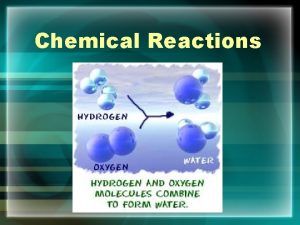
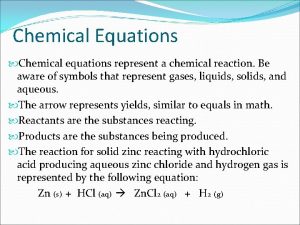
Chemical Reactions 1 Chemical Equations A chemical reaction








































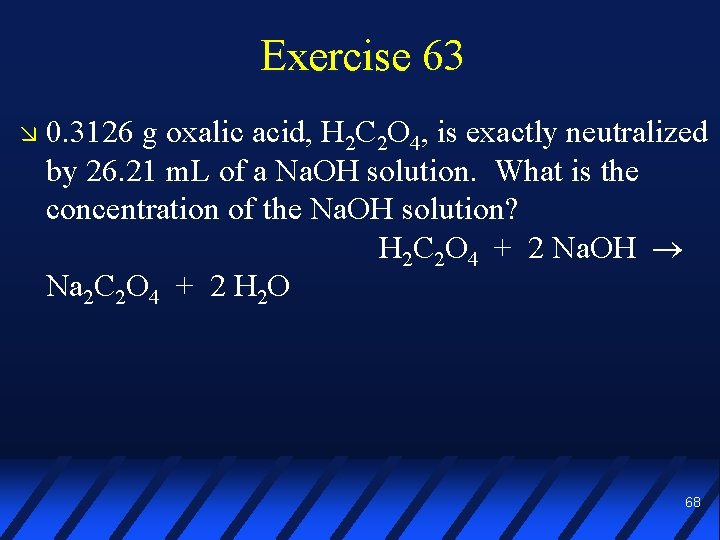


- Slides: 59

Chemical Reactions 1

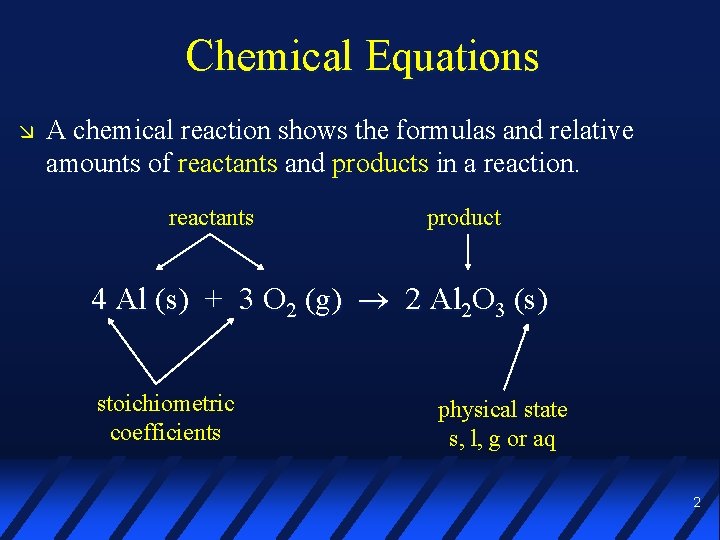
Chemical Equations A chemical reaction shows the formulas and relative amounts of reactants and products in a reaction. reactants product 4 Al (s) + 3 O 2 (g) 2 Al 2 O 3 (s) stoichiometric coefficients physical state s, l, g or aq 2

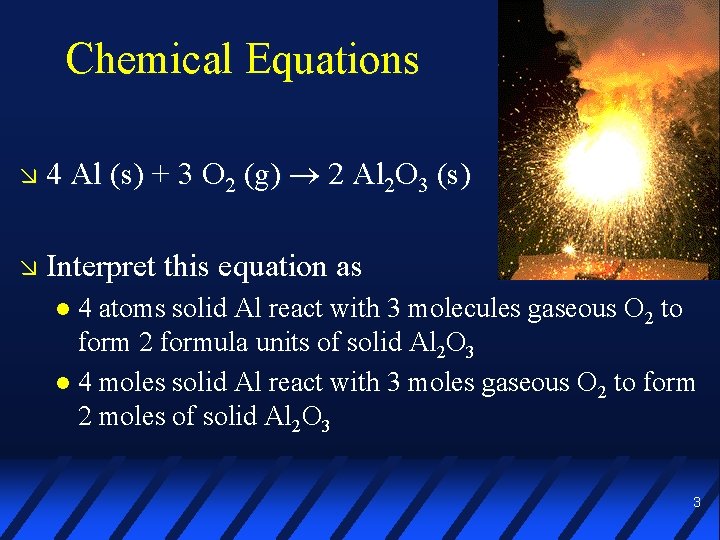
Chemical Equations 4 Al (s) + 3 O 2 (g) 2 Al 2 O 3 (s) Interpret this equation as 4 atoms solid Al react with 3 molecules gaseous O 2 to form 2 formula units of solid Al 2 O 3 4 moles solid Al react with 3 moles gaseous O 2 to form 2 moles of solid Al 2 O 3 3

