General Chemistry M R NaimiJamal Faculty of Chemistry

































- Slides: 65

General Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology


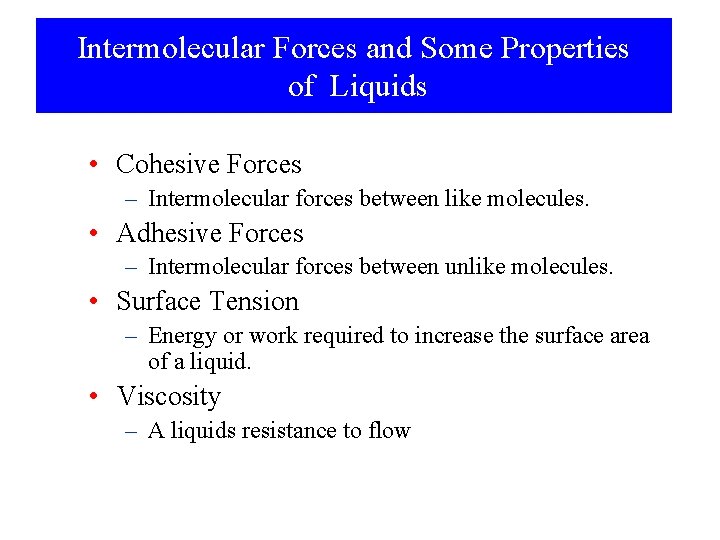
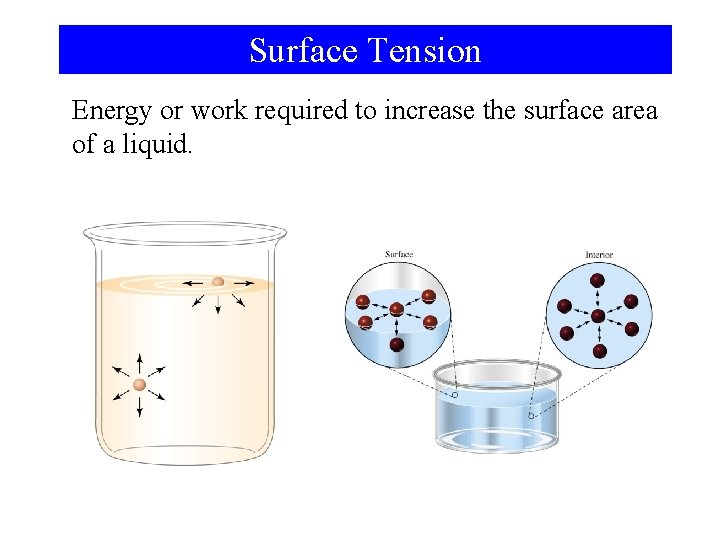
Intermolecular Forces and Some Properties of Liquids • Cohesive Forces – Intermolecular forces between like molecules. • Adhesive Forces – Intermolecular forces between unlike molecules. • Surface Tension – Energy or work required to increase the surface area of a liquid. • Viscosity – A liquids resistance to flow

Van der Waals Forces • Instantaneous dipoles. – Electrons move in an orbital to cause a polarization. • Induced dipoles. – Electrons move in response to an outside force. • Dispersion or London forces. – Instantaneous “dipole – induced dipole” attraction. – Related to polarizability.

Dipole Interactions

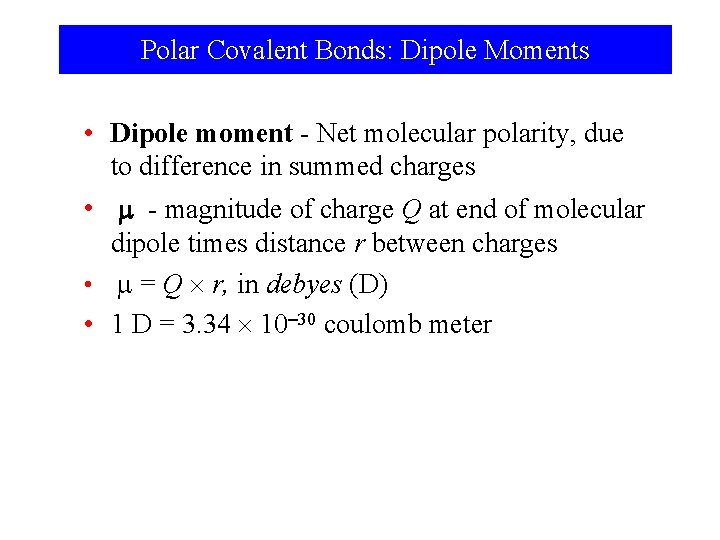
Polar Covalent Bonds: Dipole Moments • Dipole moment - Net molecular polarity, due to difference in summed charges • - magnitude of charge Q at end of molecular dipole times distance r between charges • = Q r, in debyes (D) • 1 D = 3. 34 10 30 coulomb meter

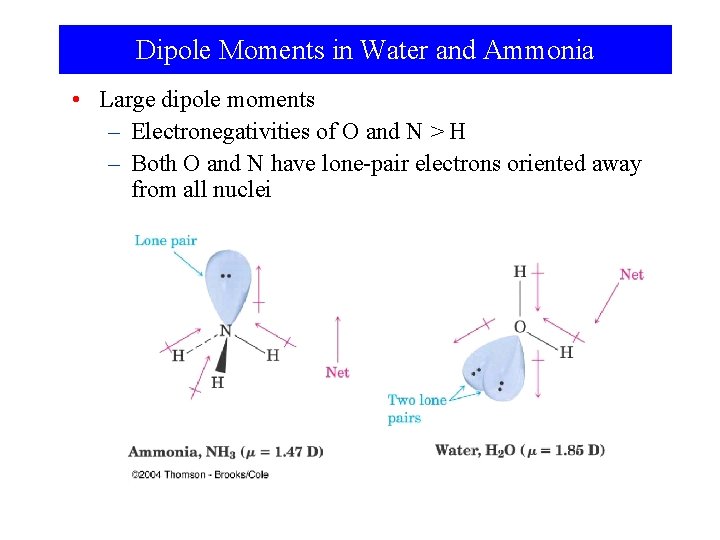
Dipole Moments in Water and Ammonia • Large dipole moments – Electronegativities of O and N > H – Both O and N have lone-pair electrons oriented away from all nuclei

Question Compare the dipole moment of NH 3 with NF 3, which one is more polar?

