Chemical Formulas and Chemical Compounds Chapter 7 Chemistry































- Slides: 47

Chemical Formulas and Chemical Compounds Chapter 7 Chemistry

7 -1 Learning Targets • Write formulas for ionic compounds and oxyanions, and polyatomic ions. • Identify the formula of ionic compounds (with polyatomic ions and oxyanions) from their name • Write the names of molecular compounds from their formulas • Identify the formula of molecular compounds from their name • Name acidic solutions

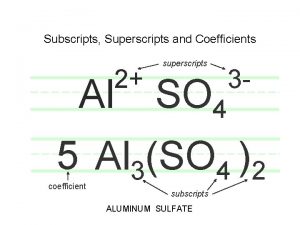
Formula • Tells the number and kinds of atoms in a compound • Subscript- number written below and to the right – Tells how many atoms are present – If there is no number, there is one atom

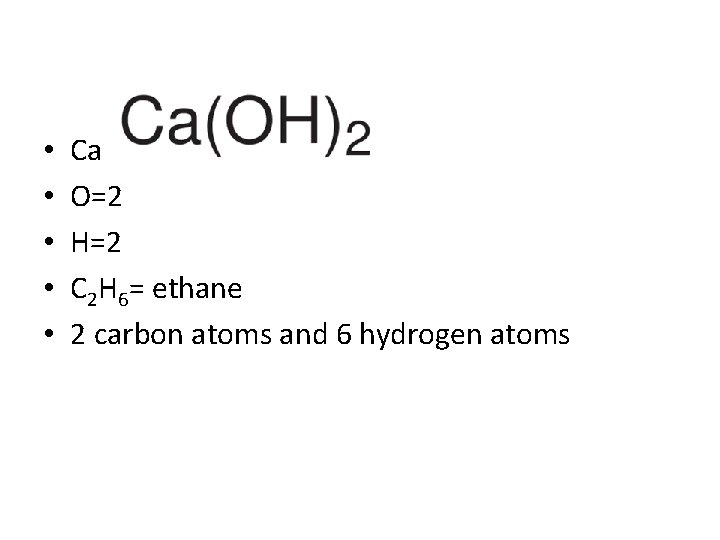
• • • Ca=1 O=2 H=2 C 2 H 6= ethane 2 carbon atoms and 6 hydrogen atoms

Monatomic ions p. 215 • Ions formed from a single atom • Naming cation: • Identified by element name • Naming anion: • Drop ending of the element name and add – ”ide” ending

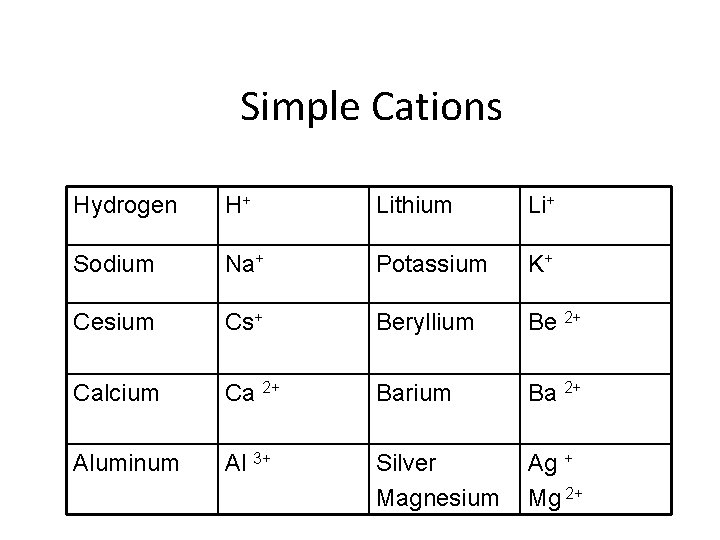
Simple Cations Hydrogen H+ Lithium Li+ Sodium Na+ Potassium K+ Cesium Cs+ Beryllium Be 2+ Calcium Ca 2+ Barium Ba 2+ Aluminum Al 3+ Silver Magnesium Ag + Mg 2+