Balancing Chemical Equations “Matter is conserved in chemical change” Antoine Lavoisier, 1789 An equation must be balanced: It must have the same number of atoms of each kind on both sides 4

The rules of the game Write the correct formulas of the reactants and products Do not change the formulas to balance the equation Put a coefficient in front of each formula so that the same number of atoms of each kind appear in both the reactants and products The coefficent multiplies through its formula 2 H 2 O shows 4 H atoms and 2 O atoms 5

Example 1 Balance this equation: N 2 + H 2 NH 3 There are 2 Ns on the left, we need 2 Ns on the right Put a coefficient of 2 in front of NH 3 N 2 + H 2 2 NH 3 Now there are 6 Hs on the right, we need 6 on the left Put a coefficient of 3 in front of H 2 N 2 + 3 H 2 2 NH 3 Balanced equation shows 2 Ns and 6 Hs on each side. 6

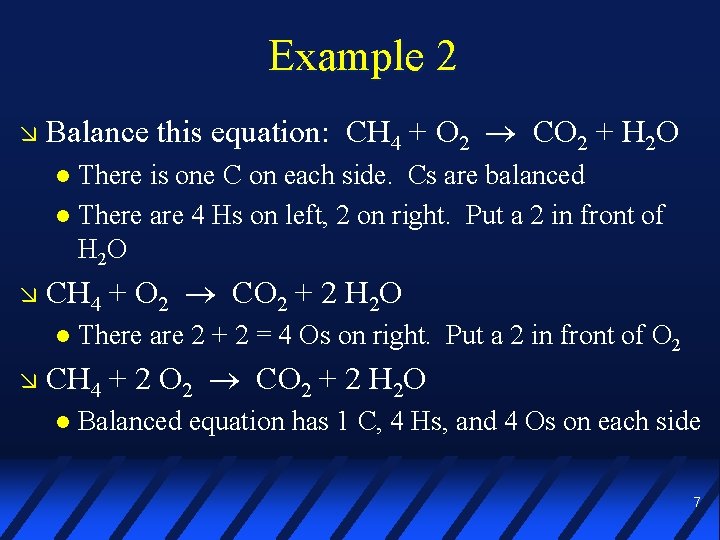
Example 2 Balance this equation: CH 4 + O 2 CO 2 + H 2 O There is one C on each side. Cs are balanced There are 4 Hs on left, 2 on right. Put a 2 in front of H 2 O CH 4 + O 2 CO 2 + 2 H 2 O There are 2 + 2 = 4 Os on right. Put a 2 in front of O 2 CH 4 + 2 O 2 CO 2 + 2 H 2 O Balanced equation has 1 C, 4 Hs, and 4 Os on each side 7

Balance these equations and list the coefficients from left to right (including coefficients of one) Hg. O (s) Hg (l) + O 2 (g) 2 Hg. O (s) 2 Hg (l) + O 2 (g) Answer 2, 2, 1 Mg (s) + HCl (aq) Mg. Cl 2 (aq) + H 2 (g) Mg (s) + 2 HCl (aq) Mg. Cl 2 (aq) + H 2 (g) Answer 1, 2, 1, 1 8

Balance these equations and list the coefficients from left to right (including coefficients of one) C 3 H 8 (g) + O 2 (g) CO 2 (g) + H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) Answer 1, 5, 3, 4 Al (s) + Cl 2 (g) Al. Cl 3 (s) 2 Al (s) + 3 Cl 2 (g) 2 Al. Cl 3 (s) Answer 2, 3, 2 9

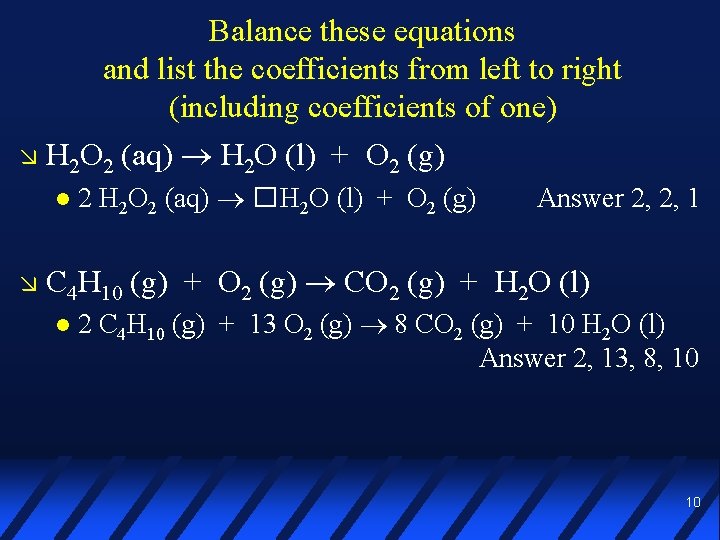
Balance these equations and list the coefficients from left to right (including coefficients of one) H 2 O 2 (aq) H 2 O (l) + O 2 (g) 2 H 2 O 2 (aq) �H 2 O (l) + O 2 (g) Answer 2, 2, 1 C 4 H 10 (g) + O 2 (g) CO 2 (g) + H 2 O (l) 2 C 4 H 10 (g) + 13 O 2 (g) 8 CO 2 (g) + 10 H 2 O (l) Answer 2, 13, 8, 10 10

