3 Chemical Compounds Leaving Certificate Chemistry Chemical Compounds







- Slides: 20

3 – Chemical Compounds Leaving Certificate Chemistry

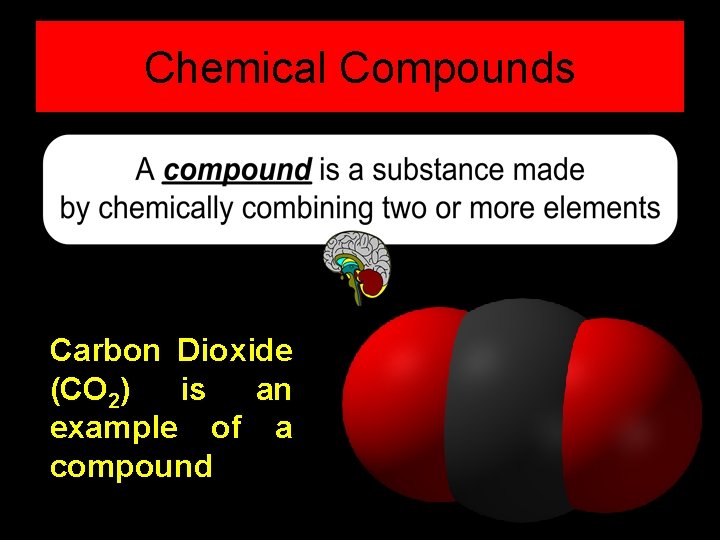
Chemical Compounds Carbon Dioxide (CO 2) is an example of a compound

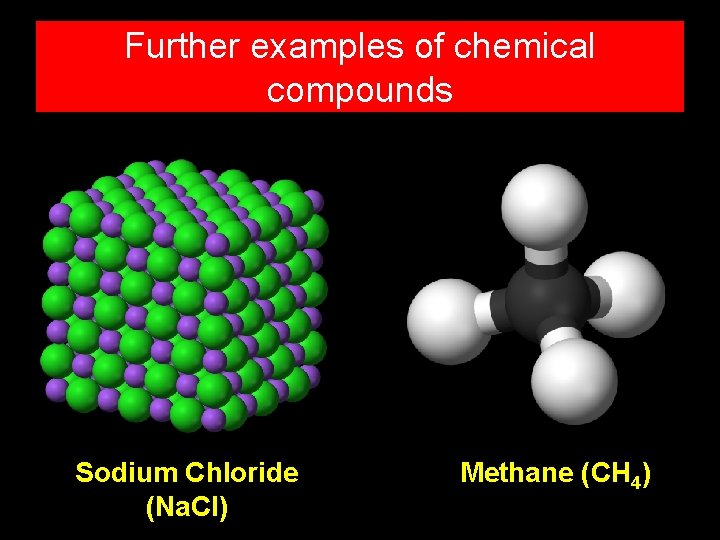
Further examples of chemical compounds Sodium Chloride (Na. Cl) Methane (CH 4)

The Noble Gases are very unreactive - They are not found combined with any other element Helium – a full outer shell of electrons Argon – a full outer shell of electrons

The uses of helium & argon Helium – used weather balloons in Argon – used in light bulbs

Valence

The Octet Rule When elements combine they do so to ensure that the outermost electron of each bonding atom has in its outer shell. 8 electrons There are exceptions: 1. Hydrogen and lithium look to have a full outer shell of 2 electrons. 2. Boron (element no. 5) forms many compounds with 6 electrons in its outer shell.