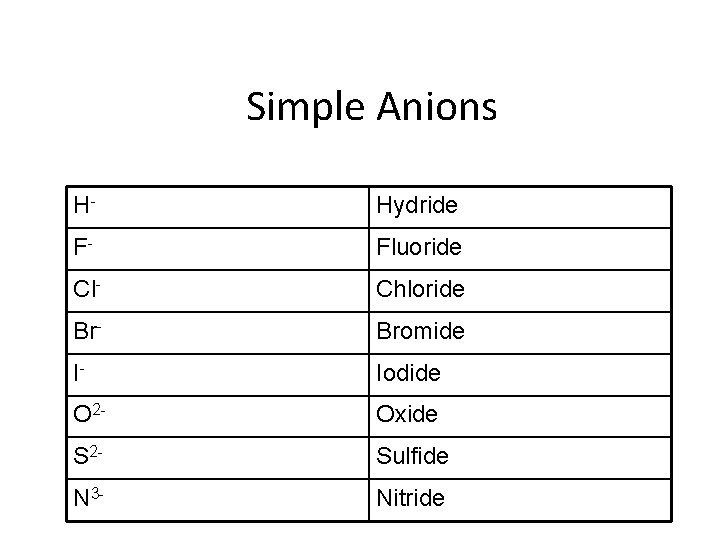
Simple Anions H- Hydride F- Fluoride Cl- Chloride Br- Bromide I- Iodide O 2 - Oxide S 2 - Sulfide N 3 - Nitride

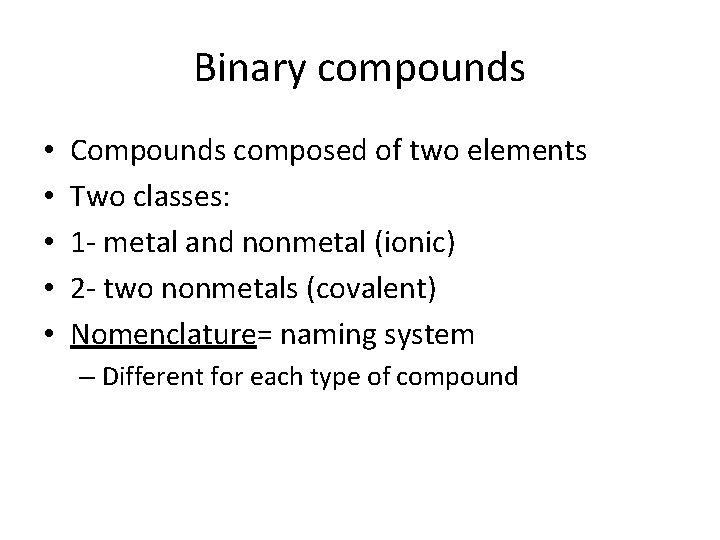
Binary compounds • • • Compounds composed of two elements Two classes: 1 - metal and nonmetal (ionic) 2 - two nonmetals (covalent) Nomenclature= naming system – Different for each type of compound

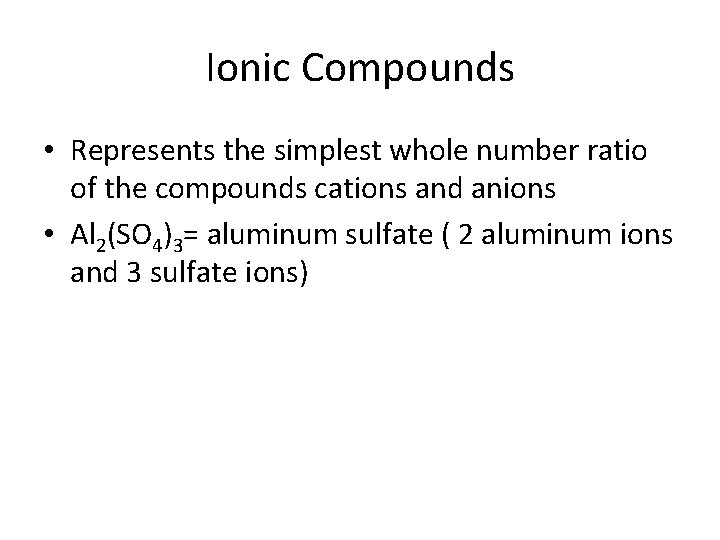
Ionic Compounds • Represents the simplest whole number ratio of the compounds cations and anions • Al 2(SO 4)3= aluminum sulfate ( 2 aluminum ions and 3 sulfate ions)