Combustion Reactions In combustion, a hydrocarbon or C–H–O fuel combines with O 2 to form CO 2 and H 2 O __ CH 4 + __ O 2 __ CO 2 + __ H 2 O 1 CH 4 + 2 O 2 1 CO 2 + 2 H 2 O Balanced equation shows 1 C, 4 H, and 4 O on each side If N or S are in the formula for the fuel, assume it is oxidized to NO 2 or SO 2 11

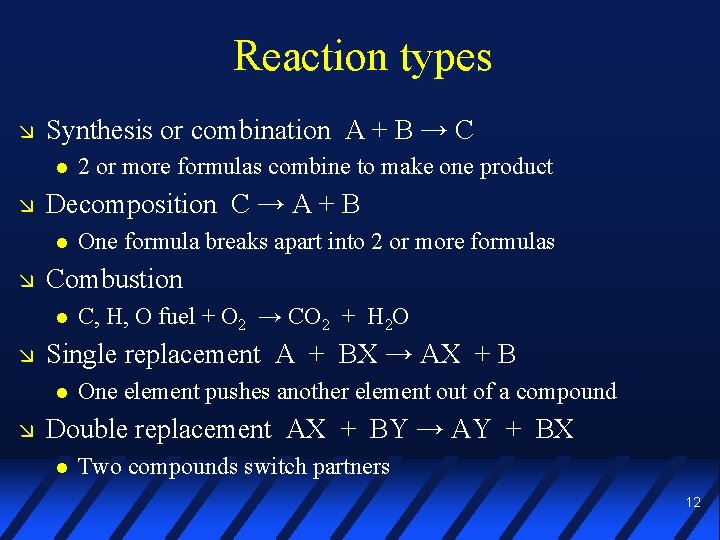
Reaction types Synthesis or combination A + B → C Decomposition C → A + B C, H, O fuel + O 2 → CO 2 + H 2 O Single replacement A + BX → AX + B One formula breaks apart into 2 or more formulas Combustion 2 or more formulas combine to make one product One element pushes another element out of a compound Double replacement AX + BY → AY + BX Two compounds switch partners 12

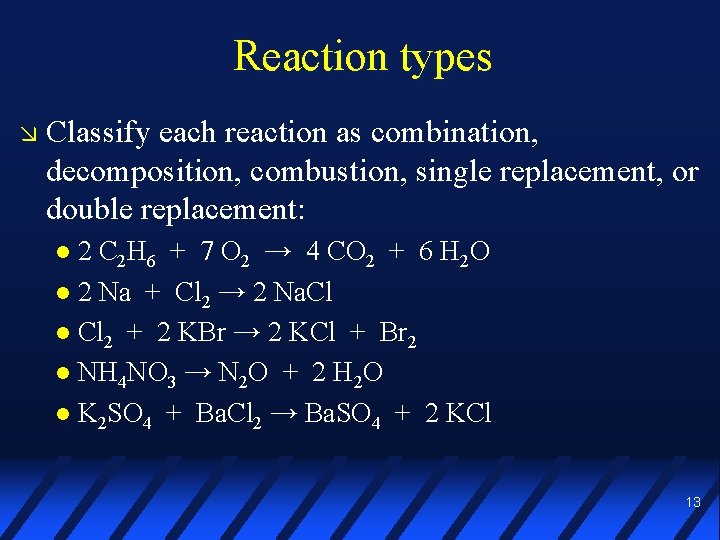
Reaction types Classify each reaction as combination, decomposition, combustion, single replacement, or double replacement: 2 C 2 H 6 + 7 O 2 → 4 CO 2 + 6 H 2 O 2 Na + Cl 2 → 2 Na. Cl 2 + 2 KBr → 2 KCl + Br 2 NH 4 NO 3 → N 2 O + 2 H 2 O K 2 SO 4 + Ba. Cl 2 → Ba. SO 4 + 2 KCl 13

Stoichiometry is chemical accounting Heart of stoichiometry is mole ratio given by coefficients of balanced equation 14

Stoichiometry is chemical accounting Heart of stoichiometry is mole ratio given by coefficients of balanced equation moles A mole ratio moles B moles A moles B 15

Example How many moles of O 2 are produced from the decomposition of 1. 76 mol KCl. O 3: 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) Question about O 2 but information for KCl. O 3 O 2 and KCl. O 3 related by coefficients of balanced equation. Coefficients are in moles: 2 mol KCl. O 3 : 3 mol O 2 16

Example How many moles of O 2 are produced from the decomposition of 1. 76 mol KCl. O 3: 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) 17

Example How many moles of CO 2 form when 0. 35 mol C 2 H 6 burn? 2 C 2 H 6 + 7 O 2 → 4 CO 2 + 6 H 2 O 18

Example How many moles of CO 2 form when 0. 35 mol C 2 H 6 burn? 2 C 2 H 6 + 7 O 2 → 4 CO 2 + 6 H 2 O 19