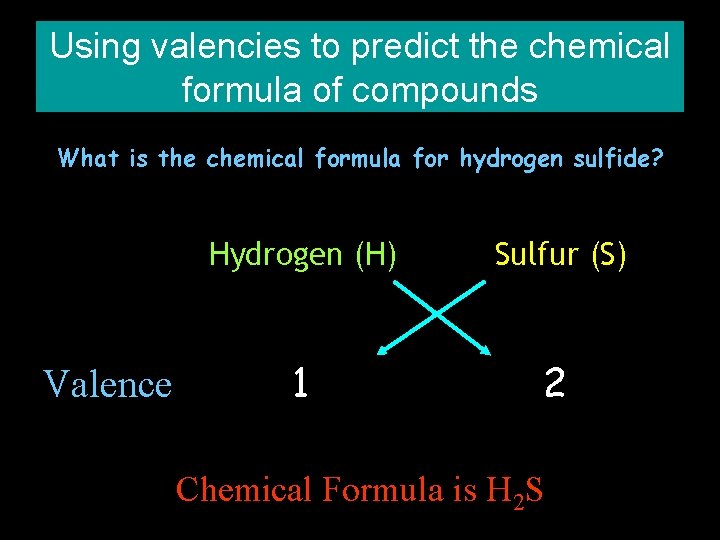
Using valencies to predict the chemical formula of compounds What is the chemical formula for hydrogen sulfide? Valence Hydrogen (H) Sulfur (S) 1 2 Chemical Formula is H 2 S

Can you… • Give the definition of a chemical compound? • Give some examples of compounds? • Tell some uses of noble gases • Say what the octet rule states and some exceptions • Define vacancy?

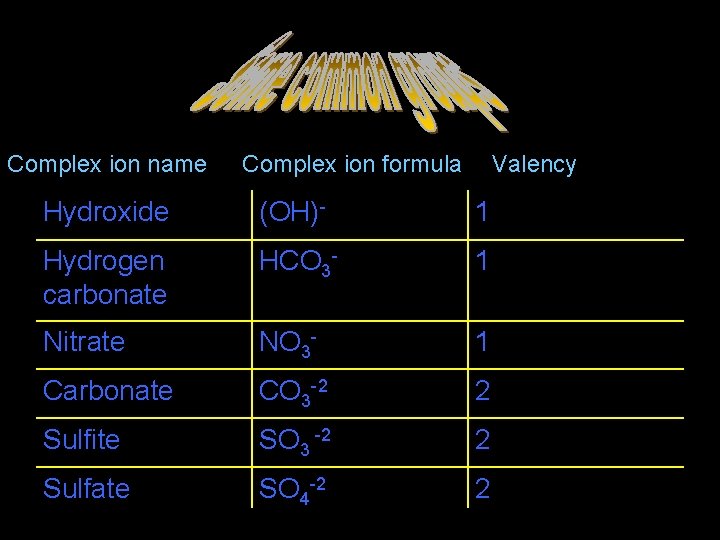
Some common groups Complex ion name Complex ion formula Valency Hydroxide (OH)- 1 Hydrogen carbonate HCO 3 - 1 Nitrate NO 3 - 1 Carbonate CO 3 -2 2 Sulfite SO 3 -2 2 Sulfate SO 4 -2 2

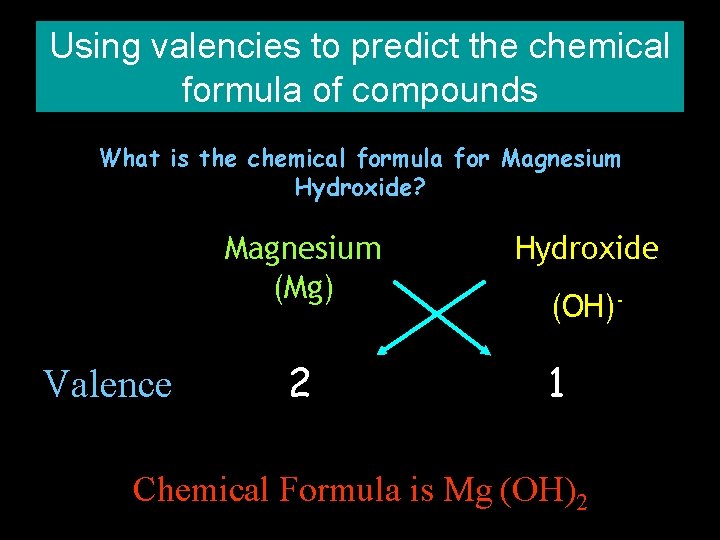
Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Hydroxide? Magnesium (Mg) Valence 2 Hydroxide (OH)- 1 Chemical Formula is Mg (OH)2