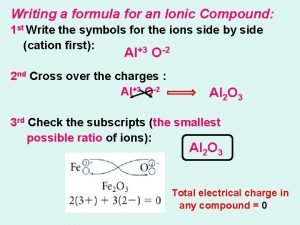
Binary Ionic compound • • Compound composed of two different elements Contains a cation and anion Always list cation first Writing formulas : – Write they symbols for the ions side by side – Cross over the charges by using absolute value of each ion’s charge as the subscript for the other ion – Subscripts need to be in smallest whole number ratio

• Naming binary ionic compounds from their formulas • Name the cation • Name the anion

Naming Binary ionic compounds • • Cs. F Cesium fluoride Al. Cl 3 Aluminum chloride Ba. O Barium oxide KF Potassium fluoride • • Li 2 O Lithium oxide Potassium iodide KI Barium bromide Ba. Br 2 Sodium phosphide Na 3 P

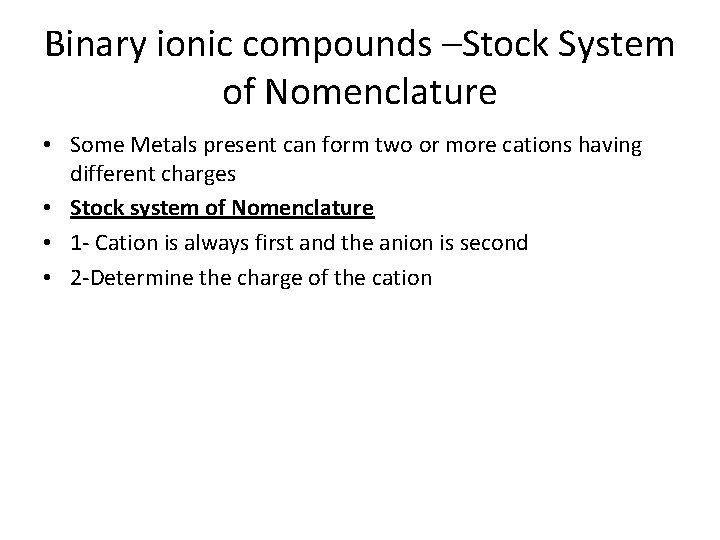
Binary ionic compounds –Stock System of Nomenclature • Some Metals present can form two or more cations having different charges • Stock system of Nomenclature • 1 - Cation is always first and the anion is second • 2 -Determine the charge of the cation

• 3 - Roman numerals are used to denote the charge of metals that can form two or more cations • Numeral enclosed in parentheses and placed after the metal name – Iron (II) “iron two” • Roman numerals never used: – For anions – For metals that form only one ion

• 4 -Anions are named by adding –ide to the root name of the element

Old system of naming • 1 -Ion with the higher charge has a name ending in –ic • 2 - Ion with lower charge has a name ending in -ous

Common Stock System Cations Fe 3+ Fe 2+ Cu + Iron (III) Iron (II) Copper (I) Ferric Ferrous Cupric Cuprous Co 3+ Co 2+ Sn 4+ Sn 2+ Ni 2+ Cobalt (III) Cobalt (II) Tin (IV) Tin (II) Nickel (II) Cobaltic Cobaltous Stannic Stannous