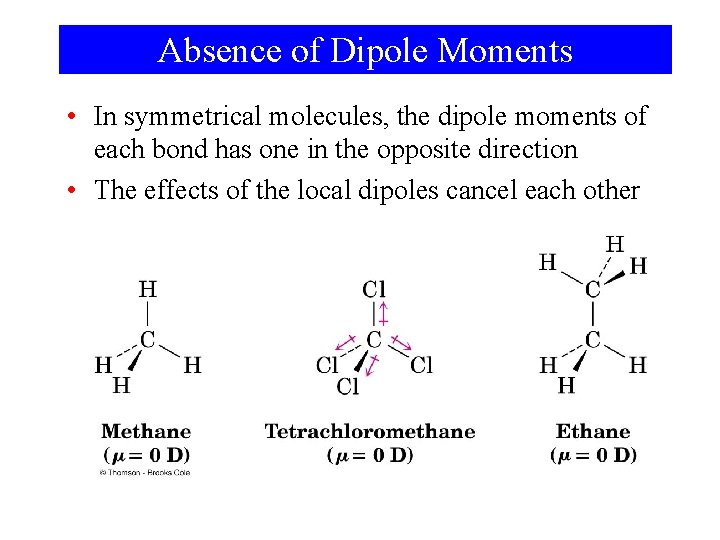
Absence of Dipole Moments • In symmetrical molecules, the dipole moments of each bond has one in the opposite direction • The effects of the local dipoles cancel each other

Phenomenon of Induction

Dispersion or London forces Instantaneous “dipole – induced dipole” attraction, related to polarizability.


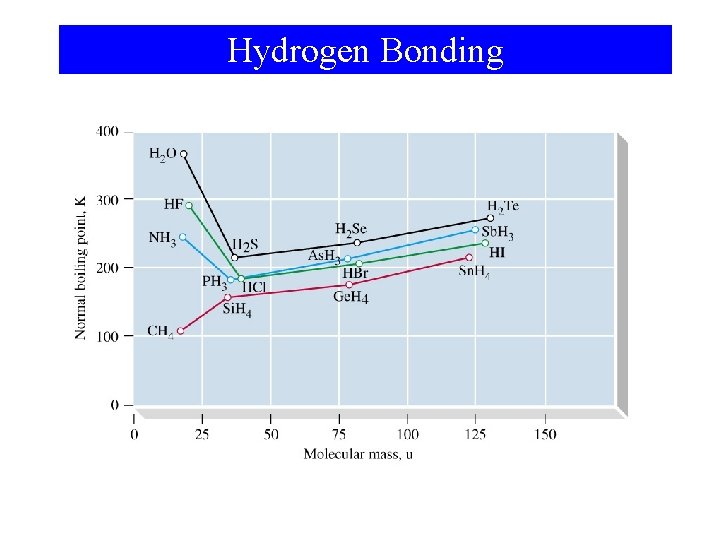
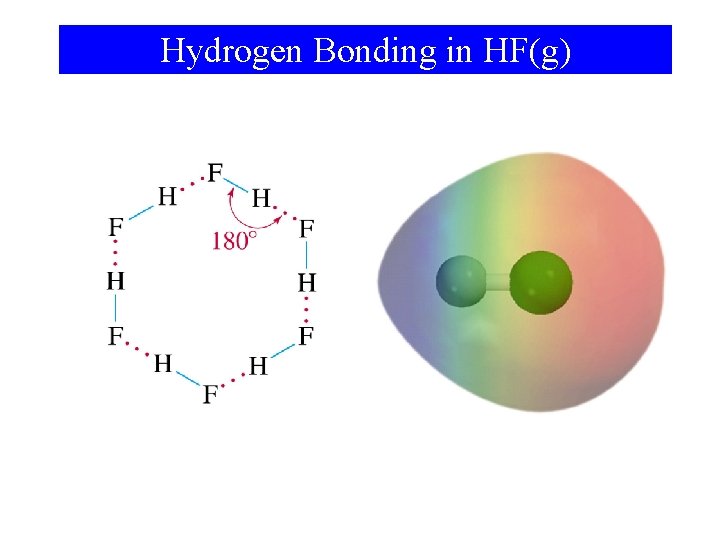
Hydrogen Bonding

Hydrogen Bonding in HF(g)

Hydrogen Bonding in Water around a molecule in the solid in the liquid

Other examples of H-Bonds

Liquids • Viscosity • Surface Tension • Evaporation


Surface Tension Energy or work required to increase the surface area of a liquid.