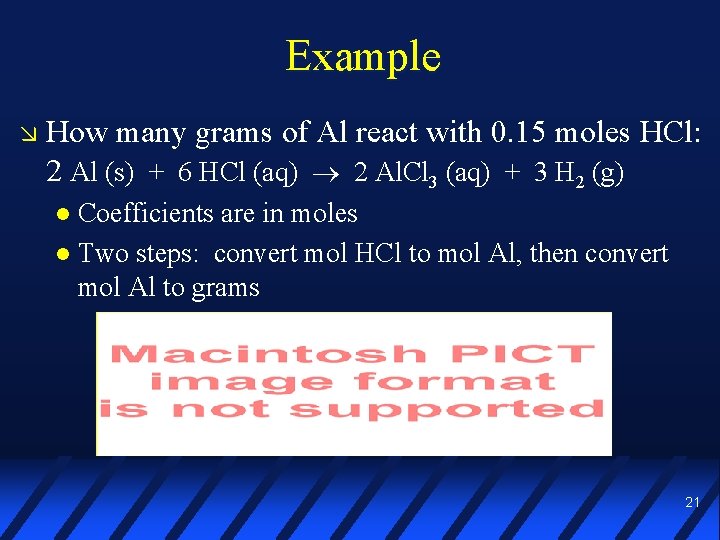
Example How many grams of Al react with 0. 15 moles HCl: 2 Al (s) + 6 HCl (aq) 2 Al. Cl 3 (aq) + 3 H 2 (g) Coefficients are in moles Two steps: convert mol HCl to mol Al, then convert mol Al to grams 20

Example How many grams of Al react with 0. 15 moles HCl: 2 Al (s) + 6 HCl (aq) 2 Al. Cl 3 (aq) + 3 H 2 (g) Coefficients are in moles Two steps: convert mol HCl to mol Al, then convert mol Al to grams 21

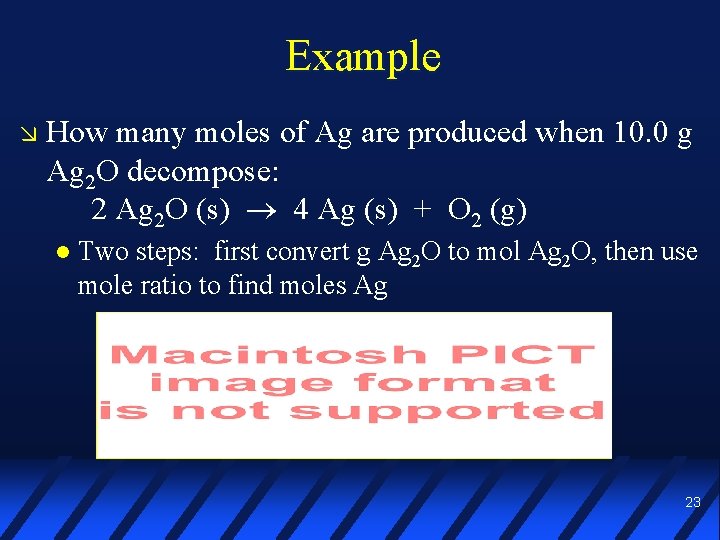
Example How many moles of Ag are produced when 10. 0 g Ag 2 O decompose: 2 Ag 2 O (s) 4 Ag (s) + O 2 (g) Two steps: first convert g Ag 2 O to mol Ag 2 O, then use mole ratio to find moles Ag 22

Example How many moles of Ag are produced when 10. 0 g Ag 2 O decompose: 2 Ag 2 O (s) 4 Ag (s) + O 2 (g) Two steps: first convert g Ag 2 O to mol Ag 2 O, then use mole ratio to find moles Ag 23

Example How many grams of H 2 (g) are needed to produce 100. 0 g of CH 3 OH: CO (g) + 2 H 2 (g) CH 3 OH (l) 25

Example How many grams of O 2 react with 268 grams of octane (C 8 H 18) in the combustion of octane? 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O 27

Example How many grams of O 2 react with 268 grams of octane (C 8 H 18) in the combustion of octane? 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O 28

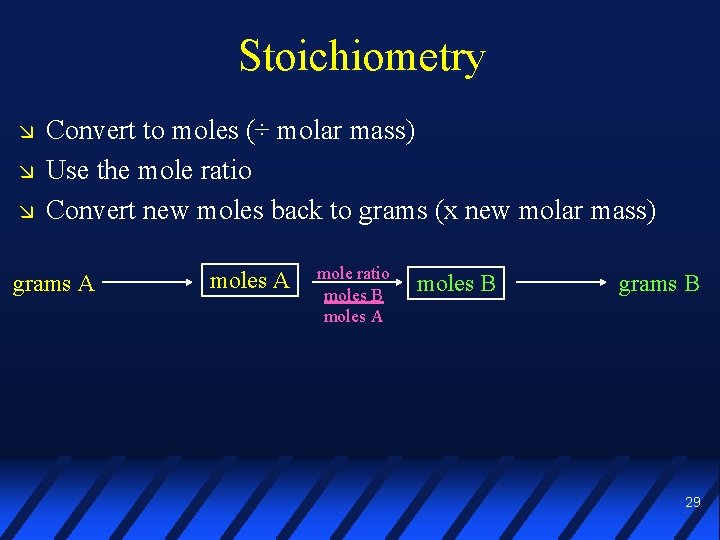
Stoichiometry Convert to moles (÷ molar mass) Use the mole ratio Convert new moles back to grams (x new molar mass) grams A mole ratio moles B moles A moles B grams B 29

