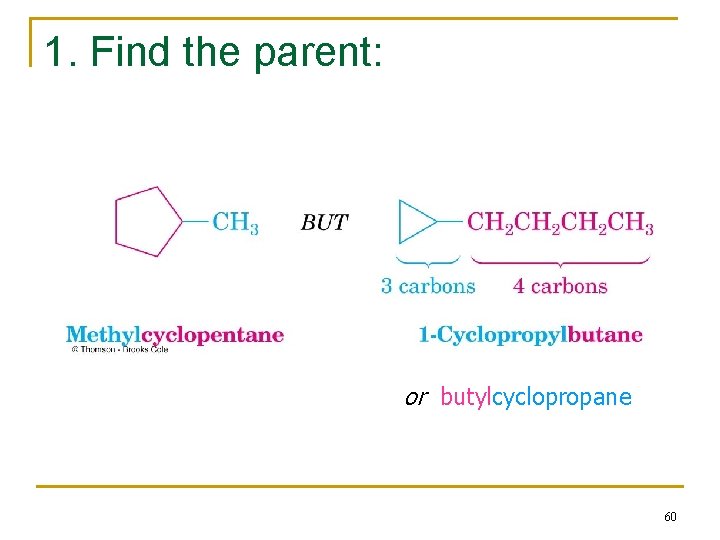
1. Find the parent: or butylcyclopropane 60

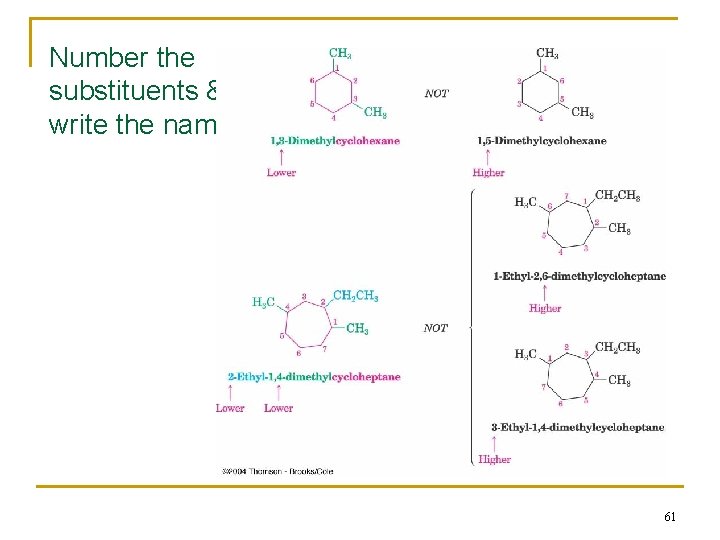
Number the substituents & write the name: 61