Surface Tension

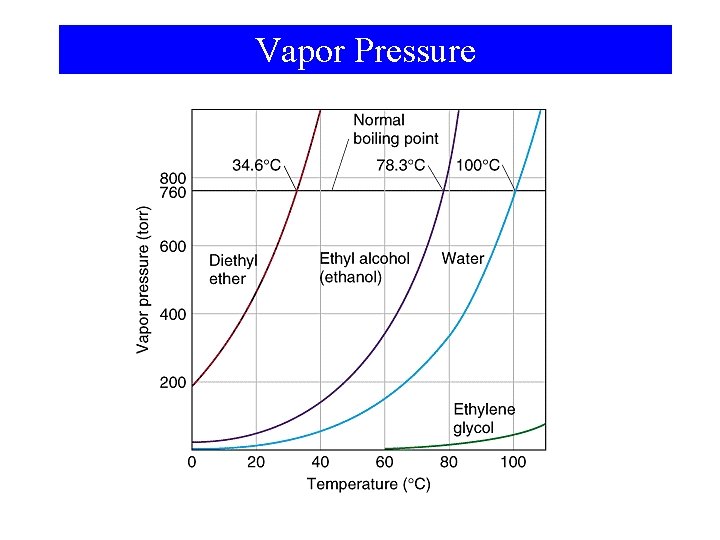
Vaporization of Liquids: Vapor Pressure

Vapor Pressure



Vapor Pressure and Boiling Point (e) (d) (c) (b) (a) 1 Ln P = -A ( )+B T ΔHvap A= R






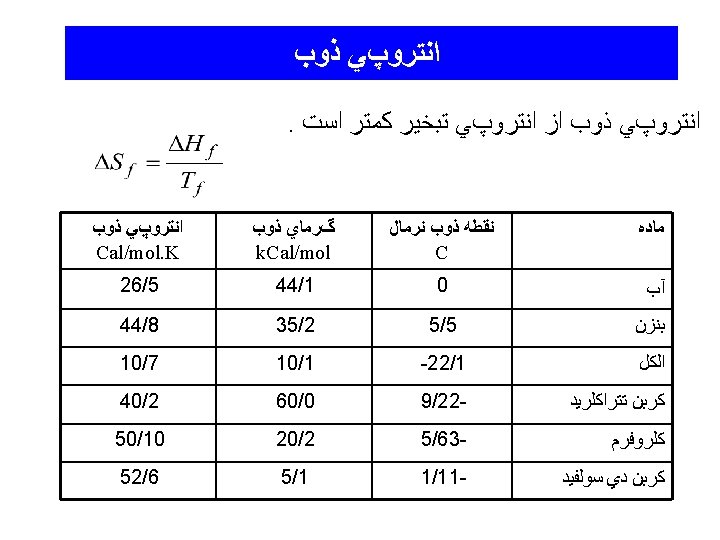
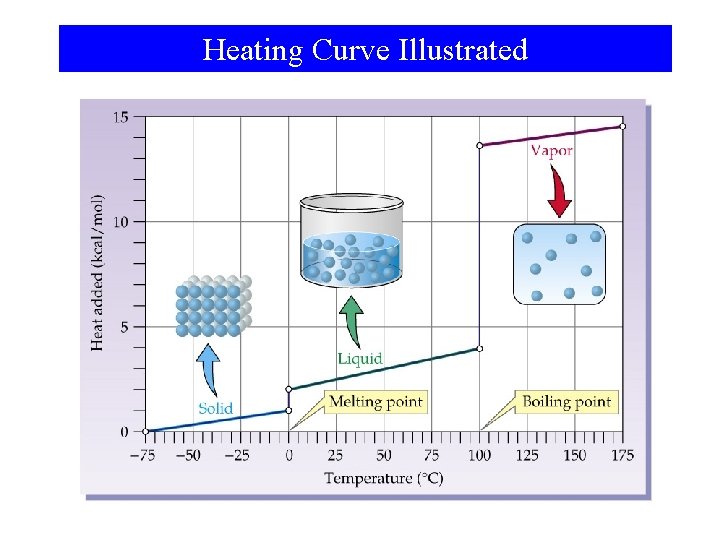
Some Properties of Solids Freezing Point Melting Point ΔHfus(H 2 O) = + 6. 01 k. J/mol


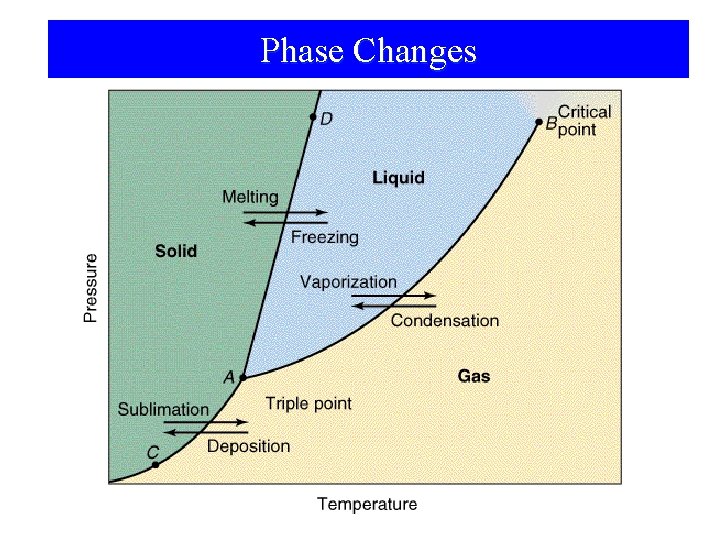
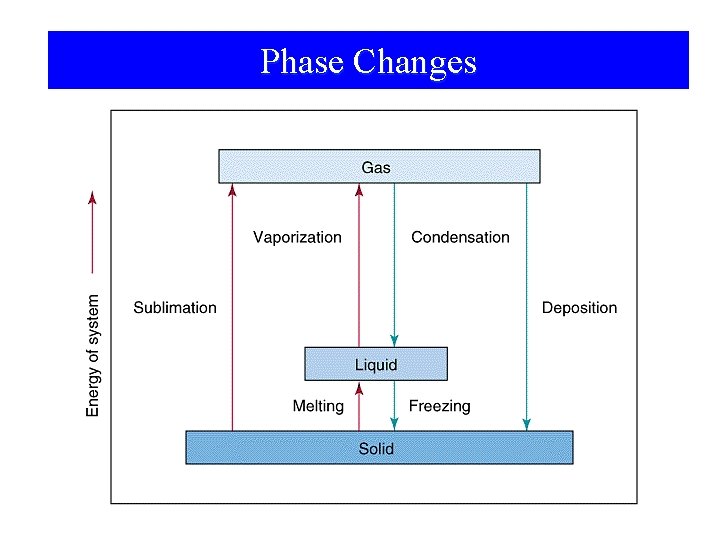
Phase Changes