Example Balance the chemical equation Identify the type of reaction Calculate how many moles of Ag. NO 3 are needed to produce 3. 17 g Ag 2 CO 3 Ag. NO 3 + K 2 CO 3 Ag 2 CO 3 + KNO 3 30

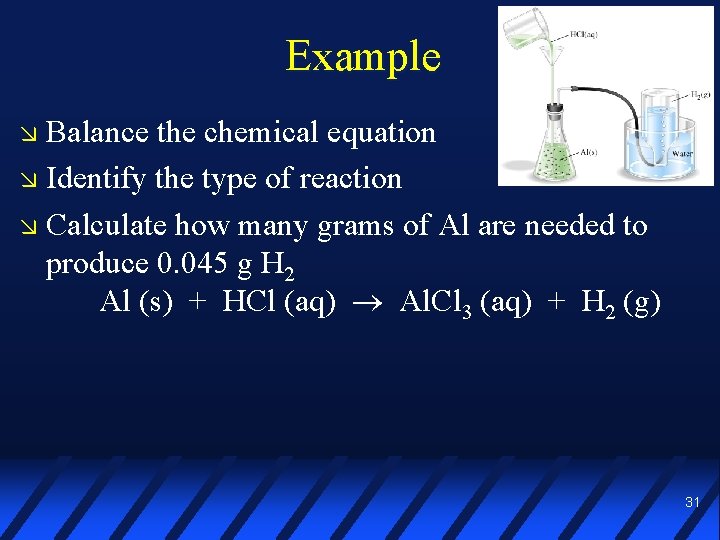
Example Balance the chemical equation Identify the type of reaction Calculate how many grams of Al are needed to produce 0. 045 g H 2 Al (s) + HCl (aq) Al. Cl 3 (aq) + H 2 (g) 31

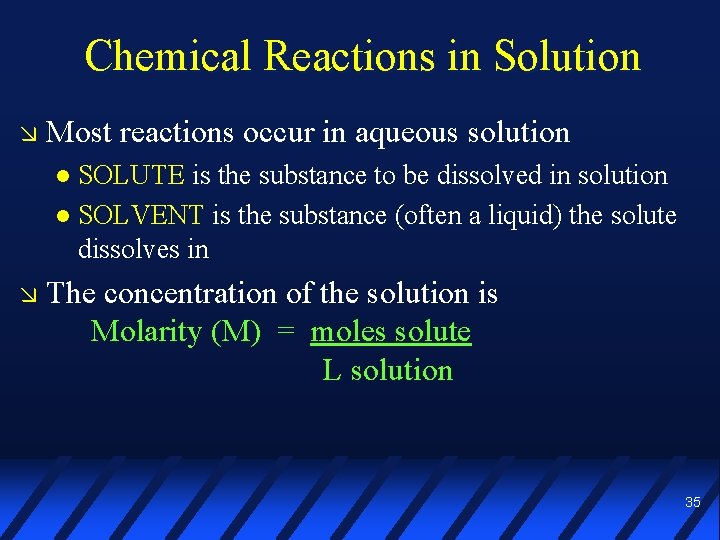
Chemical Reactions in Solution Most reactions occur in aqueous solution SOLUTE is the substance to be dissolved in solution SOLVENT is the substance (often a liquid) the solute dissolves in The concentration of the solution is Molarity (M) = moles solute L solution 35

Example 4 -8 B 15. 0 m. L of concentrated acetic acid, HC 2 H 3 O 2 (d = 1. 048 g/m. L), are dissolved in enough water to produce 500. 0 m. L of solution. What is the concentration of the solution? 37

Example 4 -9 B How many grams of Na 2 SO 4 • 10 H 2 O are needed to prepare 355 m. L of 0. 445 M Na 2 SO 4? 39

Dilution problems It is common to prepare a solution by diluting a more concentrated solution (the stock solution). The moles of solute taken from the stock solution are given by moles solute = volume x molarity All the solute taken from the stock appears in the diluted solution, so moles solute are constant: Vstock. Mstock = Vdilute. Mdilute 40

Example 4 -10 A 15. 00 m. L of 0. 450 M K 2 Cr. O 4 solution are diluted to 100. 00 m. L. What is the concentration of the dilute solution? 41

Example 4 -10 B After being left out in an open beaker, 275 m. L of 0. 105 M Na. Cl has evaporated to only 237 m. L. What is the concentration of the solution after evaporation? 42