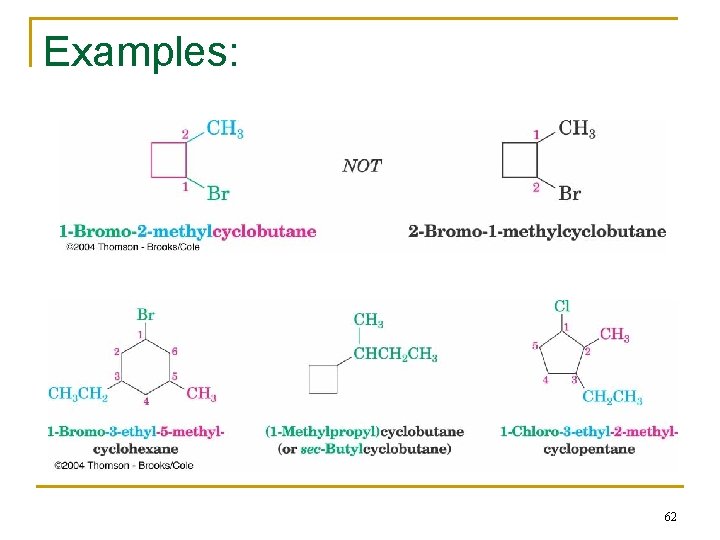
Examples: 62

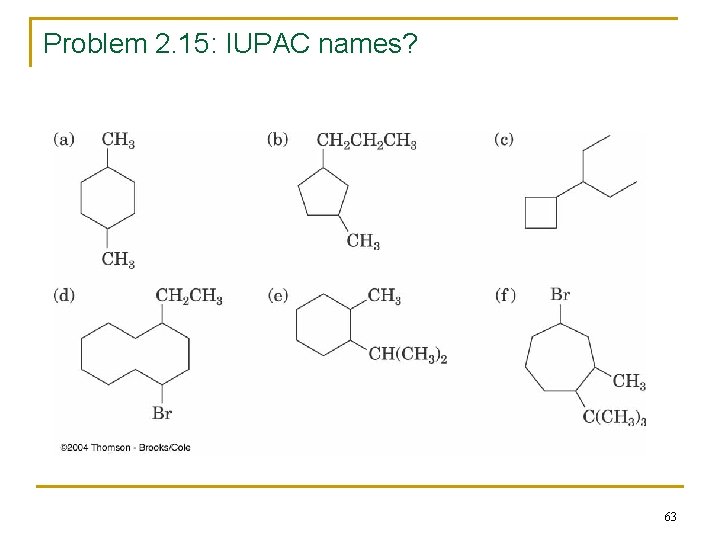
Problem 2. 15: IUPAC names? 63

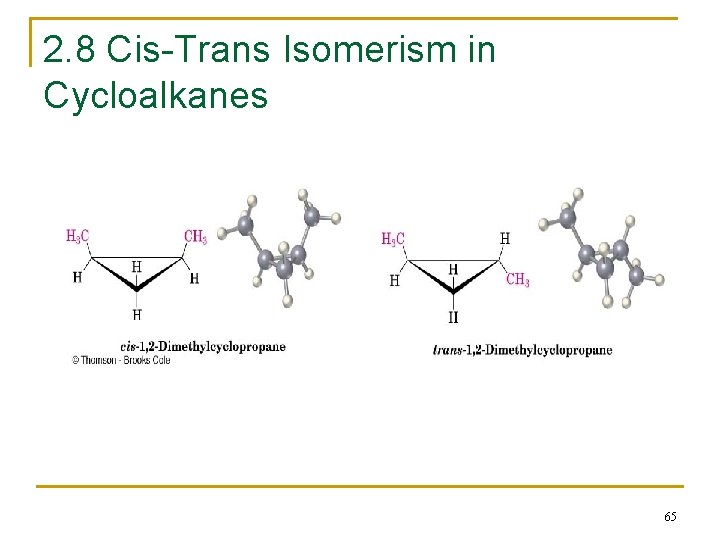
2. 8 Cis-Trans Isomerism in Cycloalkanes n Rotation about C-C bonds in cycloalkanes is limited by the ring structure n Rings have two “faces” and substituents are labeled as to their relative facial positions n There are two different 1, 2 -dimethyl-cyclopropane isomers, one with the two methyls on the same side (cis) of the ring and one with the methyls on opposite sides (trans) 64