Phase Changes • • • Sublimation: Vaporization: Melting or fusion: Deposition: Condensation: Freezing: solid gas. liquid gas. solid liquid. gas solid. gas liquid solid.

Phase Changes

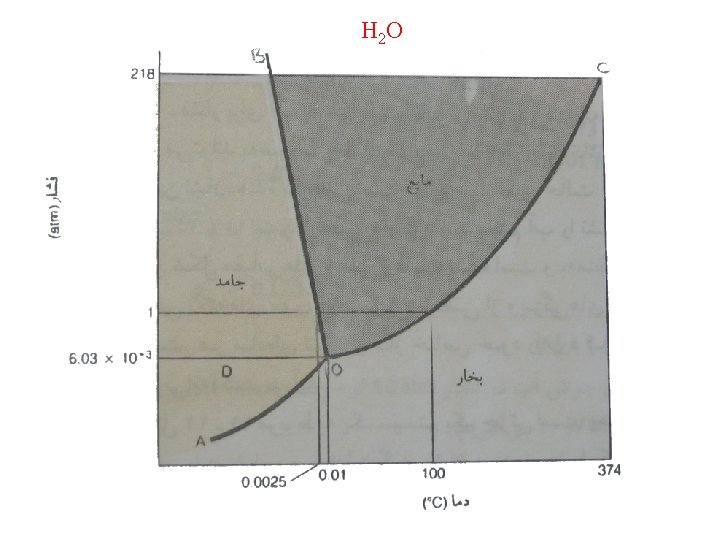
H 2 O

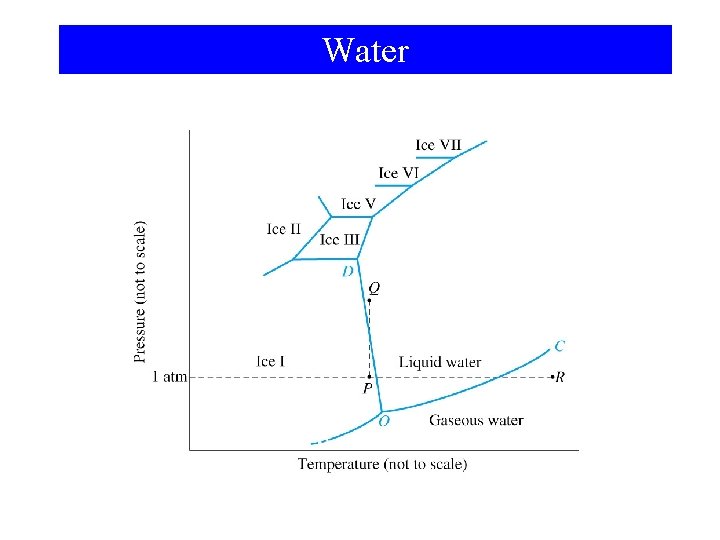
Water

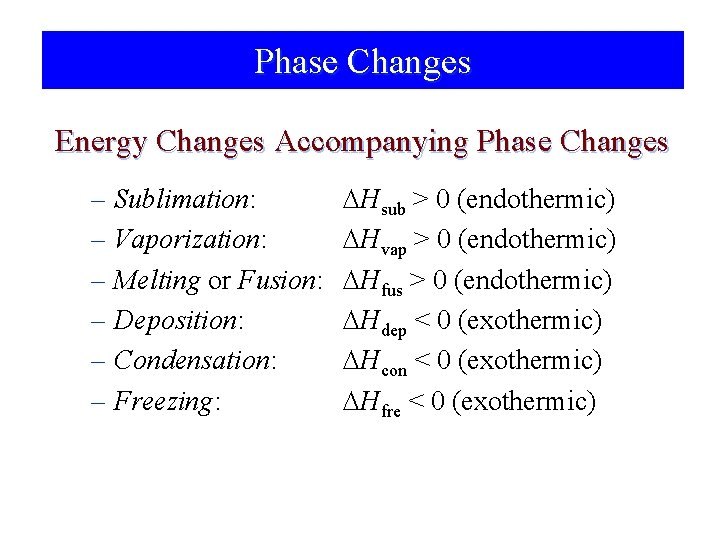
Phase Changes Energy Changes Accompanying Phase Changes – Sublimation: – Vaporization: – Melting or Fusion: – Deposition: – Condensation: – Freezing: Hsub > 0 (endothermic) Hvap > 0 (endothermic) Hfus > 0 (endothermic) Hdep < 0 (exothermic) Hcon < 0 (exothermic) Hfre < 0 (exothermic)