Practice Questions Write the formula of each of the following: 1. Aluminium hydroxide 2. Calcium carbonate 3. Magnesium nitrate 4. Sodium hydrogencarbonate 5. Potassium sulfite 6. Aluminium sulfate

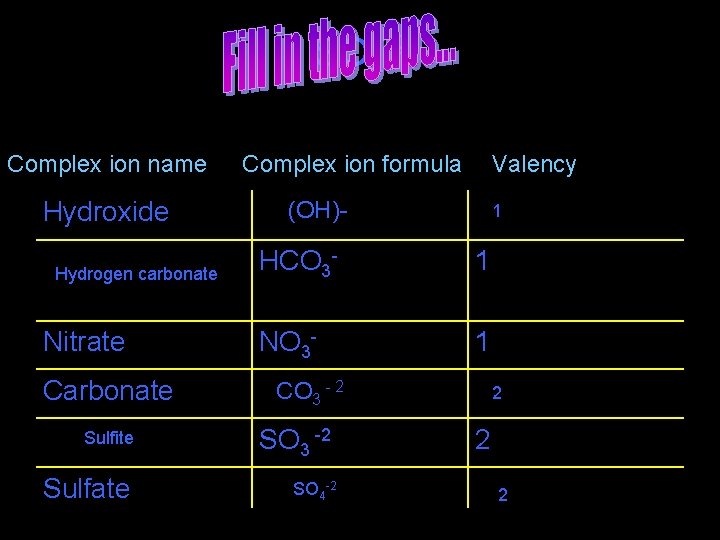
SO 4 -2 Complex ion name Hydroxide Hydrogen carbonate Nitrate Carbonate Complex ion formula Valency (OH)- 1 HCO 3 - 1 NO 3 - 1 CO 3 - 2 Sulfite SO 3 -2 Sulfate SO 4 -2 2

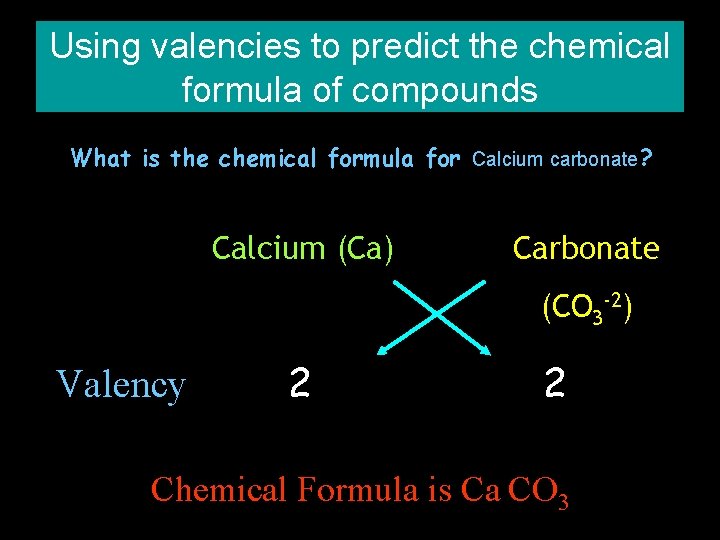
Using valencies to predict the chemical formula of compounds What is the chemical formula for Calcium carbonate? Calcium (Ca) Carbonate (CO 3 -2) Valency 2 2 Chemical Formula is Ca CO 3