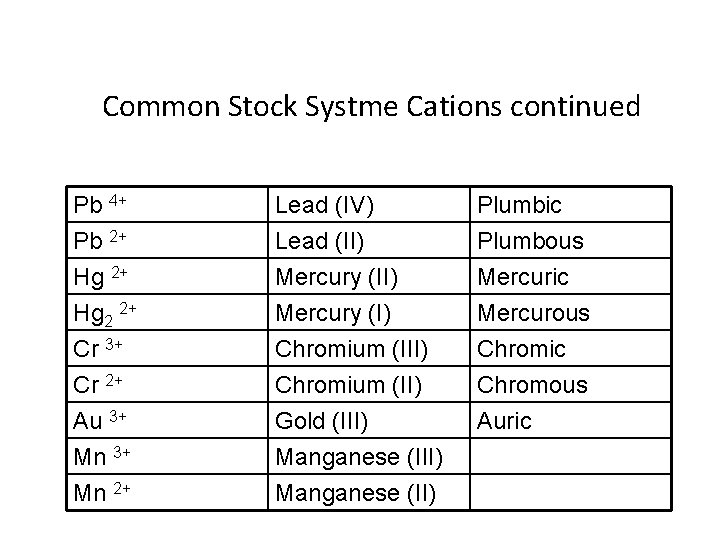
Common Stock Systme Cations continued Pb 4+ Pb 2+ Hg 2 2+ Lead (IV) Lead (II) Mercury (I) Plumbic Plumbous Mercuric Mercurous Cr 3+ Cr 2+ Au 3+ Mn 2+ Chromium (III) Chromium (II) Gold (III) Manganese (II) Chromic Chromous Auric

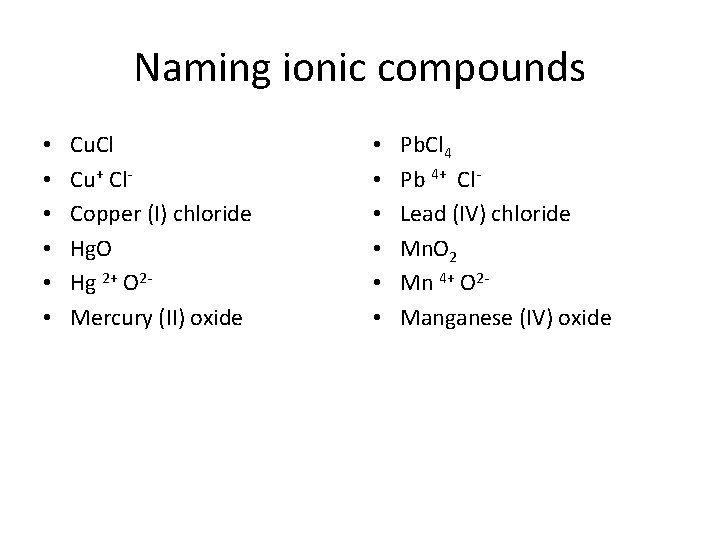
Naming ionic compounds • • • Cu. Cl Cu+ Cl. Copper (I) chloride Hg. O Hg 2+ O 2 Mercury (II) oxide • • • Pb. Cl 4 Pb 4+ Cl. Lead (IV) chloride Mn. O 2 Mn 4+ O 2 Manganese (IV) oxide

Writing formula of Ionic compound • Write they symbols for the ions side by side – Always list cation (+)first, anion (-) second • Cross over the charges by using absolute value of each ion’s charge as the subscript for the other ion • Subscripts need to be in smallest whole number ratio

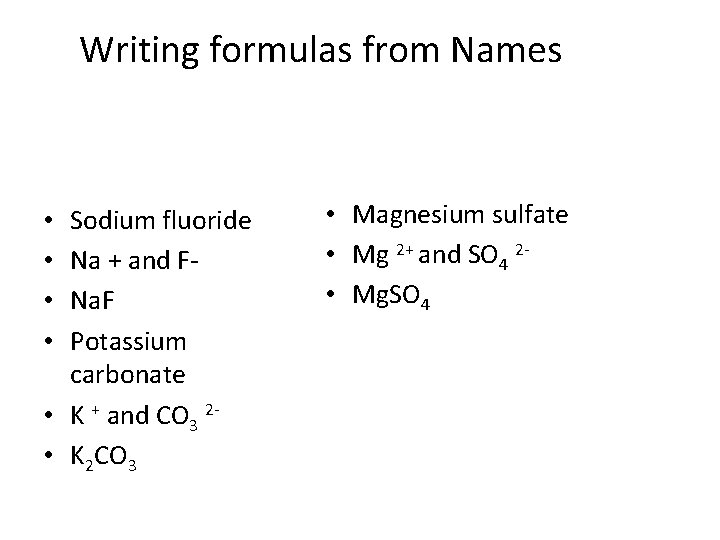
Writing formulas from Names Sodium fluoride Na + and FNa. F Potassium carbonate • K + and CO 3 2 • K 2 CO 3 • • • Magnesium sulfate • Mg 2+ and SO 4 2 • Mg. SO 4