Heating Curve Illustrated

Phase changes

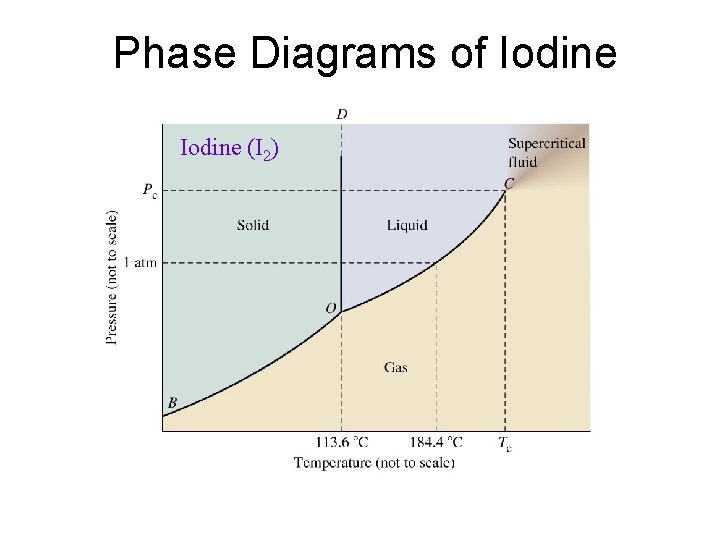
Phase Diagrams of Iodine (I 2)

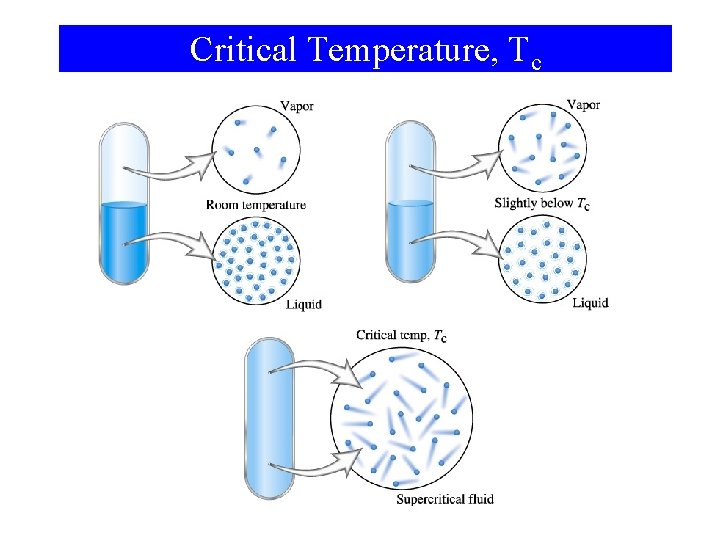
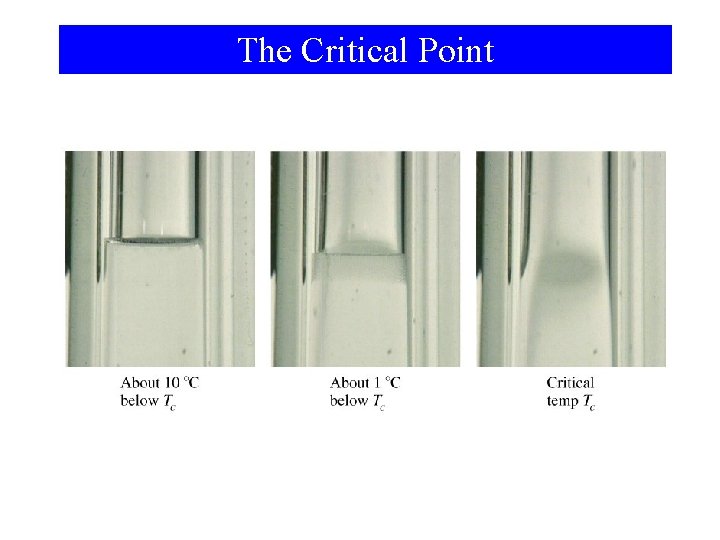
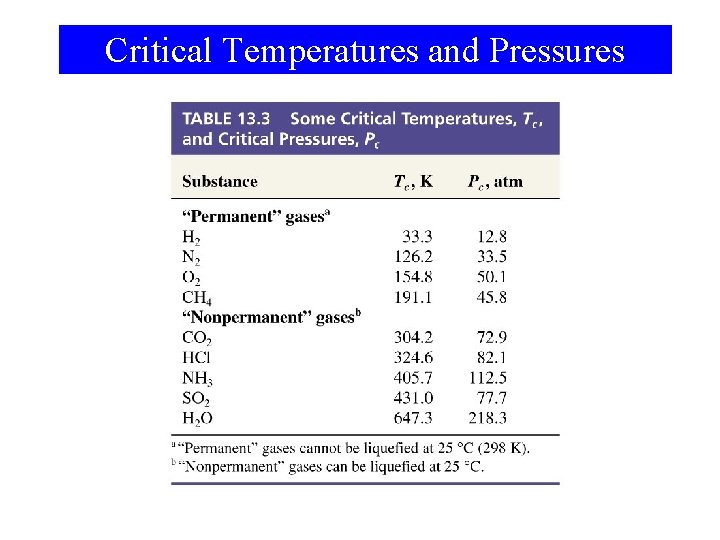
Phase Changes Critical Temperature and Pressure • Gases liquefied by increasing pressure at some temperature. • Critical temperature (Tc): The temperature, above it liquefaction of a gas using pressure is not more possible. • Critical pressure: the minimum pressure required for liquefaction at Tc.