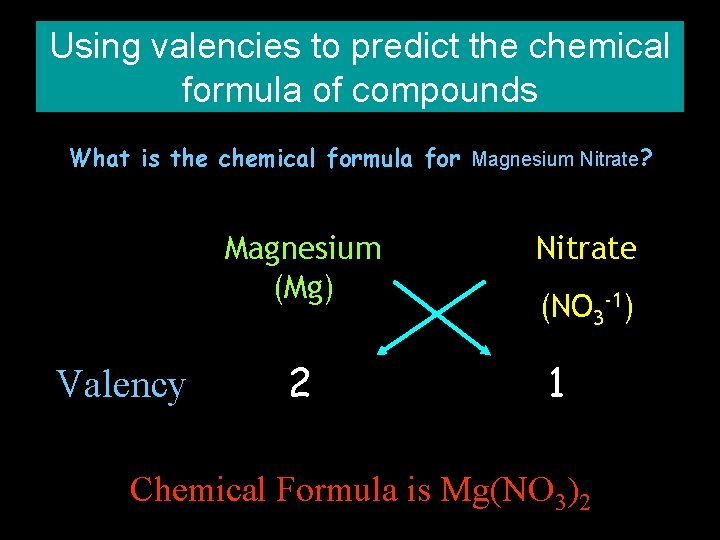
Using valencies to predict the chemical formula of compounds What is the chemical formula for Magnesium Nitrate? Magnesium (Mg) Valency 2 Nitrate (NO 3 -1) 1 Chemical Formula is Mg(NO 3)2

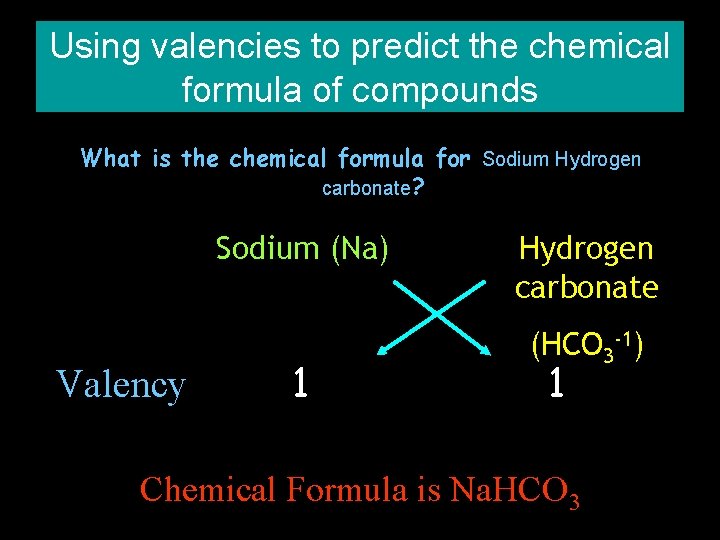
Using valencies to predict the chemical formula of compounds What is the chemical formula for Sodium Hydrogen carbonate? Sodium (Na) Valency 1 Hydrogen carbonate (HCO 3 -1) 1 Chemical Formula is Na. HCO 3

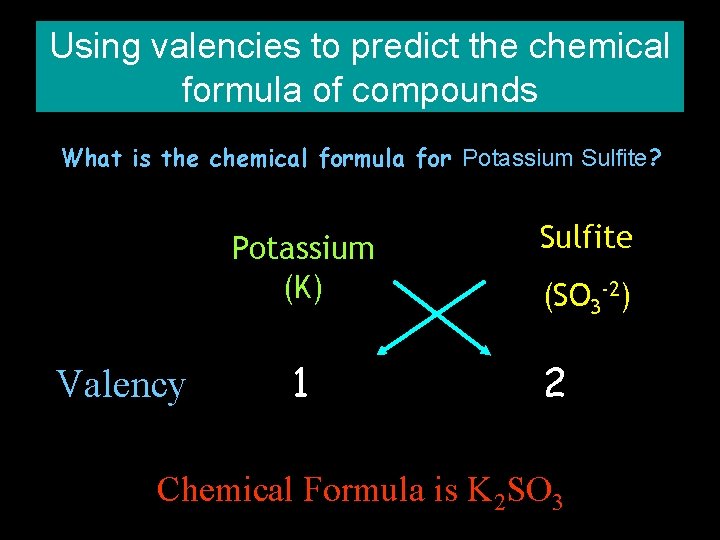
Using valencies to predict the chemical formula of compounds What is the chemical formula for Potassium Sulfite? Potassium (K) Valency 1 Sulfite (SO 3 -2) 2 Chemical Formula is K 2 SO 3

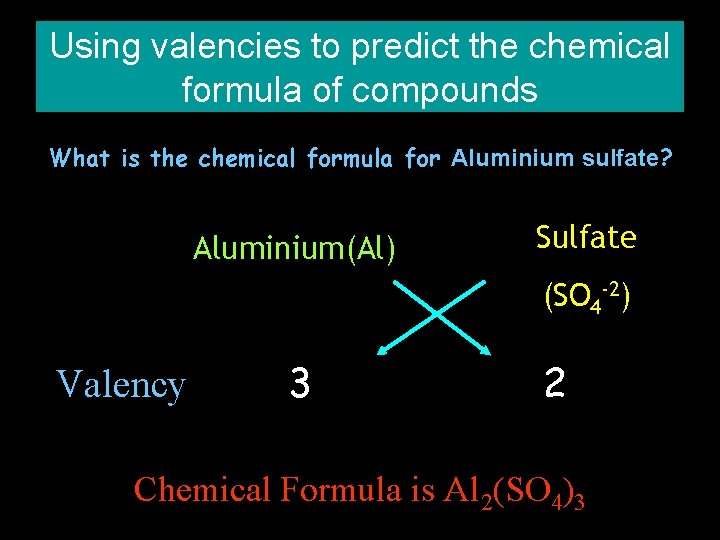
Using valencies to predict the chemical formula of compounds What is the chemical formula for Aluminium sulfate? Aluminium(Al) Sulfate (SO 4 -2) Valency 3 2 Chemical Formula is Al 2(SO 4)3

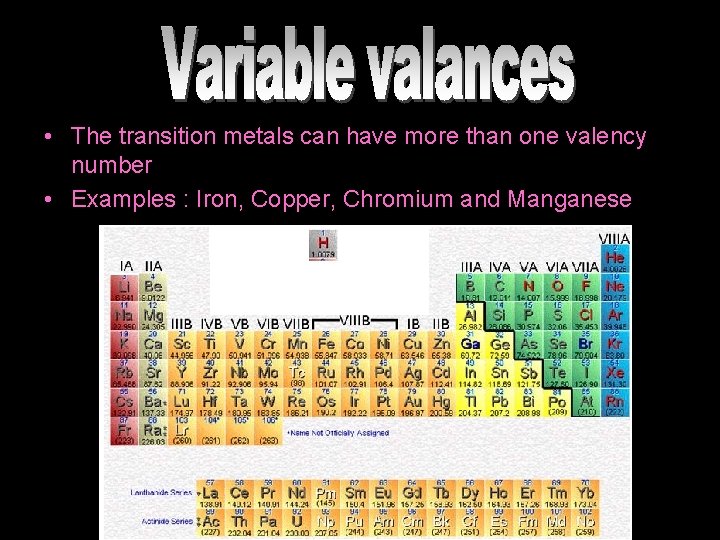
• The transition metals can have more than one valency number • Examples : Iron, Copper, Chromium and Manganese

Groups 3 -11 The d block metals Includes the following elements: Scandium (Sc) Titanium (Ti) Vanadium (V) Chromium (Cr) Manganese (Mn) Iron (Fe) They are all metals and are usually brightly coloured and act as catalysts for chemical reactions Cobalt (Co) Nickel (Ni) Copper (Co) Zinc (Zn) and others!
 History leaving cert syllabus
History leaving cert syllabus Primary leaving certificate
Primary leaving certificate Leaving certificate
Leaving certificate Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chemical formula of love
Chemical formula of love Leaving group chemistry
Leaving group chemistry Ionic, covalent and metallic bonds venn diagram
Ionic, covalent and metallic bonds venn diagram How to write formulas for ionic compounds
How to write formulas for ionic compounds Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Alkanes formula
Alkanes formula Seniority of functional groups
Seniority of functional groups Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Writing formulas criss cross method examples
Writing formulas criss cross method examples Naming compounds
Naming compounds Names of ionic compounds
Names of ionic compounds Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Covalent compound formula
Covalent compound formula Covalent compound formula
Covalent compound formula Hcn binary or ternary
Hcn binary or ternary Naming chemical compounds flowchart
Naming chemical compounds flowchart







