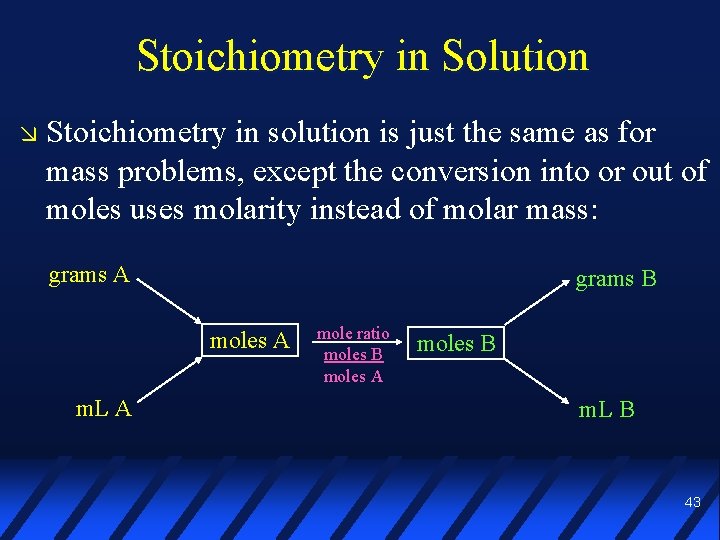
Stoichiometry in Solution Stoichiometry in solution is just the same as for mass problems, except the conversion into or out of moles uses molarity instead of molar mass: grams A grams B moles A m. L A mole ratio moles B moles A moles B m. L B 43

Example 4 -11 B K 2 Cr. O 4 (aq) + 2 Ag. NO 3 (aq) Ag 2 Cr. O 4 (s) + 2 KNO 3 (aq) How many m. L of 0. 150 M Ag. NO 3 must react with excess K 2 Cr. O 4 to produce exactly 1. 00 g Ag 2 Cr. O 4? 45

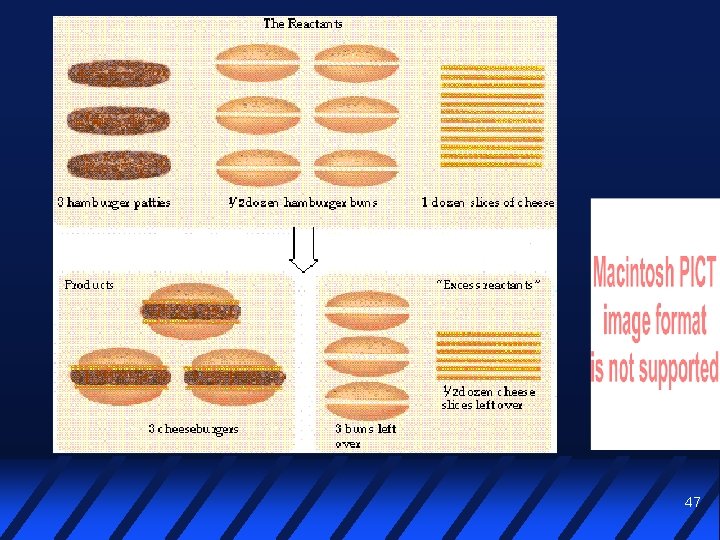
Limiting reactant In a given reaction, often there is not enough of one reactant to use up the other reactant completely The reactant in short supply LIMITS the quantity of product that can be formed 46

47

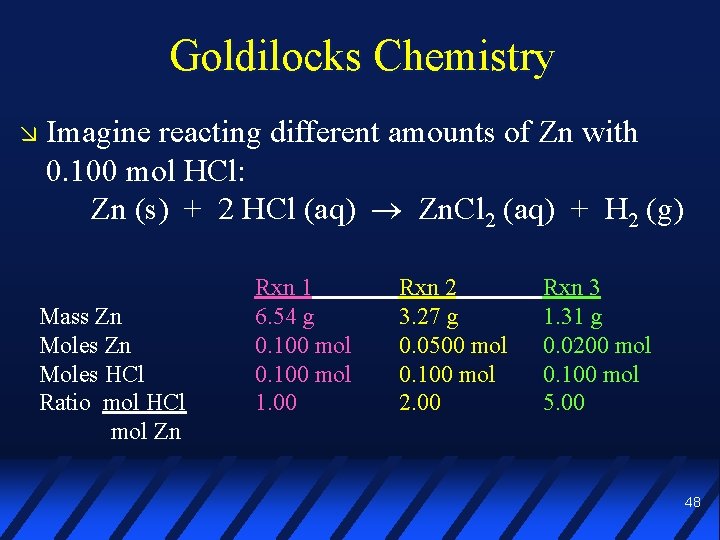
Goldilocks Chemistry Imagine reacting different amounts of Zn with 0. 100 mol HCl: Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) Mass Zn Moles HCl Ratio mol HCl mol Zn Rxn 1 6. 54 g 0. 100 mol 1. 00 Rxn 2 3. 27 g 0. 0500 mol 0. 100 mol 2. 00 Rxn 3 1. 31 g 0. 0200 mol 0. 100 mol 5. 00 48

Limiting reactant problems The easiest way to do these is to do two stoichiometry calculations Find the amount of product possible from each reactant The smaller answer is the amount of product you can actually make (you just ran out of one reactant) The reactant on which that answer was based is the limiting reactant 49