Critical Temperature, Tc

Supercritical Fluids

The Critical Point

Critical Temperatures and Pressures

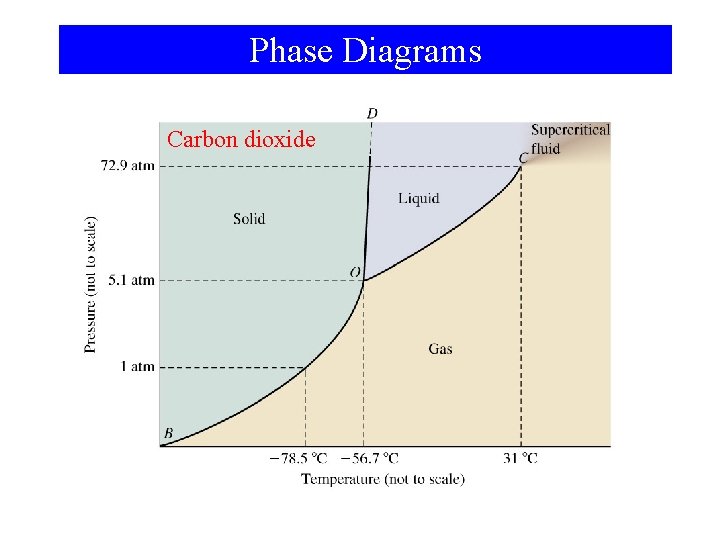
Phase Diagrams Carbon dioxide

Transition to Supercritical CO 2

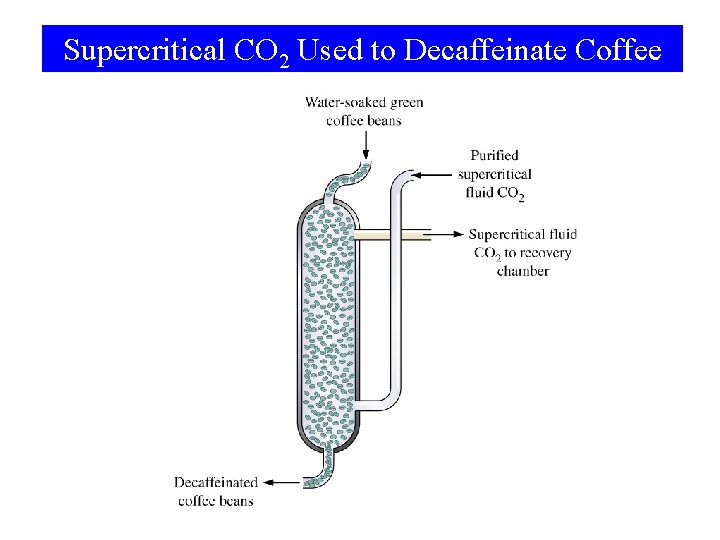
Supercritical CO 2 Used to Decaffeinate Coffee

Crystal Structures


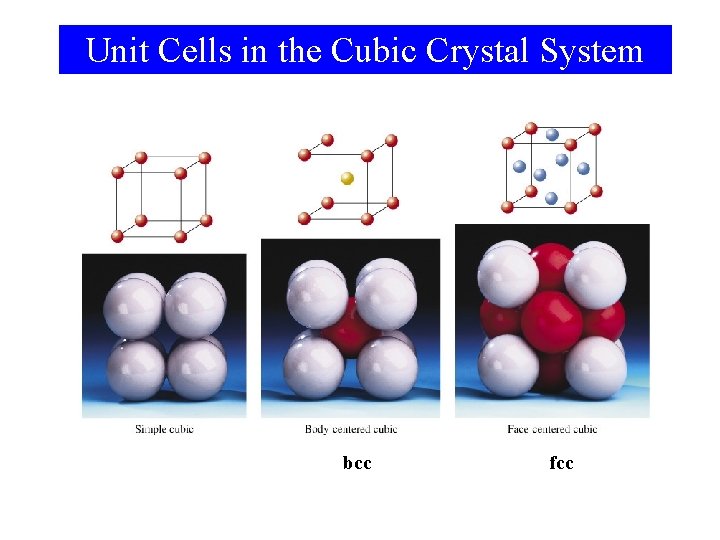
Unit Cells in the Cubic Crystal System bcc fcc

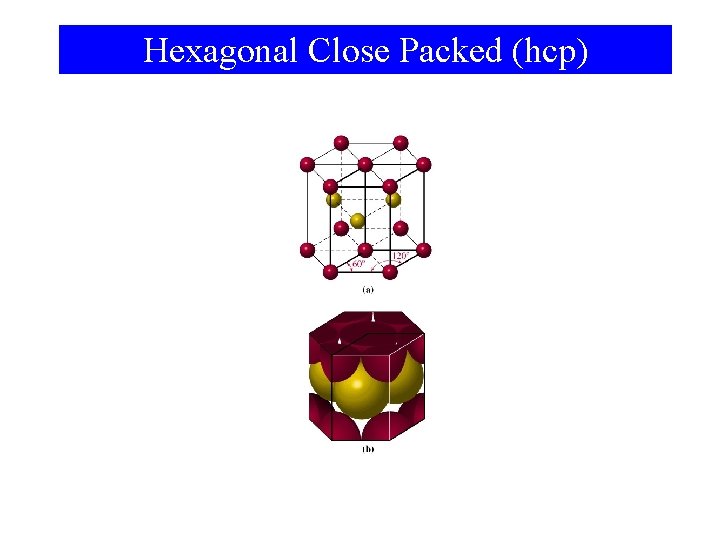
Hexagonal Close Packed (hcp)

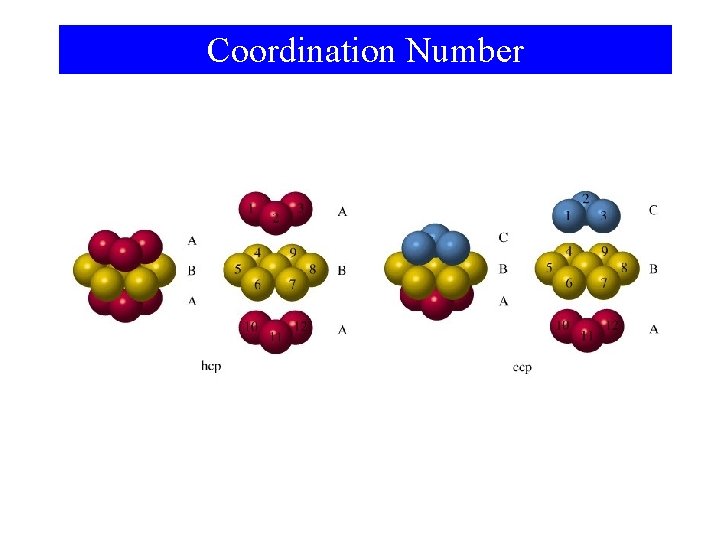
Coordination Number

Counting Cell Occupancy

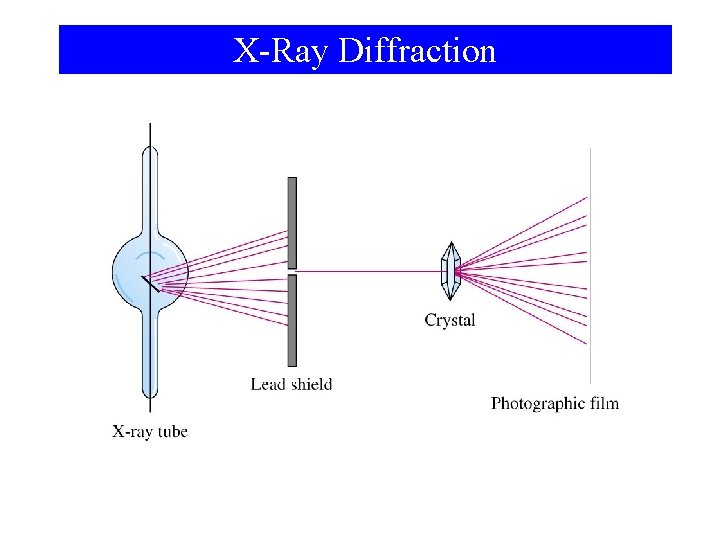
X-Ray Diffraction

X-Ray Diffraction Bragg’s equation: nλ = 2 d sin θ

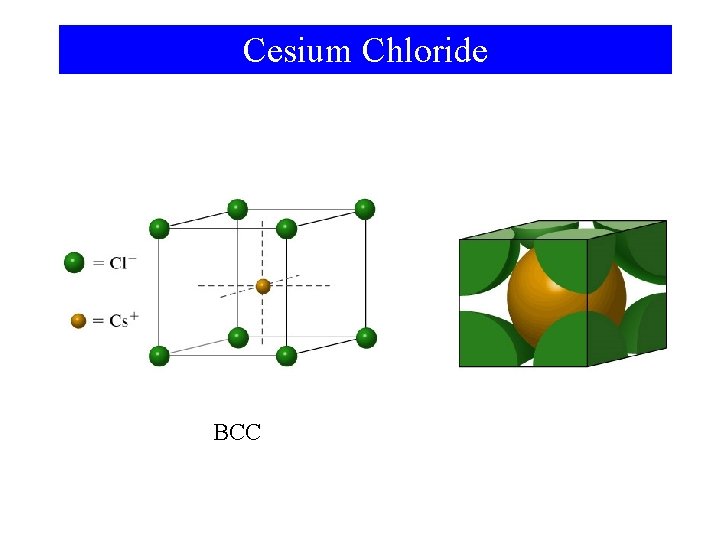
Cesium Chloride BCC

Atomic Radii from Crystal Structures

Sodium Chloride

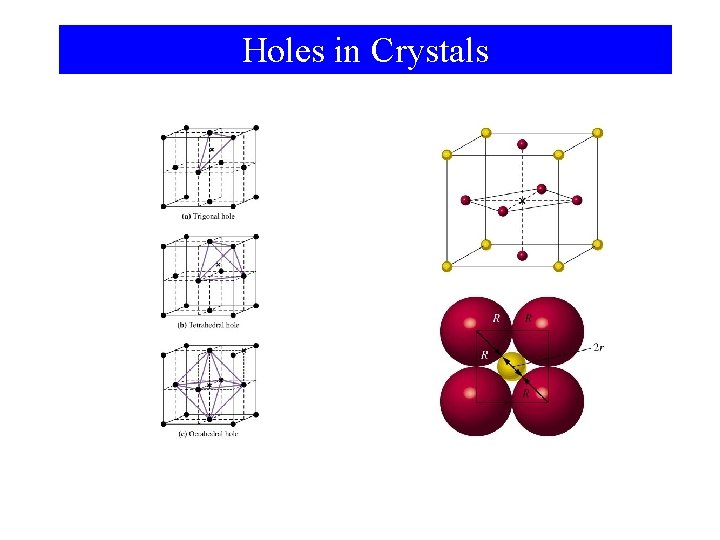
Holes in Crystals

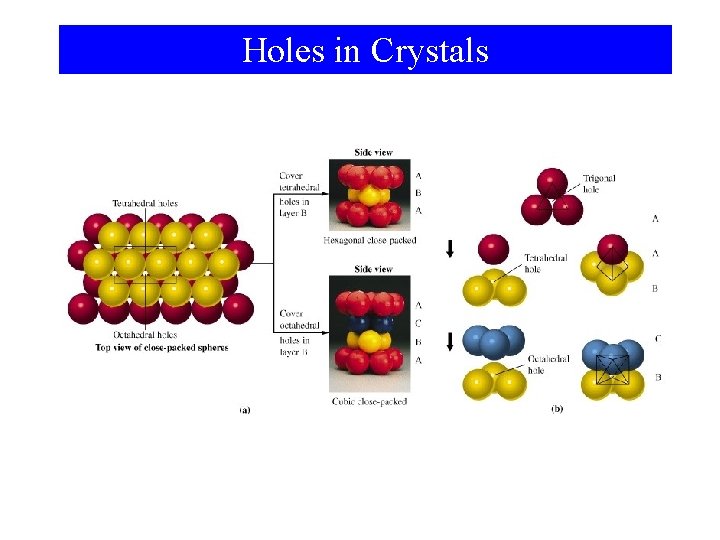
Holes in Crystals

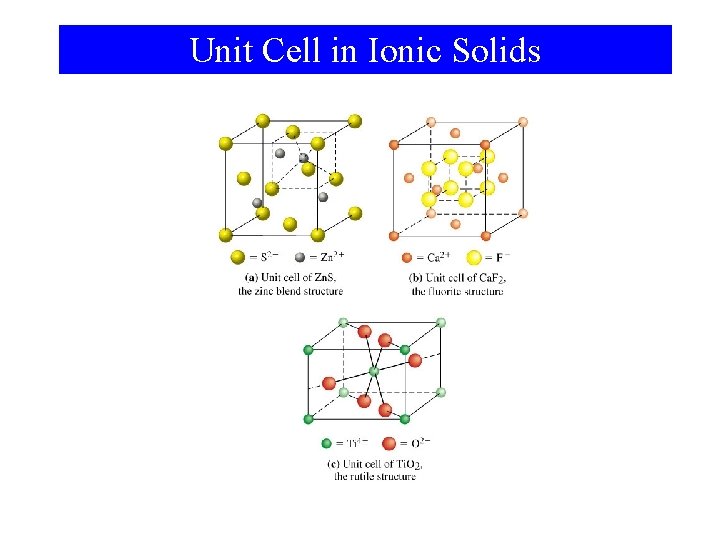
Unit Cell in Ionic Solids

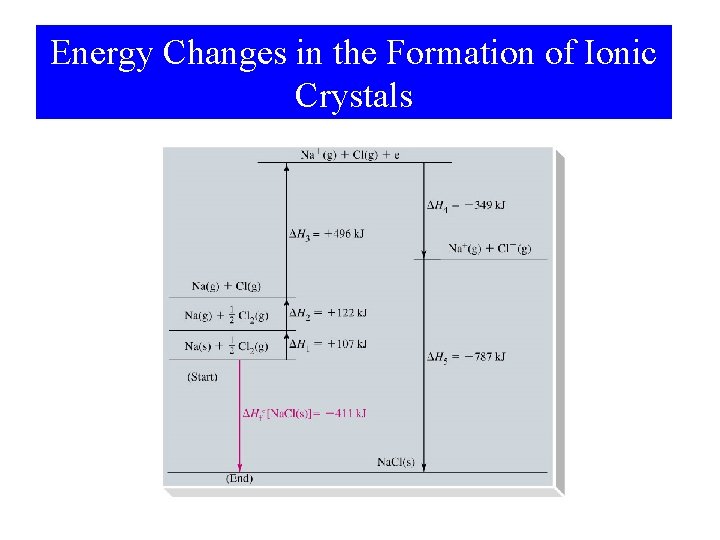
Energy Changes in the Formation of Ionic Crystals

Chapter 11 Questions 9, 14, 16, 20, 23, 28, 33, 34, 46, 53, 58, 64, 70, 74
 Keralastec
Keralastec Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science
University of bridgeport computer science Bridgeport engineering department
Bridgeport engineering department Alamo colleges faculty salary schedule
Alamo colleges faculty salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine 002
002 Semmelweis
Semmelweis Penn state neurosurgery faculty
Penn state neurosurgery faculty Mercy faculty forward
Mercy faculty forward Mrbs scholarship
Mrbs scholarship Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science Applied medical sciences
Applied medical sciences Carelli kutztown
Carelli kutztown Florida state university computer science
Florida state university computer science Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd electrical engineering
Umd electrical engineering Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Faculty 180 ecu
Faculty 180 ecu Benha faculty of engineering
Benha faculty of engineering Singularity executive program
Singularity executive program Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv bylaws
Unlv bylaws Ulm nursing faculty
Ulm nursing faculty Personal tutor ucl
Personal tutor ucl Elibrary symbiosis
Elibrary symbiosis Training and development metrics
Training and development metrics Faculty washington edu chudler
Faculty washington edu chudler Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Faculty kutztown carelli
Faculty kutztown carelli Student faculty ratio for nba
Student faculty ratio for nba Kfupm faculty housing pictures
Kfupm faculty housing pictures Ascaris lumbricoides ova
Ascaris lumbricoides ova Faculty model of training
Faculty model of training Dr veerasamy
Dr veerasamy Fulbright faculty development program
Fulbright faculty development program Faculty of engineering university of porto
Faculty of engineering university of porto Faculty of organizational sciences
Faculty of organizational sciences Charles university faculty of humanities
Charles university faculty of humanities Electrotechnical faculty belgrade
Electrotechnical faculty belgrade Elearningunideb
Elearningunideb Dmse
Dmse Electrical engineering kfupm
Electrical engineering kfupm Faculty model of training
Faculty model of training