2. 8 Cis-Trans Isomerism in Cycloalkanes 65

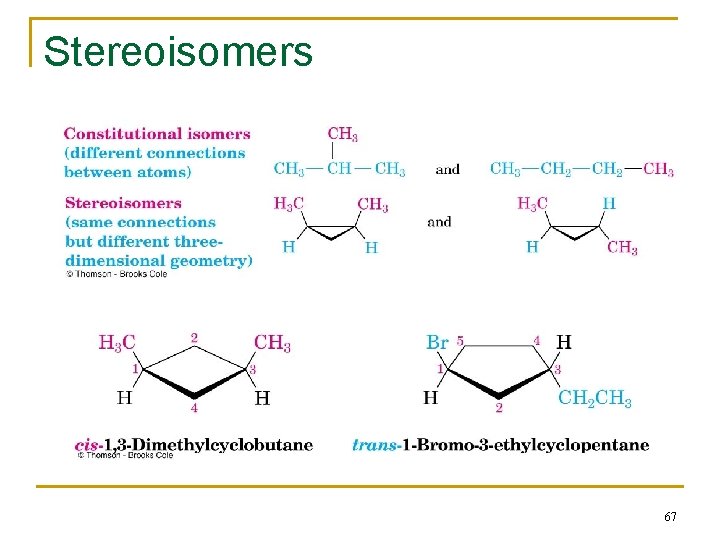
Stereoisomers n Compounds with atoms connected in the same order but which differ in three-dimensional orientation, are stereoisomers n The terms “cis” and “trans” should be used to specify stereoisomeric ring structures n Recall that constitutional isomers have atoms connected in different order 66