Example 4 -13 B 12. 2 g H 2 and 154 g O 2 are allowed to react. Identify the limiting reactant, which gas remains after the reaction, and what mass of it is left over. 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 53

Percent Yield In real experiments we often do not get the amount of product we calculate we should, because the reactants may participate in other reactions (side reactions) that produce other products (by-products) The reaction often does not go to completion. Percent yield tells the ratio of actual to theoretical amount formed. 54

Percent Yield Suppose you calculate that a reaction will produce 50. 0 g of product. This is theoretical yield. The reaction actually produces only 45. 0 g of product. This is the actual yield. Percent yield = 45. 0 g (actual) x 100 = 90. 0% 50. 0 g (theoretical) 55

Example 4 -14 B What is the percent yield if 25. 0 g P 4 reacts with 91. 5 g Cl 2 to produce 104 g PCl 3: P 4 (s) + 6 Cl 2 (g) 4 PCl 3 (l) 57

Example 4 -15 B What mass of C 6 H 11 OH should you start with to produce 45. 0 g C 6 H 10 if the reaction has 86. 2% yield and the C 6 H 11 OH is 92. 3% pure: C 6 H 11 OH (l) C 6 H 10 + H 2 O (l) 59

Exercise 26 Balance these equations by inspection (NH 4)2 Cr 2 O 7 (s) Cr 2 O 3 (s) + N 2 (g) + H 2 O (g) NO 2 (g) + H 2 O (l) HNO 3 (aq) + NO (g) H 2 S (g) + SO 2 (g) S (g) + H 2 O (g) SO 2 Cl 2 + HI H 2 S + H 2 O + HCl + I 2 60

Exercise 30 Write balanced equations for these reactions: Sulfur dioxide gas with oxygen gas to produce sulfur trioxide gas Solid calcium carbonate with water and dissolved carbon dioxide to produce aqueous calcium hydrogen carbonate Ammonia gas and nitrogen monoxide gas to produce nitrogen gas and water vapor 61

Exercise 32 3 Fe (s) + 4 H 2 O (g) Fe 3 O 4 (s) + H 2 (g) How many moles of H 2 can be produced from 42. 7 g Fe and excess steam? How many grams of H 2 O are consumed in the conversion of 63. 5 g Fe to Fe 3 O 4? If 7. 36 mol H 2 are produced, how many grams of Fe 3 O 4 must also be produced? 62

Exercise 36 Silver oxide decomposes above 300 °C to yield metallic silver and oxygen gas. 3. 13 g impure silver oxide yields 0. 187 g O 2. Assuming there is no other source of O 2, what is the % Ag 2 O by mass in the original sample? 63

Exercise 42 How many grams of CO 2 are produced in the complete combustion of 406 g of a bottled gas that consists of 72. 7% C 3 H 8 (propane) and 27. 3% C 4 H 10 (butane), by mass? 64

Exercise 45 What are the molarities of these solutes? 150. 0 g sucrose (C 12 H 22 O 11) in 250. 0 m. L aqueous solution 98. 3 mg of 97. 9% pure urea, CO(NH 2)2, in 5. 00 m. L aqueous solution 12. 5. 0 m. L methanol (CH 3 OH, density = 0. 792 g/m. L) in 15. 0 L aqueous solution 65

Exercise 52 After 25. 0 m. L of aqueous HCl solution is diluted to 500. 0 m. L, the concentration of the diluted solution is found to be 0. 085 M HCl. What was the concentration of the original HCl solution? 66

Exercise 56 Ca(OH)2 (s) + 2 HCl (aq) Ca. Cl 2 (aq) + 2 H 2 O (l) How many grams of Ca(OH)2 will react completely with 415 m. L of 0. 477 M HCl? How many kilograms of Ca(OH)2 will react with 324 L of an HCl solution that is 24. 28% HCl by mass, density = 1. 12 g/m. L? 67
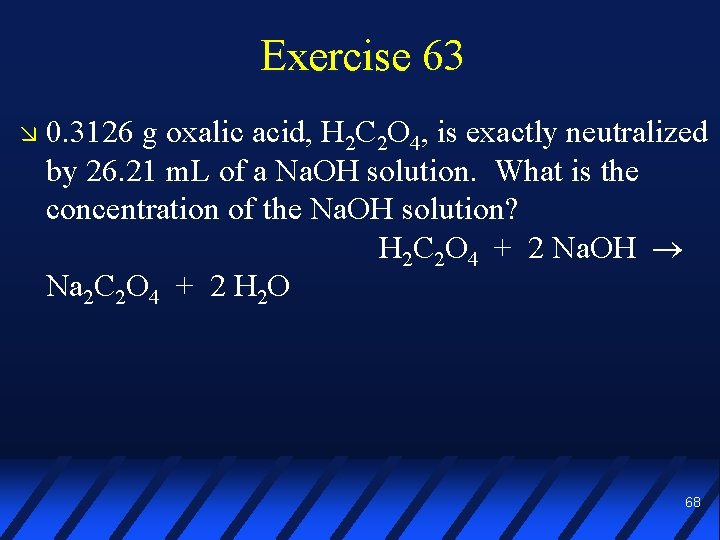
Exercise 63 0. 3126 g oxalic acid, H 2 C 2 O 4, is exactly neutralized by 26. 21 m. L of a Na. OH solution. What is the concentration of the Na. OH solution? H 2 C 2 O 4 + 2 Na. OH Na 2 C 2 O 4 + 2 H 2 O 68