• • • Aluminum hydroxide Al+3 OHAl (OH)3 Strontium bromide Sr+2 Br Sr. Br 2 • • • Iron (III) chloride Fe+3 Cl. Fe. Cl 3 Copper (II) iodide Cu+2 I – Cu. I 2

Polyatomic ions • Charged entities made of several atoms bound together covalently with a charge • Assigned special names the MUST be memorized p. 220

Oxyanions • Polyatomic ion containing an element and different numbers of oxygen atoms • 1 -the one with the smaller number of oxygen atoms ends in – ite • 2 -the one with the larger number of oxygen atoms ends in – ate • Example: Sulfite SO 32 - Sulfate SO 42 • More than two oxyanions make up a series, hypo- (less than) and per- (more than)

• • Cl. O- Hypochlorite Cl. O 2 - Chlorite Cl. O 3 - Chlorate Cl. O 4 - Perchlorate

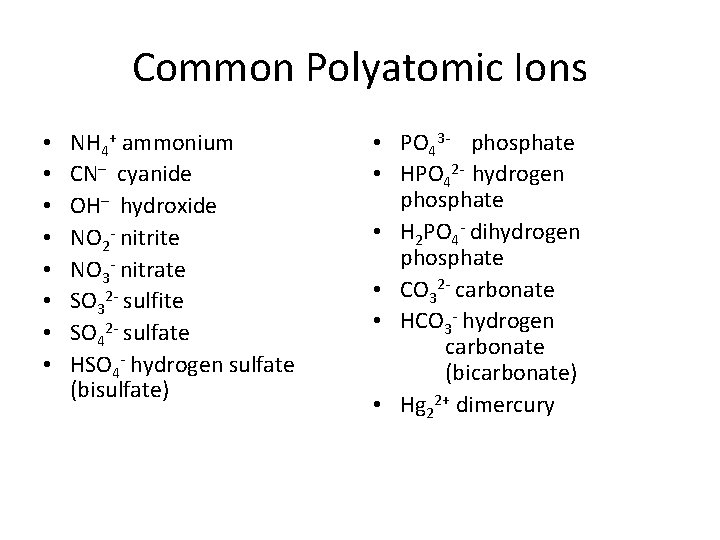
Common Polyatomic Ions • • NH 4+ ammonium CN– cyanide OH– hydroxide NO 2 - nitrite NO 3 - nitrate SO 32 - sulfite SO 42 - sulfate HSO 4 - hydrogen sulfate (bisulfate) • PO 43 - phosphate • HPO 42 - hydrogen phosphate • H 2 PO 4 - dihydrogen phosphate • CO 32 - carbonate • HCO 3 - hydrogen carbonate (bicarbonate) • Hg 22+ dimercury

Common Polyatomic Ions • • C 2 H 3 O 2 - acetate Mn. O 4 - permanganate Cr 2 O 72 - dichromate Cr. O 42 - chromate O 22 - peroxide As. O 4 3 - arsenate C 2 O 4 2 - oxalate S 2 O 3 2 - thiosulfate • • Br. O 3 - bromate IO 3 - iodate IO 4 - periodate Cl. O- hypochlorite Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate

The sentence below can be used to remember some of the polyatomic ions • Nick the Camel ate a Clam Supper in Phoenix • Nick - N with 3 consonants and 1 vowel therefore NO 3 -1 Camel - C with 3 consonants and 2 vowels, therefore CO 3 -2 Clam - Cl with 3 consonants and 2 vowels, therefore Cl. O 3 -1 Supper - S with 4 consonants and 2 vowels, therefore SO 4 -2 Phoenix - P with 4 consonants and 3 vowels, therefore PO 4 -3

• Naming compounds with polyatomic ions: – Same as for monatomic ions • Writing formulas including polyatomic ions: – Use parentheses when you need more than on polyatomic ion – Parentheses are never used for monatomic ions regardless of how many are in the formula

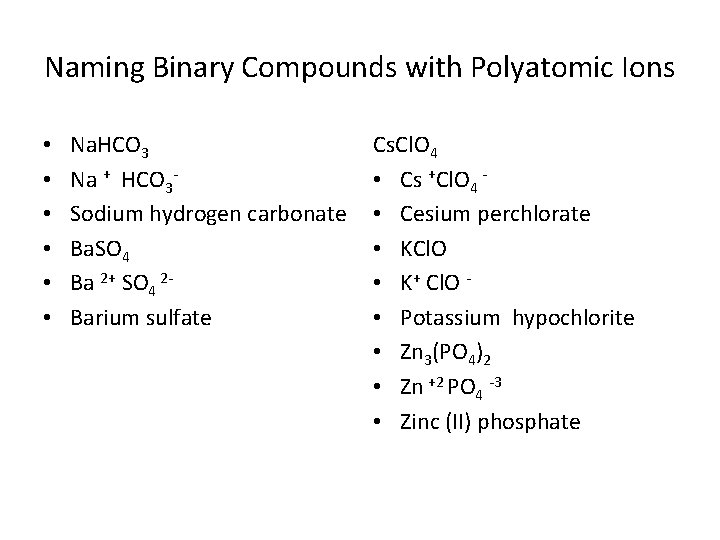
Naming Binary Compounds with Polyatomic Ions • • • Na. HCO 3 Na + HCO 3 Sodium hydrogen carbonate Ba. SO 4 Ba 2+ SO 4 2 Barium sulfate Cs. Cl. O 4 • Cs +Cl. O 4 • Cesium perchlorate • KCl. O • K+ Cl. O • Potassium hypochlorite • Zn 3(PO 4)2 • Zn +2 PO 4 -3 • Zinc (II) phosphate

Writing Formulas from Names • • • Calcium chlorate Ca +2 Cl. O 3 Ca(Cl. O 3)2 Ammonium sulfate NH 4 +1 SO 4 -2 (NH 4)2 SO 4 Vanadium (V) fluoride V +5 FVF 5 • • • Rubidium peroxide Rb +1 O 2 -2 Rb 2 O 2 Aluminum hydroxide Al +3 OHAl(OH)3 Calcium carbonate Ca +2 CO 3 -2 Ca. CO 3


Binary Molecular Compounds • Contain only nonmetals covalently bonded

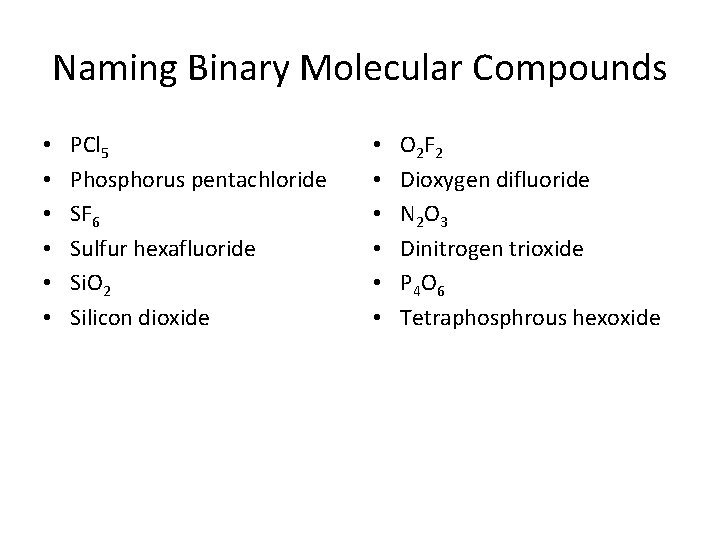
Naming Binary Molecular Compounds • 1 - First element in the formula is named first using the full element name • 2 - Second element is named as if an anion (“ide”)

• 3 - Prefixes are used to denote the number of atoms present • 4 - Prefix mono- is never used with the first element (drop o or a at end of prefix when word following begins with a vowel) • Second element ALWAYS gets a prefix

Prefixes • • 1 -mono 2 - di 3 - tri 4 - tetra 5 -penta 6 - hexa 7 - hepta • 8 - octa • 9 -nona • 10 - deca

Naming Binary Molecular Compounds • • • PCl 5 Phosphorus pentachloride SF 6 Sulfur hexafluoride Si. O 2 Silicon dioxide • • • O 2 F 2 Dioxygen difluoride N 2 O 3 Dinitrogen trioxide P 4 O 6 Tetraphosphrous hexoxide

Writing Formulas from Names • • Nitrogen trichloride NCl 3 Difluorine monoxide F 2 O • • Sulfur dichloride SCl 2 nitrogen dioxide NO 2 Diphosphorus trioxide P 2 O 3 Carbon tetrachloride CCl 4

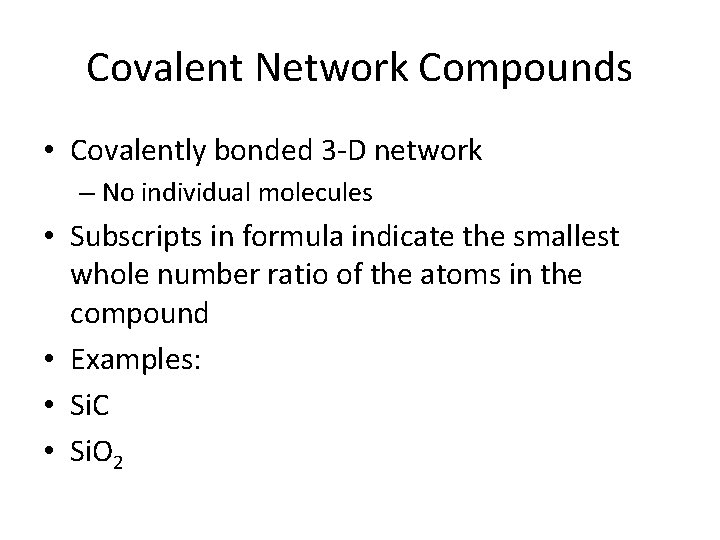
Covalent Network Compounds • Covalently bonded 3 -D network – No individual molecules • Subscripts in formula indicate the smallest whole number ratio of the atoms in the compound • Examples: • Si. C • Si. O 2

Acids • • Substances that produce H+ ions In water solution are acids Sour taste Chemical formula is H followed by anion

Naming Acids • 1 - if anion has no O, name it hydro- (root element) –ic • Example: HCl hydrochloric acid • 2 - If anion has O: anion end sin –ate , acid ends in -ic • Anion end in –ite, acid ends in –ous • Example: H 2 SO 4 (sulfate anion) sulfuric acid • HNO 2 (nitrite) nitrous acid

Common Acids • • • HF hydrofluoric acid HCl hydrochloric acid HBr hydrobromic acid HI hydroiodic acid HCN hydrocyanic acid H 2 S hydrosulfuric acid

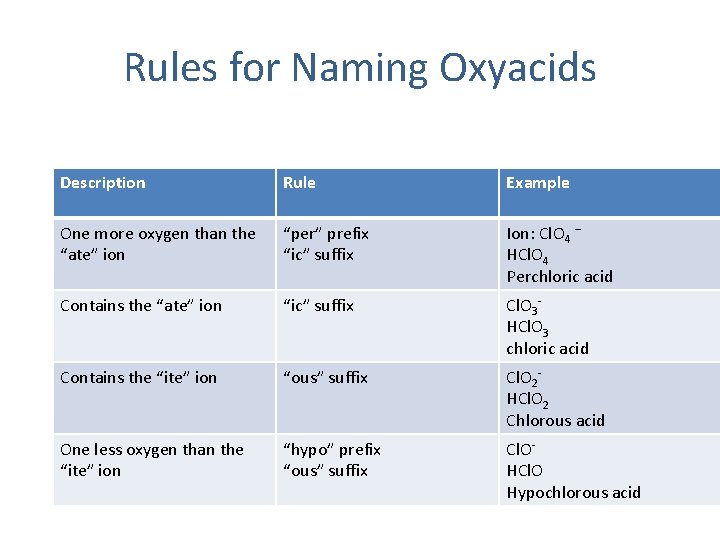
Oxyacid • Acid that contains hydrogen, oxygen, and third element (usually a nonmetal)

Rules for Naming Oxyacids Description Rule Example One more oxygen than the “ate” ion “per” prefix “ic” suffix Ion: Cl. O 4 – HCl. O 4 Perchloric acid Contains the “ate” ion “ic” suffix Cl. O 3 HCl. O 3 chloric acid Contains the “ite” ion “ous” suffix Cl. O 2 HCl. O 2 Chlorous acid One less oxygen than the “ite” ion “hypo” prefix “ous” suffix Cl. OHCl. O Hypochlorous acid

Naming Acids • • HCl. O 4 HCl. O 3 HCl. O 2 HCl. O » Anion Acid perchlorate chlorite hypochlorite perchloric acid chlorous acid hypochlorous acid

Common Acids • • • HNO 3 nitric acid HNO 2 nitrous acid H 2 SO 4 sulfuric acid H 2 SO 3 sulfurous acid H 3 PO 4 phosphoric acid HC 2 H 3 O 2 acetic acid

Salt • Ionic compound composed of a cation and the anion from an acid
 Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Modern chemistry chapter 7 test
Modern chemistry chapter 7 test Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Naming hydrates
Naming hydrates Tetraphosphorus octoxide formula
Tetraphosphorus octoxide formula Ionic covalent and metallic bonds
Ionic covalent and metallic bonds Naming compounds and writing formulas
Naming compounds and writing formulas Section 3 writing formulas and naming compounds answer key
Section 3 writing formulas and naming compounds answer key Monatomic formula
Monatomic formula Tetraiodine nonaoxide formula
Tetraiodine nonaoxide formula Section 3 writing formulas and naming compounds
Section 3 writing formulas and naming compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Chemical names and formulas chapter 9
Chemical names and formulas chapter 9 A chemical formula shows
A chemical formula shows Binary compound
Binary compound Which two formulas represent compounds
Which two formulas represent compounds Technicolor atoms flame test lab answers
Technicolor atoms flame test lab answers Writing formulas for ionic compounds worksheet
Writing formulas for ionic compounds worksheet Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Ionic compounds
Ionic compounds 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems Met eth prop
Met eth prop Priority when naming organic compounds
Priority when naming organic compounds Condensed structure of cyclohexane
Condensed structure of cyclohexane Chemical formulas
Chemical formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Writing and naming chemical formulas
Writing and naming chemical formulas Counting atoms worksheet answer key
Counting atoms worksheet answer key Mol formula
Mol formula Covalent bond formula
Covalent bond formula Li+ and po43- formula
Li+ and po43- formula Review naming ionic compounds
Review naming ionic compounds Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Deca silicon trinitride formula
Deca silicon trinitride formula Chemical formula covalent compounds
Chemical formula covalent compounds Hcn binary or ternary
Hcn binary or ternary Naming chemical compounds flowchart
Naming chemical compounds flowchart Chemical compounds
Chemical compounds Subscript
Subscript How to write an ionic compound formula
How to write an ionic compound formula Formula in chemistry
Formula in chemistry Criss cross rule
Criss cross rule Barium nitride formula
Barium nitride formula Empirical and molecular formula quiz
Empirical and molecular formula quiz Counting atoms
Counting atoms Ib chemistry functional groups
Ib chemistry functional groups