Stereoisomers 67

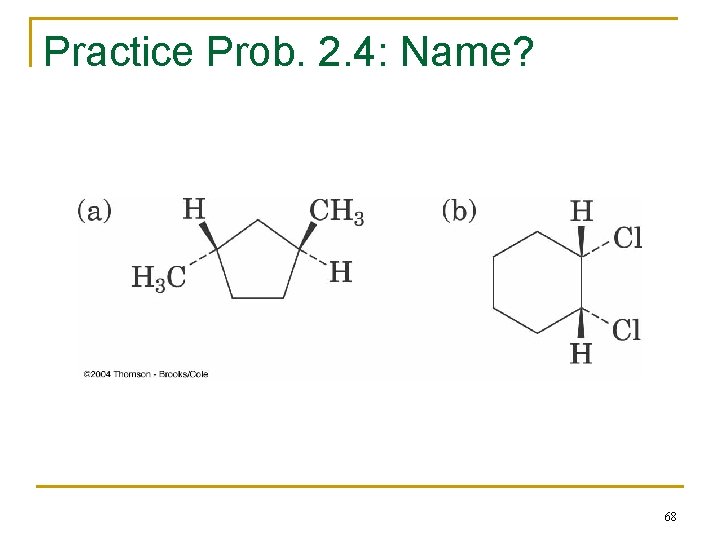
Practice Prob. 2. 4: Name? 68

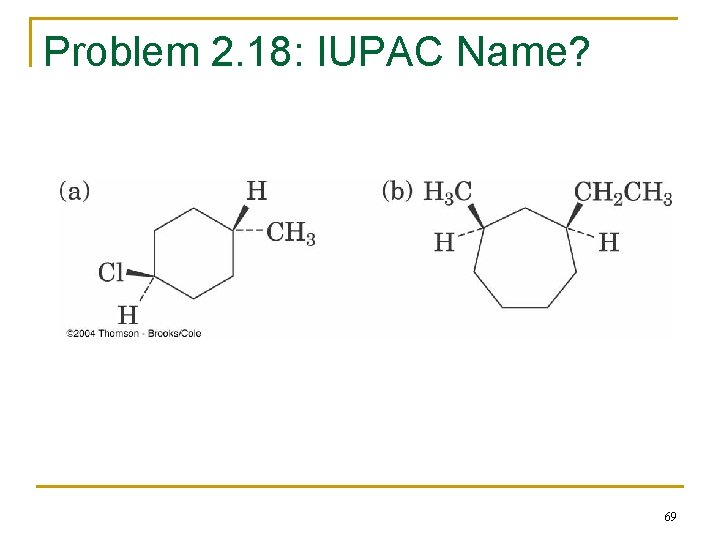
Problem 2. 18: IUPAC Name? 69

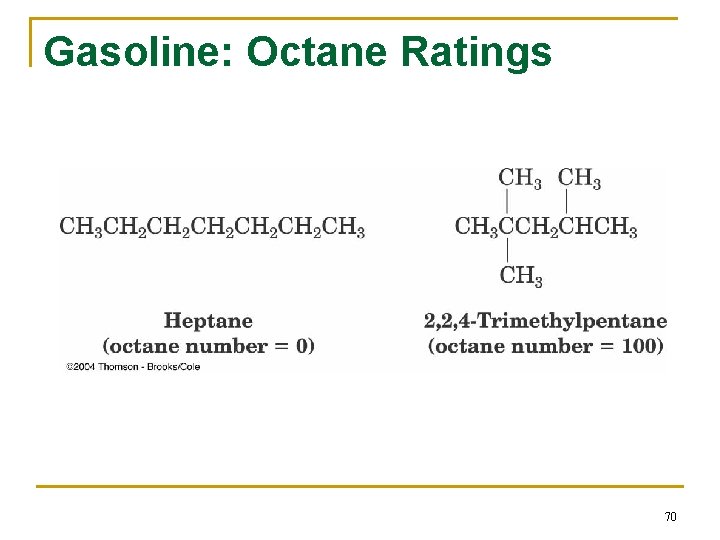
Gasoline: Octane Ratings 70

Chapter 2, Questions 30, 34, 35, 36, 37 71
 Inorganic vs organic chemistry
Inorganic vs organic chemistry Functional groups ib chemistry
Functional groups ib chemistry Nit calicut chemistry department
Nit calicut chemistry department Organic chemistry prefix
Organic chemistry prefix Organic chemistry
Organic chemistry Organic chemistry stuart warren
Organic chemistry stuart warren Brooklyn college organic chemistry
Brooklyn college organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Polarimetry organic chemistry
Polarimetry organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Iodine test for starch
Iodine test for starch Canola oil
Canola oil Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry
Organic chemistry Nbs chemistry
Nbs chemistry Organic chemistry
Organic chemistry Electrophilic addition hbr
Electrophilic addition hbr Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Chapter 7 organic chemistry
Chapter 7 organic chemistry Organic chemistry
Organic chemistry Ee organic chemistry
Ee organic chemistry Acidic cleavage of ethers
Acidic cleavage of ethers Organic chemistry class 11 notes
Organic chemistry class 11 notes Importance of lipids
Importance of lipids All structural isomers of hexane
All structural isomers of hexane Organic chemistry topic 11
Organic chemistry topic 11 Alkene alcohol naming
Alkene alcohol naming Bu organic chemistry
Bu organic chemistry Why is ceramic wool heated in cracking
Why is ceramic wool heated in cracking Is alkane an organic compound
Is alkane an organic compound Objective lab report example
Objective lab report example Structure and bonding in organic chemistry
Structure and bonding in organic chemistry Conjugation organic chemistry
Conjugation organic chemistry Ester organic chemistry
Ester organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry second edition david klein
Organic chemistry second edition david klein Organic composition definition
Organic composition definition Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry
Organic chemistry Alkyl group example
Alkyl group example High resolution
High resolution Organic chemistry introduction
Organic chemistry introduction Organic chemistry
Organic chemistry Macromolecule cheat sheet
Macromolecule cheat sheet Organic synthesis via enolates
Organic synthesis via enolates This name
This name Met et prop but pent hex hept oct
Met et prop but pent hex hept oct Ario organic chemistry
Ario organic chemistry Hybridisation
Hybridisation Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry
Organic chemistry What is organic chemistry like
What is organic chemistry like The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms What is chemistry
What is chemistry Ario practice problems
Ario practice problems Alkane organic chemistry
Alkane organic chemistry Oxidation of carbohydrates
Oxidation of carbohydrates C-c-c-c-c chemistry
C-c-c-c-c chemistry What is the displayed formula
What is the displayed formula Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry
Organic chemistry Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report Hono organic chemistry
Hono organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry
Organic chemistry Organic chemistry wade
Organic chemistry wade Organic chemistry
Organic chemistry Mindup mind map
Mindup mind map Organic chemistry
Organic chemistry