Exercise 70 Chlorine can be generated by heating calcium hypochlorite and hydrochloric acid to form chlorine gas, calcium chloride, and water. If 50. 0 g Ca(OCl)2 and 275 m. L 6. 00 M HCl react, how many grams of Cl 2 gas form? Which reactant is left over, and how much (in grams)? 69

Exercise 72 2 C 6 H 5 NO 2 + 4 C 6 H 14 O 4 (C 6 H 5 N)2 + 4 C 6 H 12 O 4 + 4 H 2 O nitrobenzene triethylene glycol azobenzene If 0. 10 L nitrobenzene (d = 1. 20 g/m. L) react with 0. 30 L triethylene glycol (d = 1. 12 g/m. L) to form 55 g azobenzene, find Theoretical yield Actual yield Percent yield 70

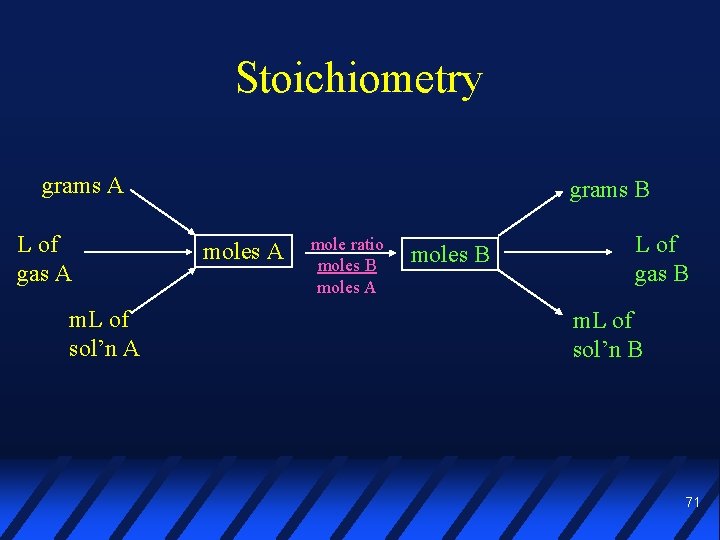
Stoichiometry grams A L of gas A m. L of sol’n A grams B moles A mole ratio moles B moles A moles B L of gas B m. L of sol’n B 71

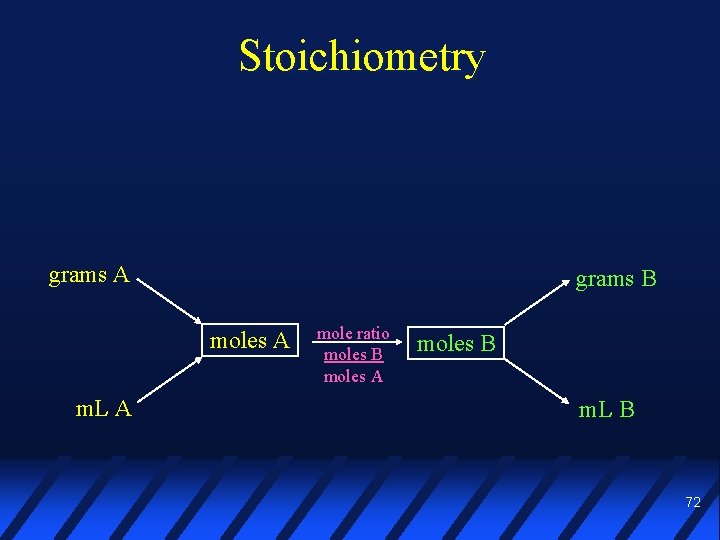
Stoichiometry grams A grams B moles A m. L A mole ratio moles B moles A moles B m. L B 72
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of reactions
Types of reactions Toxic reactions chemical equations answer key
Toxic reactions chemical equations answer key Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Unit 5 chemical reactions
Unit 5 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Synthesis reaction
Synthesis reaction Toxic reactions chemical equations
Toxic reactions chemical equations Half time equation
Half time equation Redox half reactions
Redox half reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 1 chemical changes
Section 1 chemical changes Translating chemical equations
Translating chemical equations Rate law
Rate law E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Stoichiometry mole island diagram
Stoichiometry mole island diagram Balancing redox reactions
Balancing redox reactions Identify types of reactions
Identify types of reactions 4 types of chemical reactions
4 types of chemical reactions How to identify type of reaction
How to identify type of reaction Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Combination reaction equation
Combination reaction equation Unit 11 chemical reactions
Unit 11 chemical reactions Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life What are five chemical changes
What are five chemical changes Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Rate constant and equilibrium constant
Rate constant and equilibrium constant Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Examples of double replacement reactions
Examples of double replacement reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Iupac nomenclature of esters
Iupac nomenclature of esters Chemical reactions summary
Chemical reactions summary Chemical reactions in bread
Chemical reactions in bread Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions