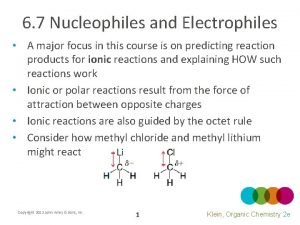
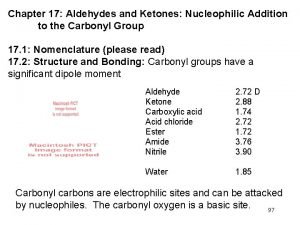
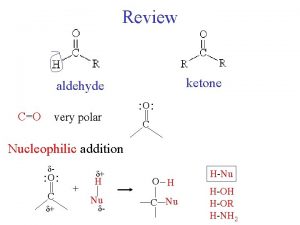
Nucleophilic Substitution and elimination Substitution Process Nucleophiles have

Nucleophilic Substitution and -elimination
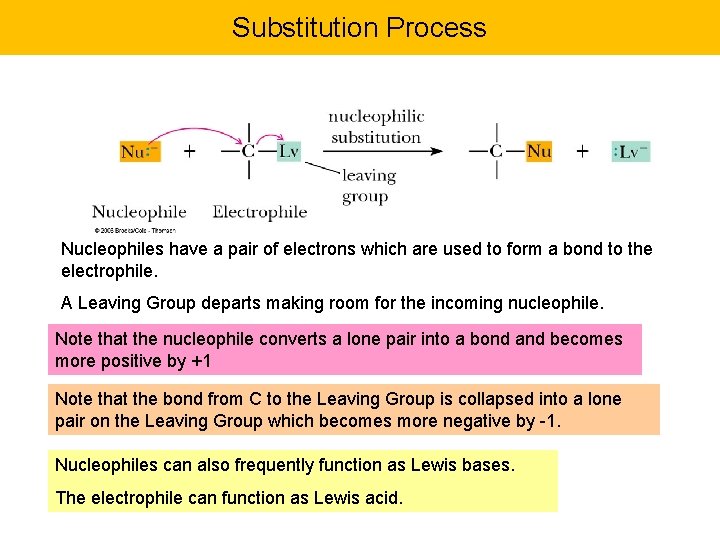
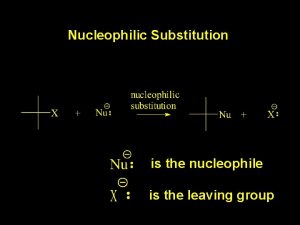
Substitution Process Nucleophiles have a pair of electrons which are used to form a bond to the electrophile. A Leaving Group departs making room for the incoming nucleophile. Note that the nucleophile converts a lone pair into a bond and becomes more positive by +1 Note that the bond from C to the Leaving Group is collapsed into a lone pair on the Leaving Group which becomes more negative by -1. Nucleophiles can also frequently function as Lewis bases. The electrophile can function as Lewis acid.
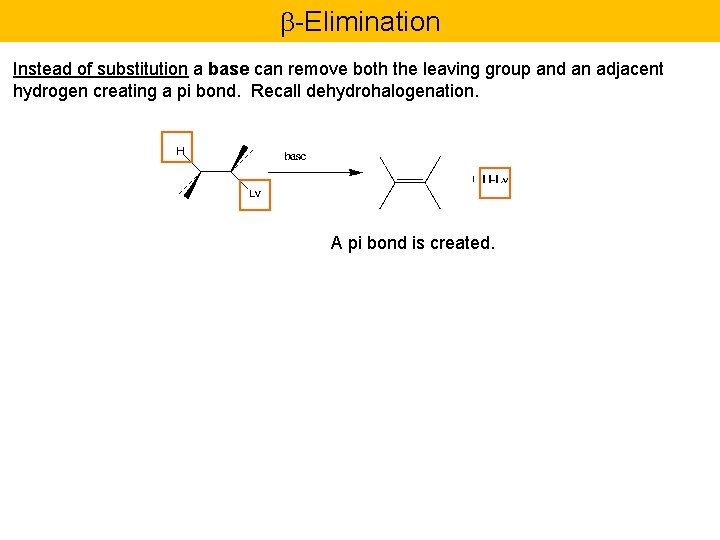
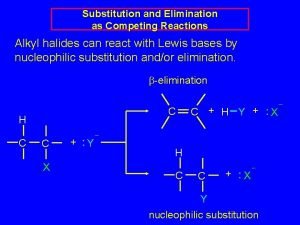
-Elimination Instead of substitution a base can remove both the leaving group and an adjacent hydrogen creating a pi bond. Recall dehydrohalogenation. A pi bond is created.
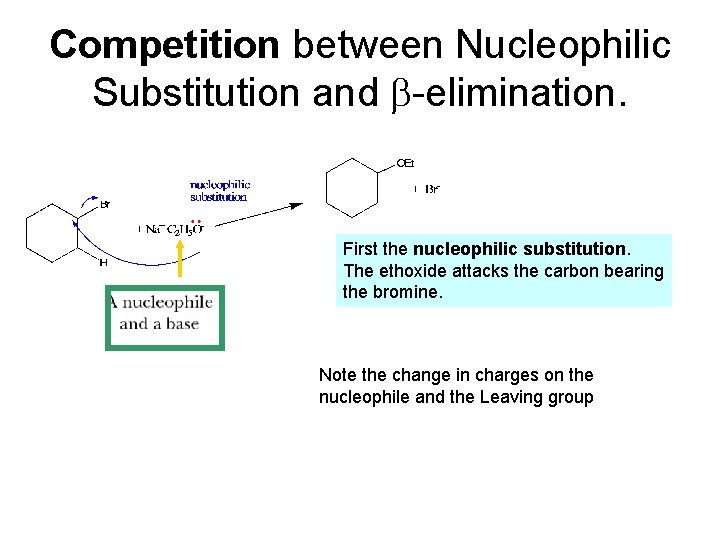
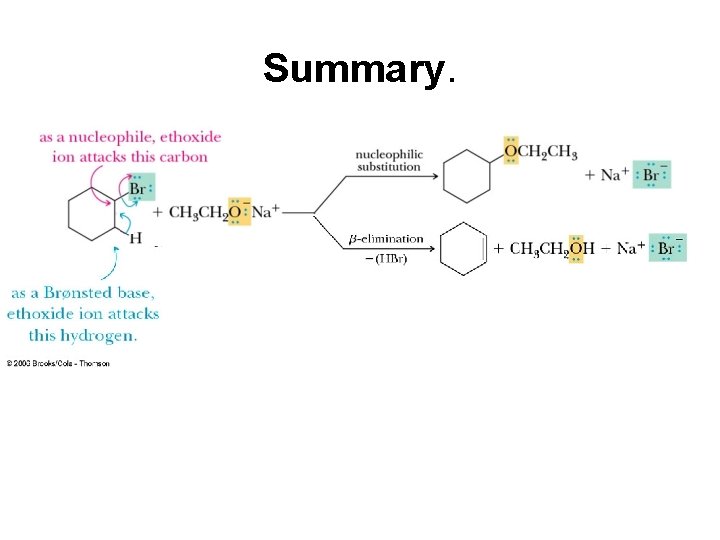
Competition between Nucleophilic Substitution and -elimination. First the nucleophilic substitution. The ethoxide attacks the carbon bearing the bromine. Note the change in charges on the nucleophile and the Leaving group
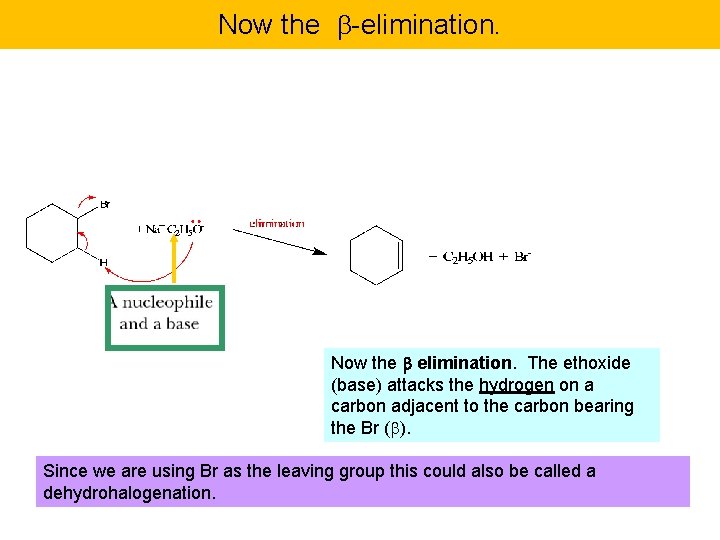
Now the -elimination. Now the b elimination. The ethoxide (base) attacks the hydrogen on a carbon adjacent to the carbon bearing the Br ( ). Since we are using Br as the leaving group this could also be called a dehydrohalogenation.
Summary.
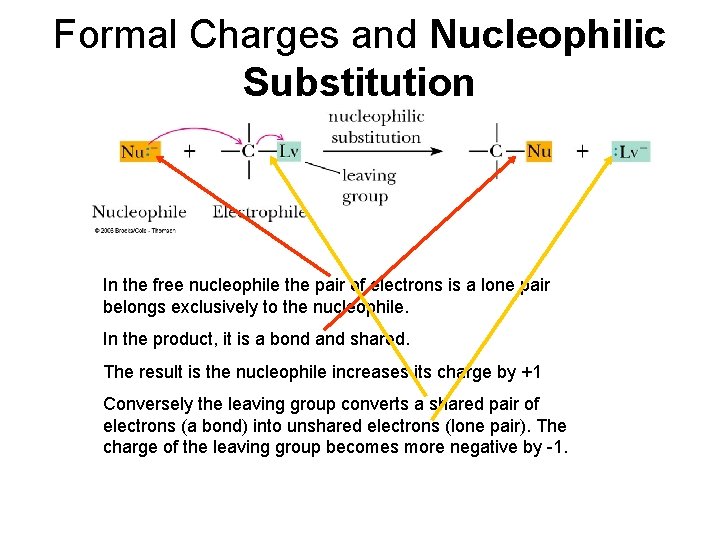
Formal Charges and Nucleophilic Substitution In the free nucleophile the pair of electrons is a lone pair belongs exclusively to the nucleophile. In the product, it is a bond and shared. The result is the nucleophile increases its charge by +1 Conversely the leaving group converts a shared pair of electrons (a bond) into unshared electrons (lone pair). The charge of the leaving group becomes more negative by -1.
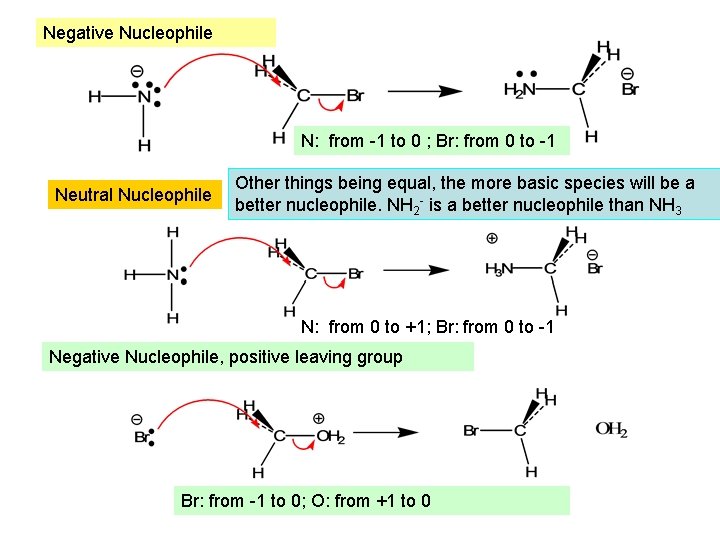
Negative Nucleophile N: from -1 to 0 ; Br: from 0 to -1 Neutral Nucleophile Other things being equal, the more basic species will be a better nucleophile. NH 2 - is a better nucleophile than NH 3 N: from 0 to +1; Br: from 0 to -1 Negative Nucleophile, positive leaving group Br: from -1 to 0; O: from +1 to 0
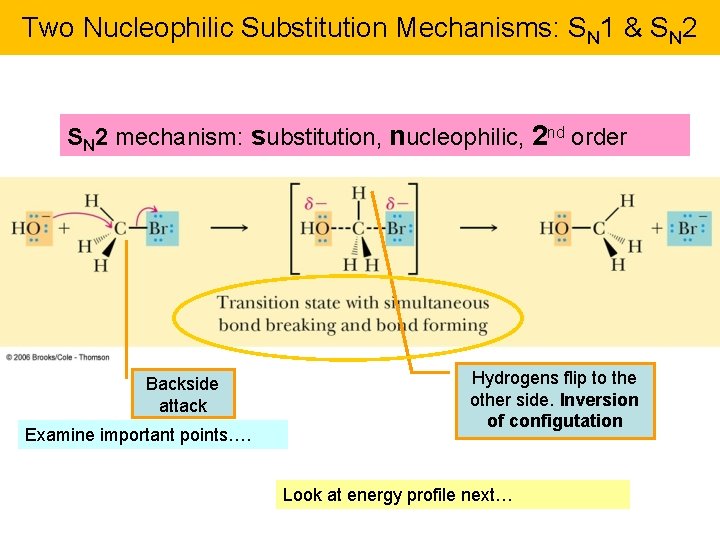
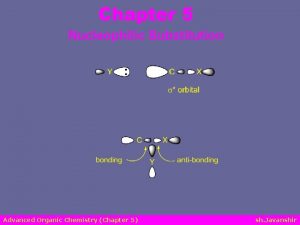
Two Nucleophilic Substitution Mechanisms: SN 1 & SN 2 mechanism: substitution, nucleophilic, 2 nd order Backside attack Examine important points…. Hydrogens flip to the other side. Inversion of configutation Look at energy profile next…
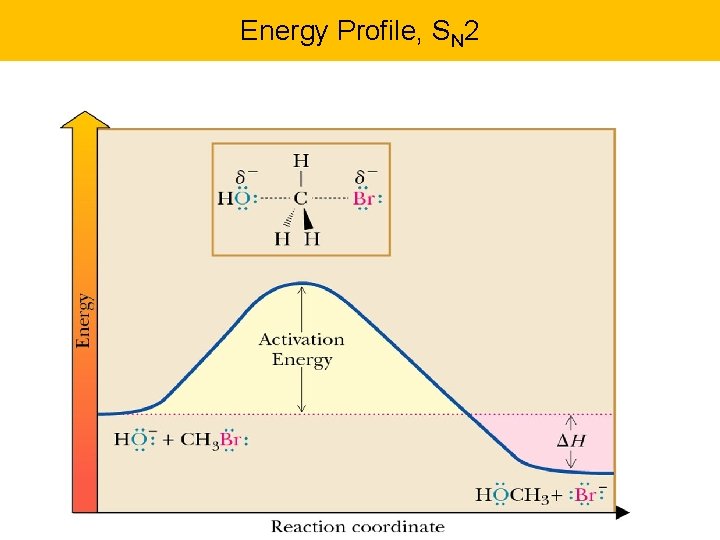
Energy Profile, SN 2
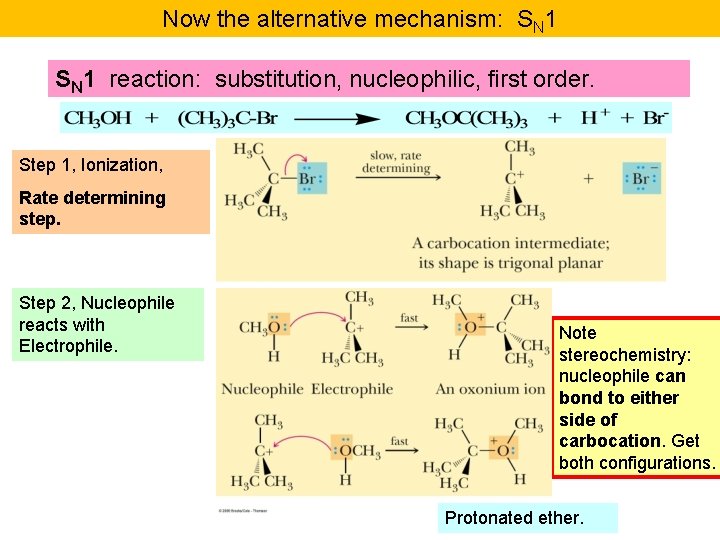
Now the alternative mechanism: SN 1 reaction: substitution, nucleophilic, first order. Step 1, Ionization, Rate determining step. Step 2, Nucleophile reacts with Electrophile. Note stereochemistry: nucleophile can bond to either side of carbocation. Get both configurations. Protonated ether.
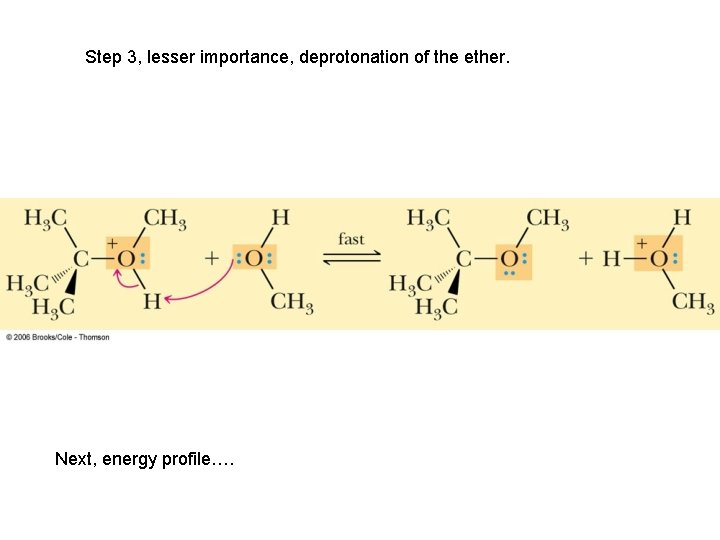
Step 3, lesser importance, deprotonation of the ether. Next, energy profile….
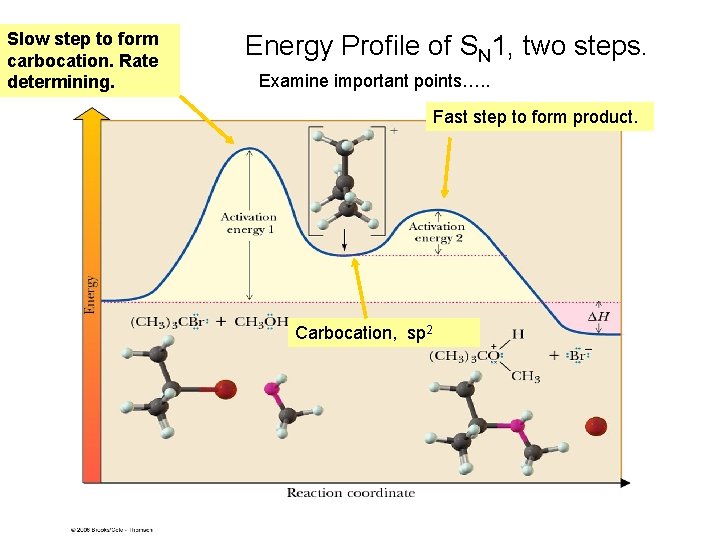
Slow step to form carbocation. Rate determining. Energy Profile of SN 1, two steps. Examine important points…. . Fast step to form product. Carbocation, sp 2
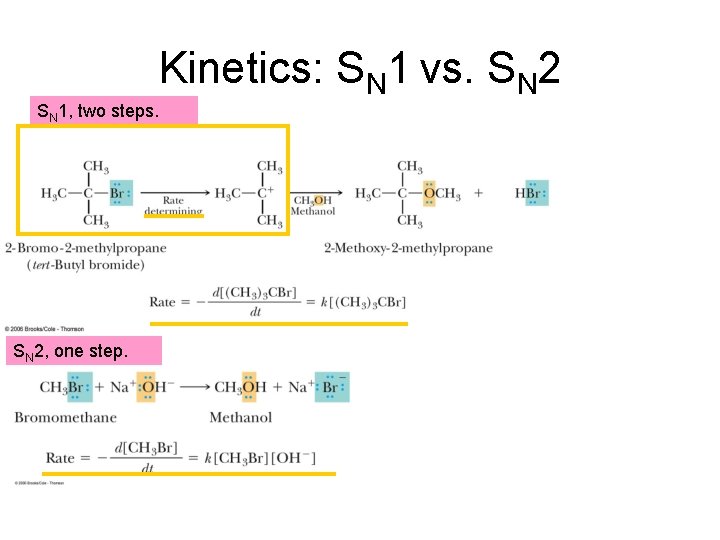
Kinetics: SN 1 vs. SN 2 SN 1, two steps. SN 2, one step.
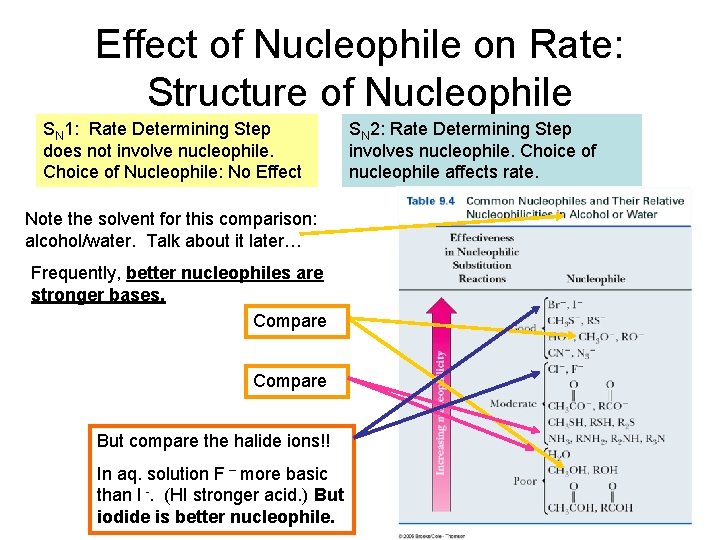
Effect of Nucleophile on Rate: Structure of Nucleophile SN 1: Rate Determining Step does not involve nucleophile. Choice of Nucleophile: No Effect Note the solvent for this comparison: alcohol/water. Talk about it later… Frequently, better nucleophiles are stronger bases. Compare But compare the halide ions!! In aq. solution F – more basic than I -. (HI stronger acid. ) But iodide is better nucleophile. SN 2: Rate Determining Step involves nucleophile. Choice of nucleophile affects rate.

We need to discuss Solvents Classifications Polar vs non-polar solvents, quantified by dielectric constant. Polar solvents reduce interaction of positive and negative ions. Water > Et. OH > Acetic acid > hexane
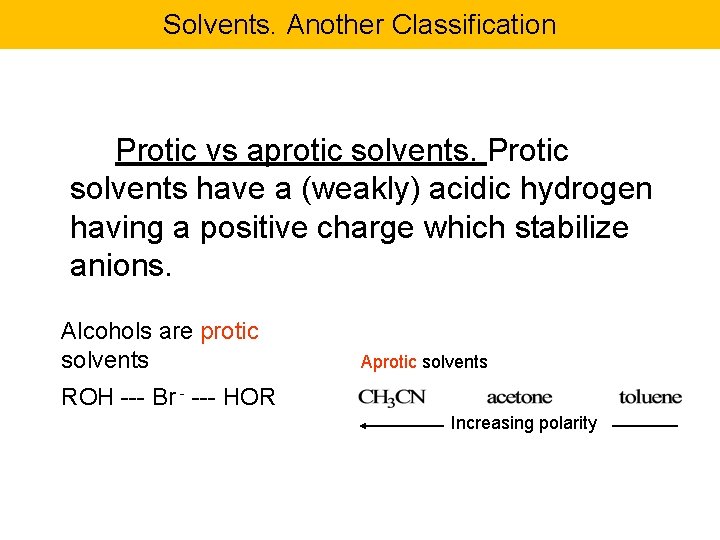
Solvents. Another Classification Protic vs aprotic solvents. Protic solvents have a (weakly) acidic hydrogen having a positive charge which stabilize anions. Alcohols are protic solvents Aprotic solvents ROH --- Br - --- HOR Increasing polarity
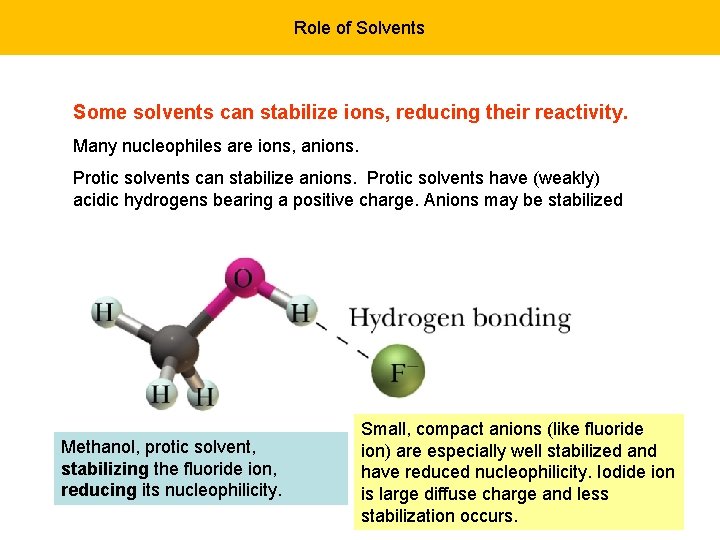
Role of Solvents Some solvents can stabilize ions, reducing their reactivity. Many nucleophiles are ions, anions. Protic solvents can stabilize anions. Protic solvents have (weakly) acidic hydrogens bearing a positive charge. Anions may be stabilized Methanol, protic solvent, stabilizing the fluoride ion, reducing its nucleophilicity. Small, compact anions (like fluoride ion) are especially well stabilized and have reduced nucleophilicity. Iodide ion is large diffuse charge and less stabilization occurs.
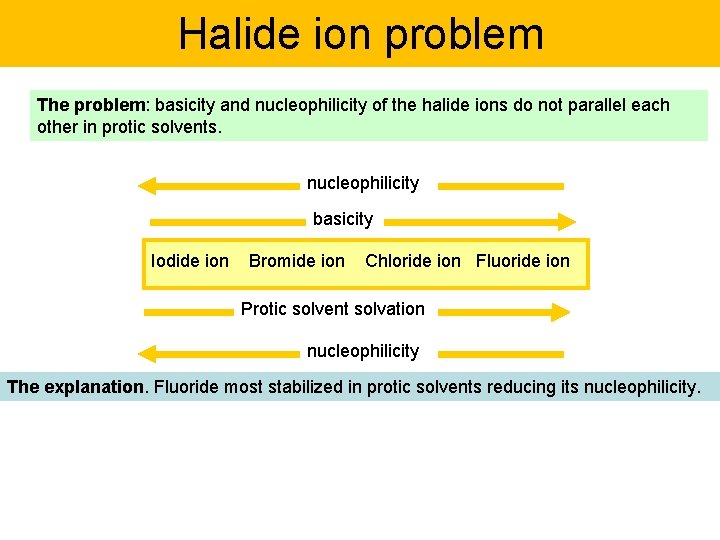
Halide ion problem The problem: basicity and nucleophilicity of the halide ions do not parallel each other in protic solvents. nucleophilicity basicity Iodide ion Bromide ion Chloride ion Fluoride ion Protic solvent solvation nucleophilicity The explanation. Fluoride most stabilized in protic solvents reducing its nucleophilicity.
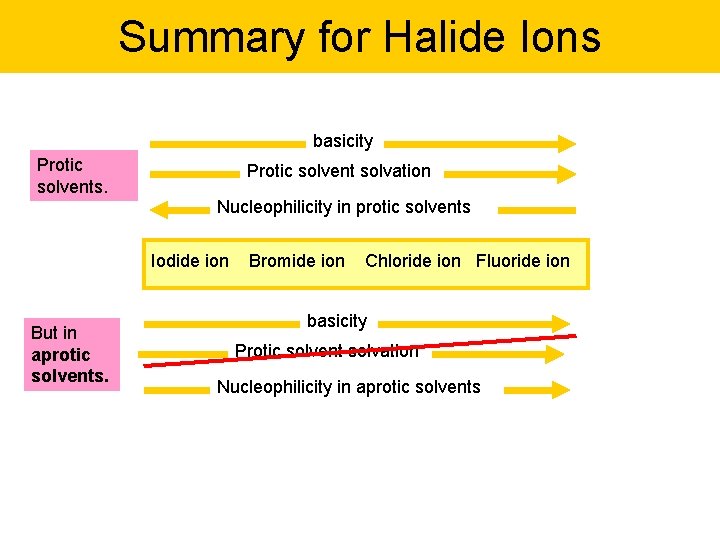
Summary for Halide Ions basicity Protic solvents. Protic solvent solvation Nucleophilicity in protic solvents Iodide ion But in aprotic solvents. Bromide ion Chloride ion Fluoride ion basicity Protic solvent solvation Nucleophilicity in aprotic solvents
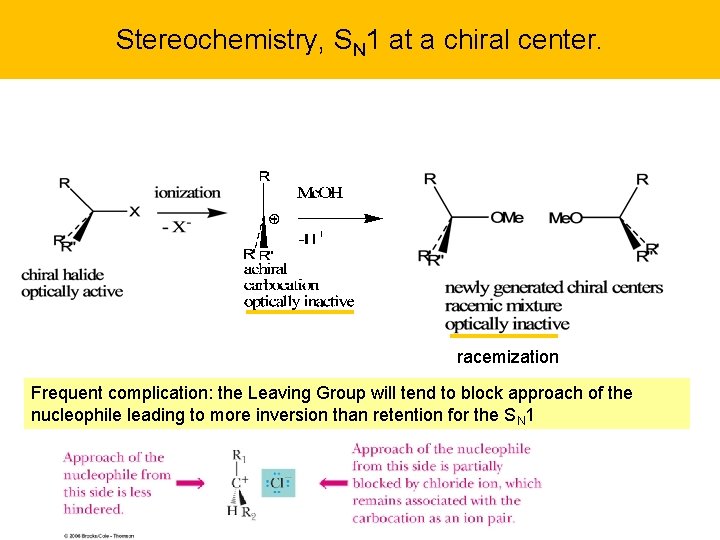
Stereochemistry, SN 1 at a chiral center. racemization Frequent complication: the Leaving Group will tend to block approach of the nucleophile leading to more inversion than retention for the SN 1
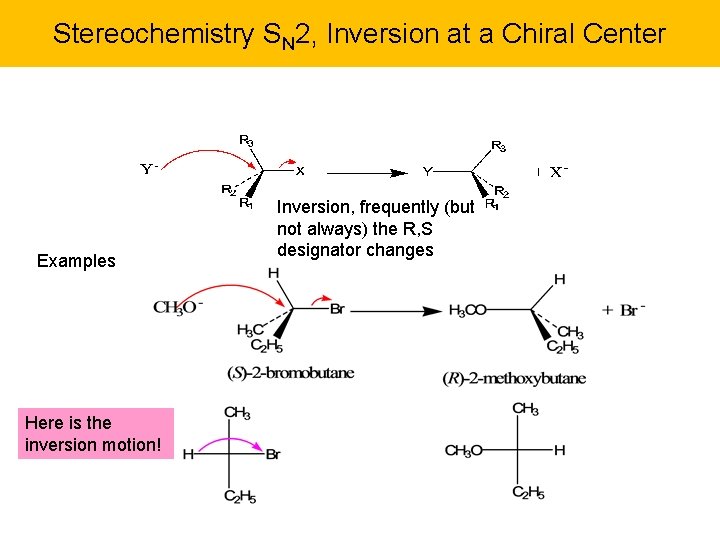
Stereochemistry SN 2, Inversion at a Chiral Center Examples Here is the inversion motion! Inversion, frequently (but not always) the R, S designator changes
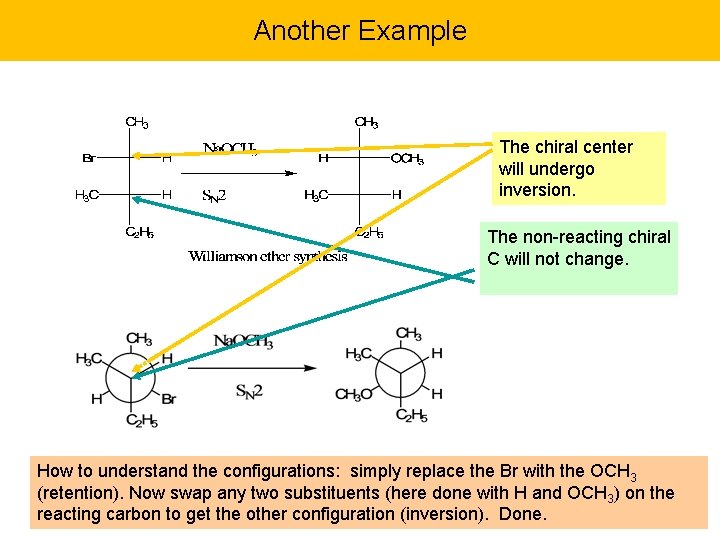
Another Example The chiral center will undergo inversion. The non-reacting chiral C will not change. How to understand the configurations: simply replace the Br with the OCH 3 (retention). Now swap any two substituents (here done with H and OCH 3) on the reacting carbon to get the other configuration (inversion). Done.
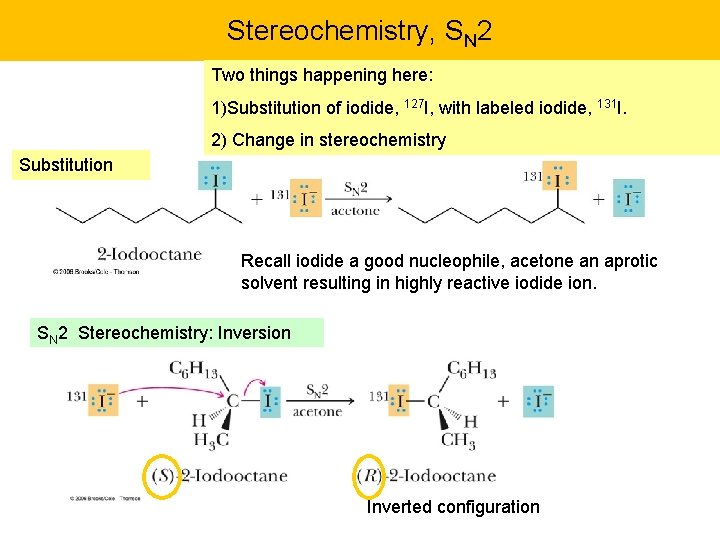
Stereochemistry, SN 2 Two things happening here: 1)Substitution of iodide, 127 I, with labeled iodide, 131 I. 2) Change in stereochemistry Substitution Recall iodide a good nucleophile, acetone an aprotic solvent resulting in highly reactive iodide ion. SN 2 Stereochemistry: Inversion Inverted configuration
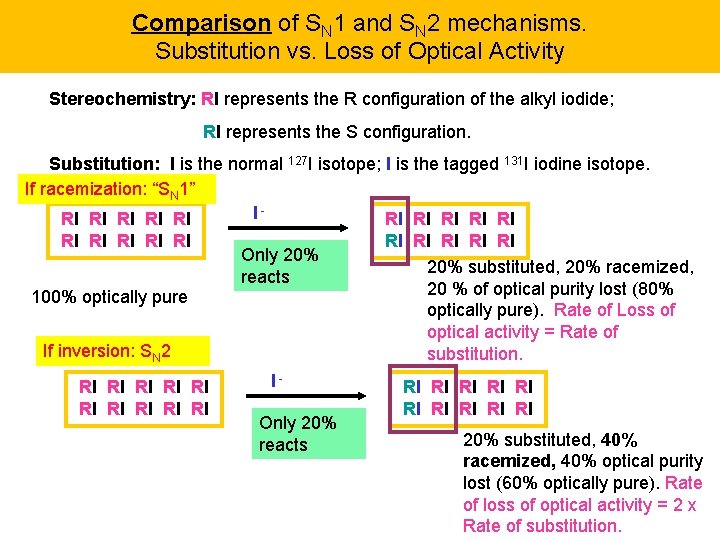
Comparison of SN 1 and SN 2 mechanisms. Substitution vs. Loss of Optical Activity Stereochemistry: RI represents the R configuration of the alkyl iodide; RI represents the S configuration. Substitution: I is the normal 127 I isotope; I is the tagged 131 I iodine isotope. If racemization: “SN 1” IRI RI RI RI Only 20% reacts 100% optically pure If inversion: SN 2 RI RI RI IOnly 20% reacts RI RI RI 20% substituted, 20% racemized, 20 % of optical purity lost (80% optically pure). Rate of Loss of optical activity = Rate of substitution. RI RI RI 20% substituted, 40% racemized, 40% optical purity lost (60% optically pure). Rate of loss of optical activity = 2 x Rate of substitution.
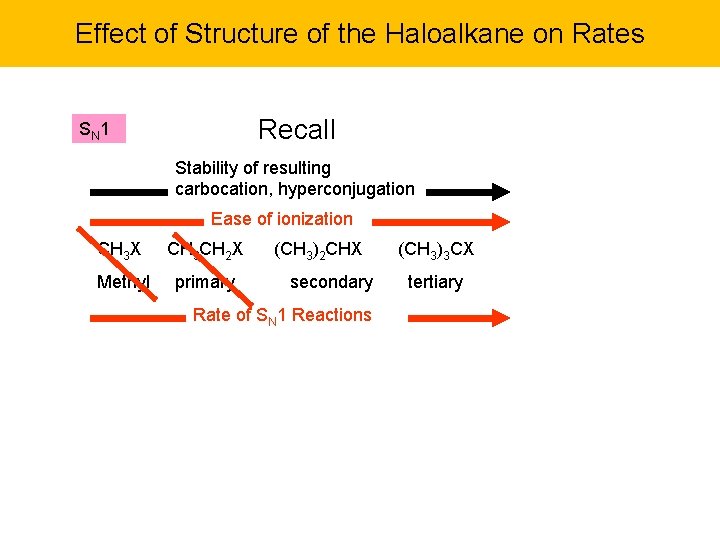
Effect of Structure of the Haloalkane on Rates Recall SN 1 Stability of resulting carbocation, hyperconjugation Ease of ionization CH 3 X CH 3 CH 2 X Methyl primary (CH 3)2 CHX secondary Rate of SN 1 Reactions (CH 3)3 CX tertiary
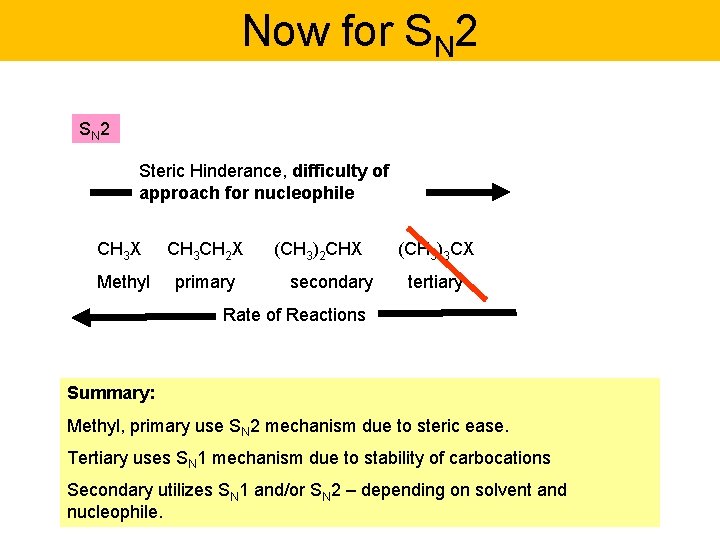
Now for SN 2 Steric Hinderance, difficulty of approach for nucleophile CH 3 X CH 3 CH 2 X Methyl primary (CH 3)2 CHX secondary (CH 3)3 CX tertiary Rate of Reactions Summary: Methyl, primary use SN 2 mechanism due to steric ease. Tertiary uses SN 1 mechanism due to stability of carbocations Secondary utilizes SN 1 and/or SN 2 – depending on solvent and nucleophile.
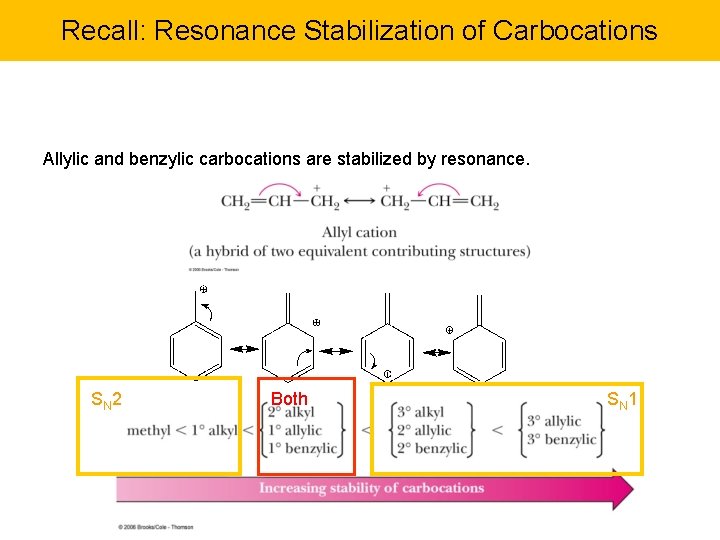
Recall: Resonance Stabilization of Carbocations Allylic and benzylic carbocations are stabilized by resonance. SN 2 Both SN 1
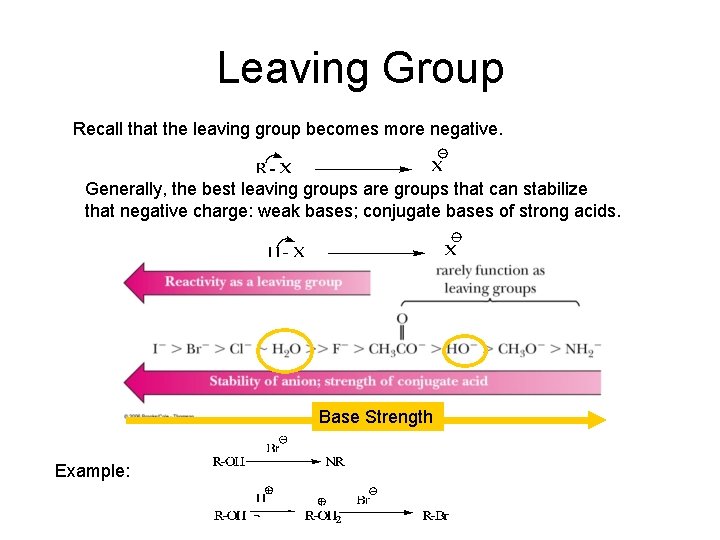
Leaving Group Recall that the leaving group becomes more negative. Generally, the best leaving groups are groups that can stabilize that negative charge: weak bases; conjugate bases of strong acids. Base Strength Example:
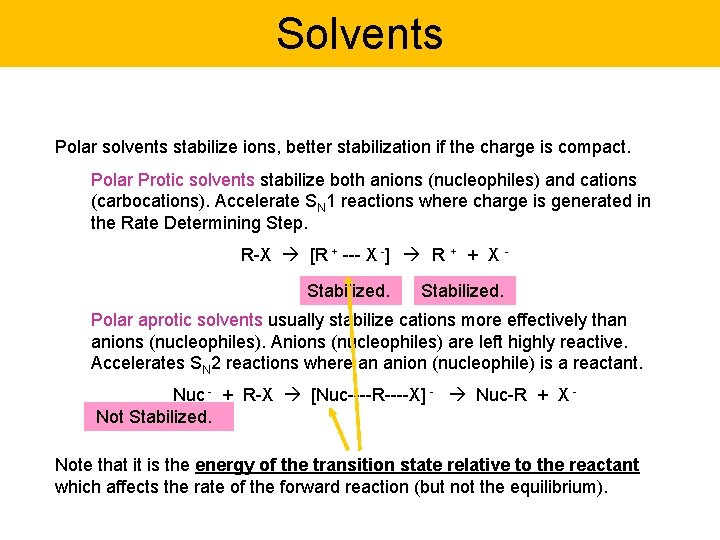
Solvents Polar solvents stabilize ions, better stabilization if the charge is compact. Polar Protic solvents stabilize both anions (nucleophiles) and cations (carbocations). Accelerate SN 1 reactions where charge is generated in the Rate Determining Step. R-X [R + --- X -] R + + X Stabilized. Polar aprotic solvents usually stabilize cations more effectively than anions (nucleophiles). Anions (nucleophiles) are left highly reactive. Accelerates SN 2 reactions where an anion (nucleophile) is a reactant. Nuc - + R-X [Nuc----R----X] - Nuc-R + X Not Stabilized. Note that it is the energy of the transition state relative to the reactant which affects the rate of the forward reaction (but not the equilibrium).
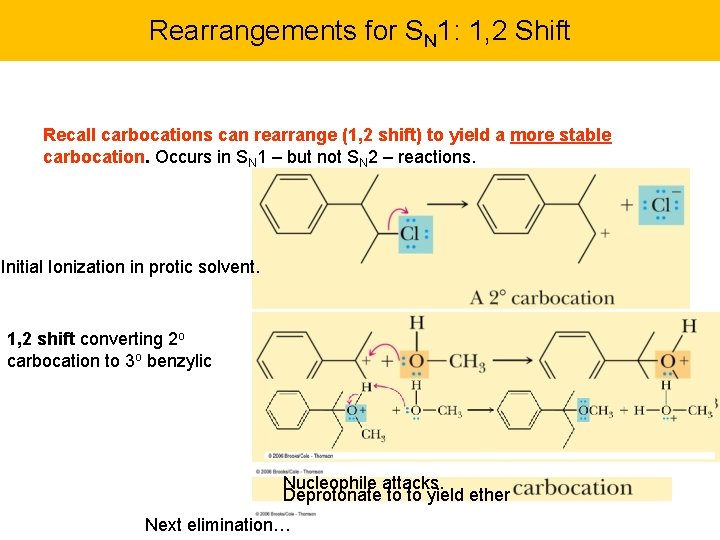
Rearrangements for SN 1: 1, 2 Shift Recall carbocations can rearrange (1, 2 shift) to yield a more stable carbocation. Occurs in SN 1 – but not SN 2 – reactions. Initial Ionization in protic solvent. 1, 2 shift converting 2 o carbocation to 3 o benzylic Nucleophile attacks. Deprotonate to to yield ether Next elimination…
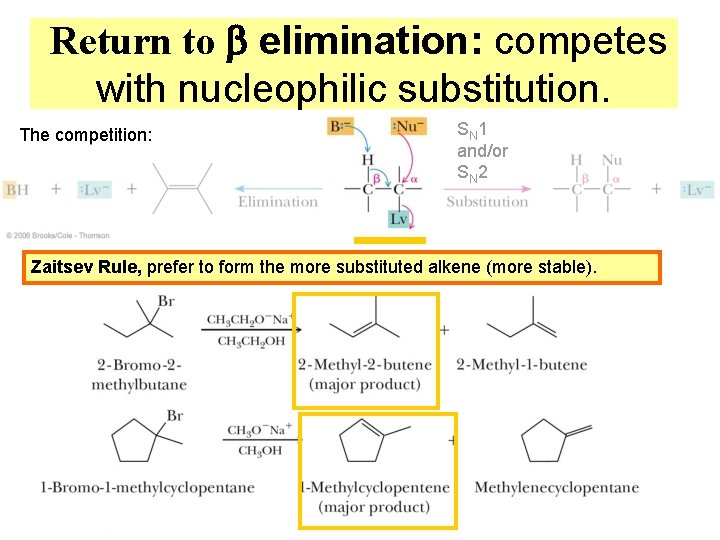
Return to b elimination: competes with nucleophilic substitution. The competition: SN 1 and/or SN 2 Zaitsev Rule, prefer to form the more substituted alkene (more stable).
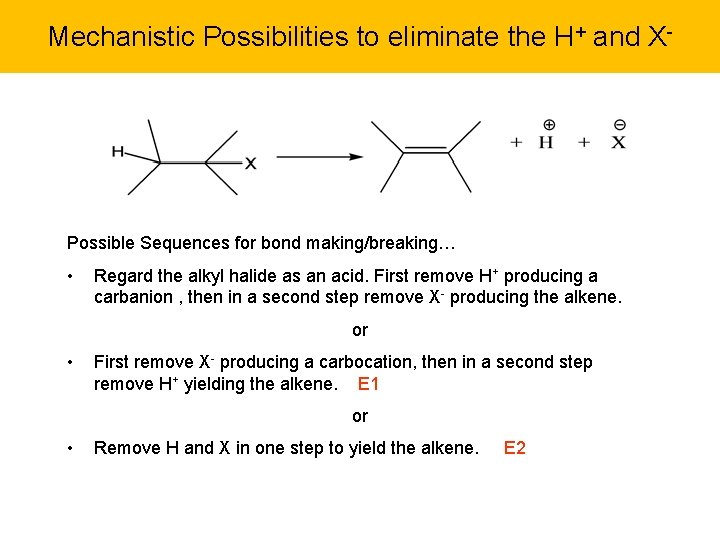
Mechanistic Possibilities to eliminate the H+ and X- Possible Sequences for bond making/breaking… • Regard the alkyl halide as an acid. First remove H+ producing a carbanion , then in a second step remove X- producing the alkene. or • First remove X- producing a carbocation, then in a second step remove H+ yielding the alkene. E 1 or • Remove H and X in one step to yield the alkene. E 2
• There are two idealized mechanisms for elimination reactions • E 1 mechanism: at one extreme, breaking of the RLv bond to give a carbocation is complete before reaction with base to break the C-H bond – only R-Lv is involved in the rate-determining step (as in SN 1) • E 2 mechanism: at the other extreme, breaking of the R-Lv and C-H bonds is concerted (same time) – both R-Lv and base are involved in the rate-determining step (as in SN 2)
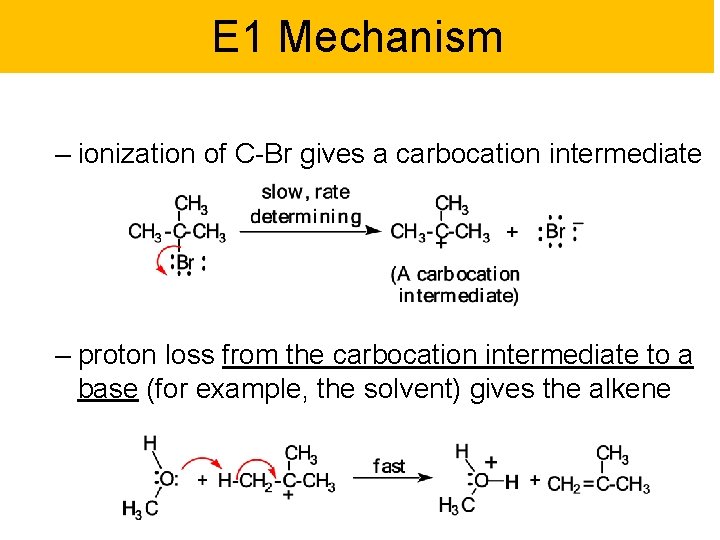
E 1 Mechanism – ionization of C-Br gives a carbocation intermediate – proton loss from the carbocation intermediate to a base (for example, the solvent) gives the alkene
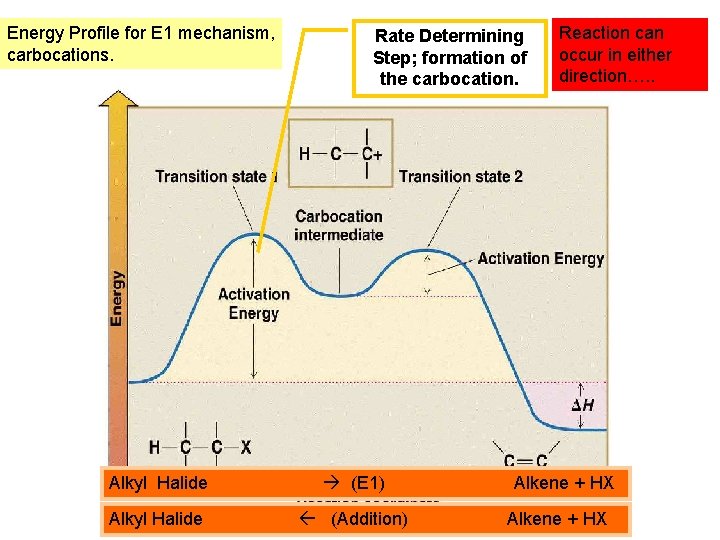
Energy Profile for E 1 mechanism, carbocations. Rate Determining Step; formation of the carbocation. Alkyl Halide (E 1) Alkyl Halide (Addition) Reaction can occur in either direction…. . Alkene + HX
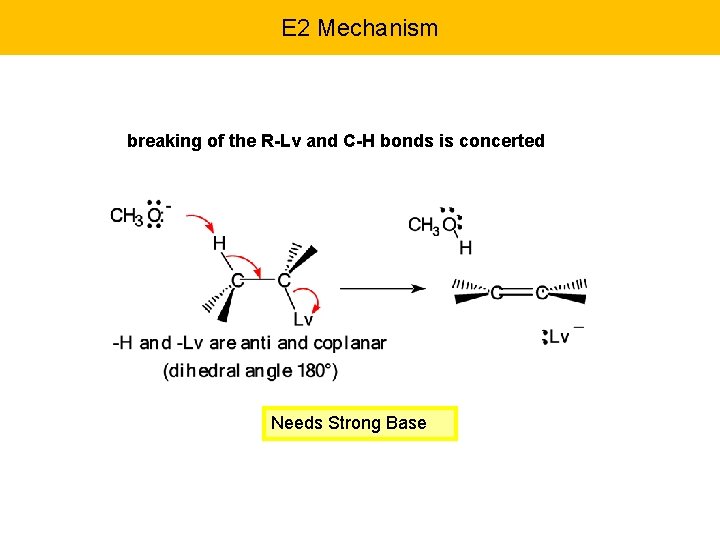
E 2 Mechanism breaking of the R-Lv and C-H bonds is concerted Needs Strong Base
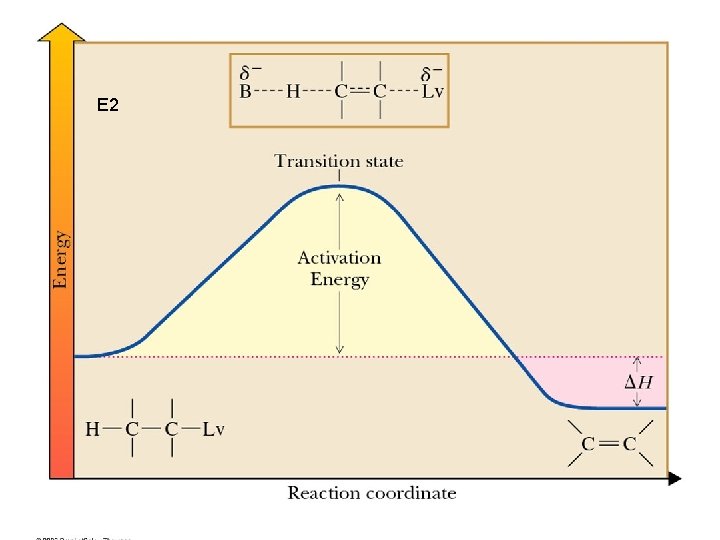
E 2
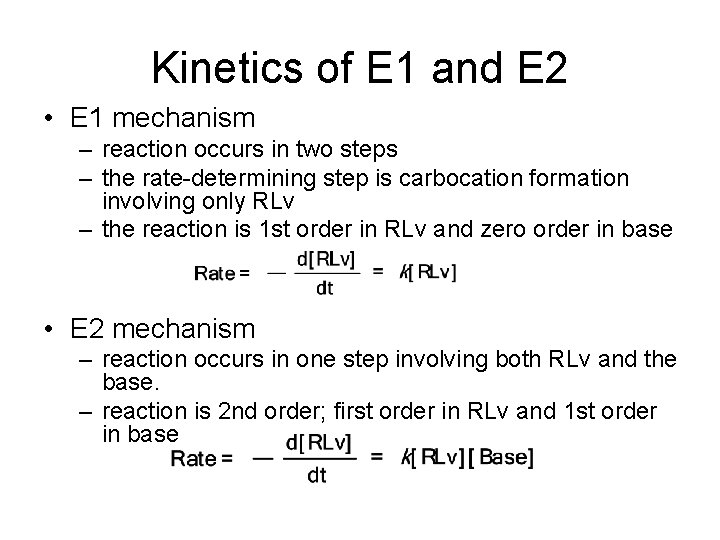
Kinetics of E 1 and E 2 • E 1 mechanism – reaction occurs in two steps – the rate-determining step is carbocation formation involving only RLv – the reaction is 1 st order in RLv and zero order in base • E 2 mechanism – reaction occurs in one step involving both RLv and the base. – reaction is 2 nd order; first order in RLv and 1 st order in base

Regioselectivity of E 1/E 2 • E 1: major product is the more stable alkene (more substituted, more resonance) • E 2: with strong base, the major product is the more stable (more substituted, more resonance) alkene Special notes about sterically hindered bases such as tert-butoxide, (CH 3)3 CO -. E 2 – anti-Zaitsev: with a strong, sterically hindered base the major product is often the less stable (less substituted) alkene. Reason: hydrogens on less substituted carbons are more accessible. Also E 2 vs SN 2: In competition of SN 2 vs E 2: steric bulk in either the alkyl halide or the base/nucleophile prevents the SN 2 reaction and favors the E 2.
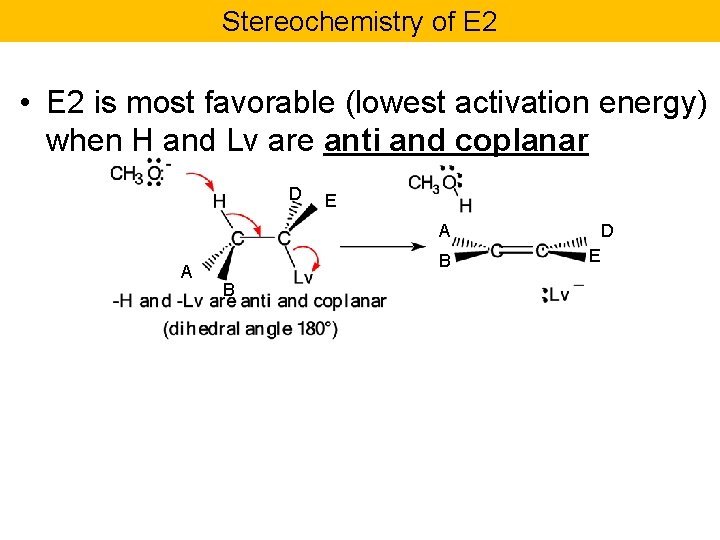
Stereochemistry of E 2 • E 2 is most favorable (lowest activation energy) when H and Lv are anti and coplanar D E A A B B D E
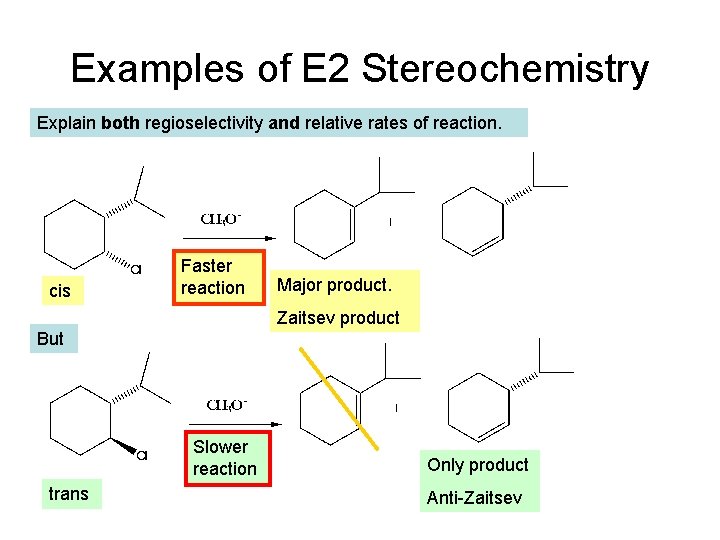
Examples of E 2 Stereochemistry Explain both regioselectivity and relative rates of reaction. cis Faster reaction Major product. Zaitsev product But Slower reaction trans Only product Anti-Zaitsev
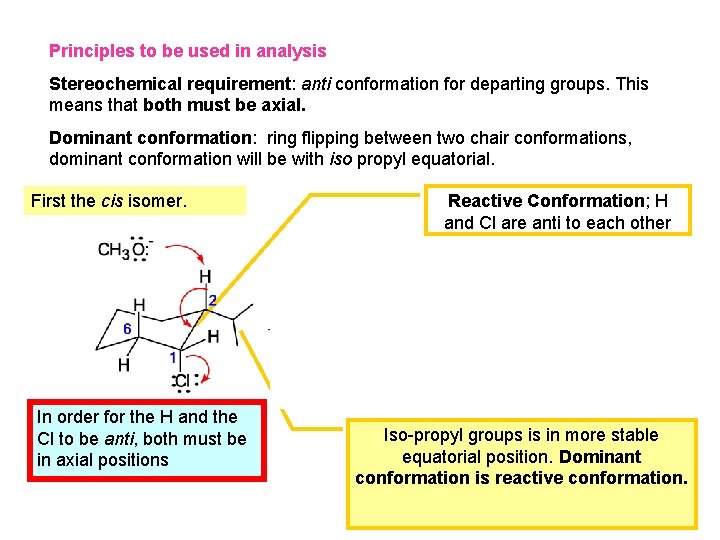
Principles to be used in analysis Stereochemical requirement: anti conformation for departing groups. This means that both must be axial. Dominant conformation: ring flipping between two chair conformations, dominant conformation will be with iso propyl equatorial. First the cis isomer. In order for the H and the Cl to be anti, both must be in axial positions Reactive Conformation; H and Cl are anti to each other Iso-propyl groups is in more stable equatorial position. Dominant conformation is reactive conformation.
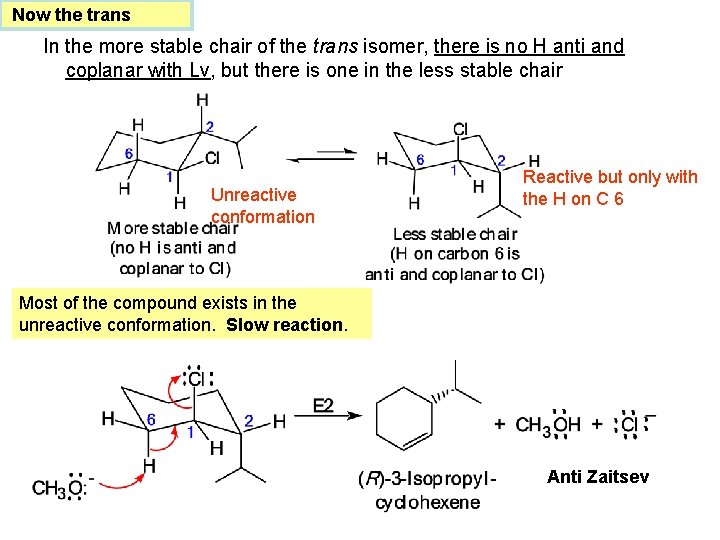
Now the trans In the more stable chair of the trans isomer, there is no H anti and coplanar with Lv, but there is one in the less stable chair Unreactive conformation Reactive but only with the H on C 6 Most of the compound exists in the unreactive conformation. Slow reaction. Anti Zaitsev
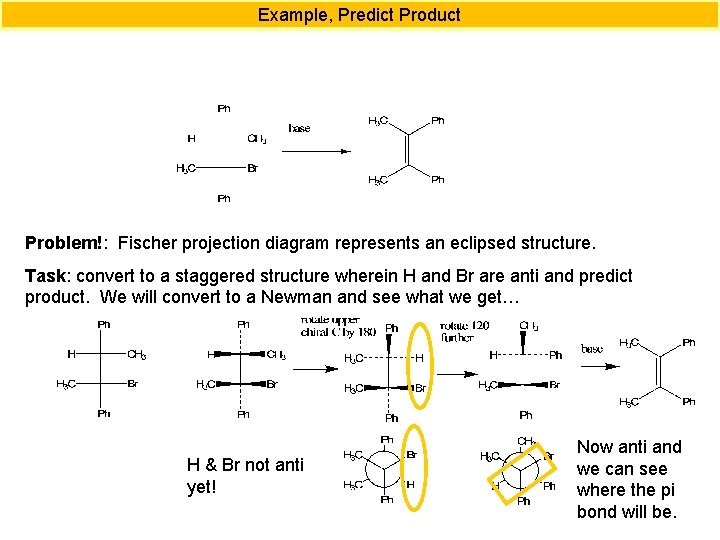
Example, Predict Product Problem!: Fischer projection diagram represents an eclipsed structure. Task: convert to a staggered structure wherein H and Br are anti and predict product. We will convert to a Newman and see what we get… H & Br not anti yet! Now anti and we can see where the pi bond will be.
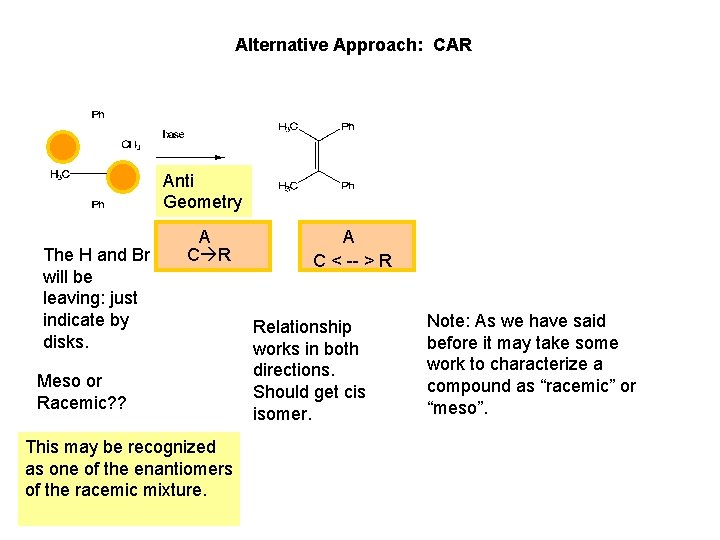
Alternative Approach: CAR Anti Geometry The H and Br will be leaving: just indicate by disks. A C R Meso or Racemic? ? This may be recognized as one of the enantiomers of the racemic mixture. A C < -- > R Relationship works in both directions. Should get cis isomer. Note: As we have said before it may take some work to characterize a compound as “racemic” or “meso”.
E 1 or E 2 (Carbocation)
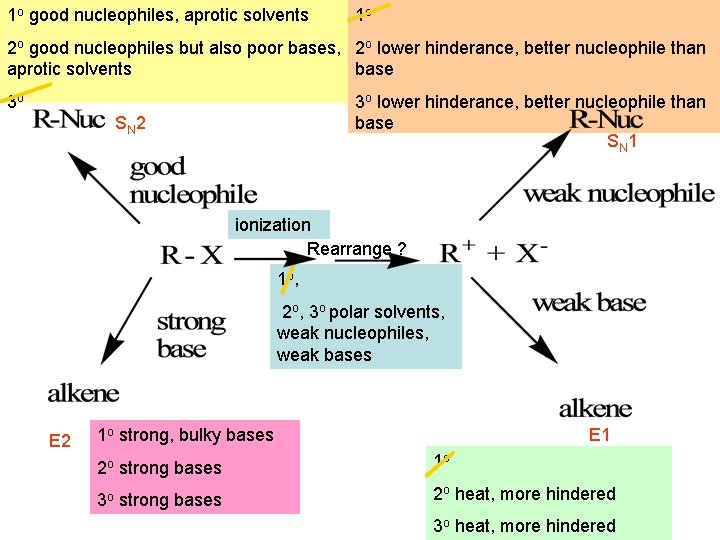
1 o good nucleophiles, aprotic solvents 1 o 2 o good nucleophiles but also poor bases, 2 o lower hinderance, better nucleophile than base aprotic solvents 3 o lower hinderance, better nucleophile than base SN 1 30 SN 2 ionization Rearrange ? 1 o , 2 o, 3 o polar solvents, weak nucleophiles, weak bases E 2 1 o strong, bulky bases E 1 2 o strong bases 1 o 3 o strong bases 2 o heat, more hindered 3 o heat, more hindered
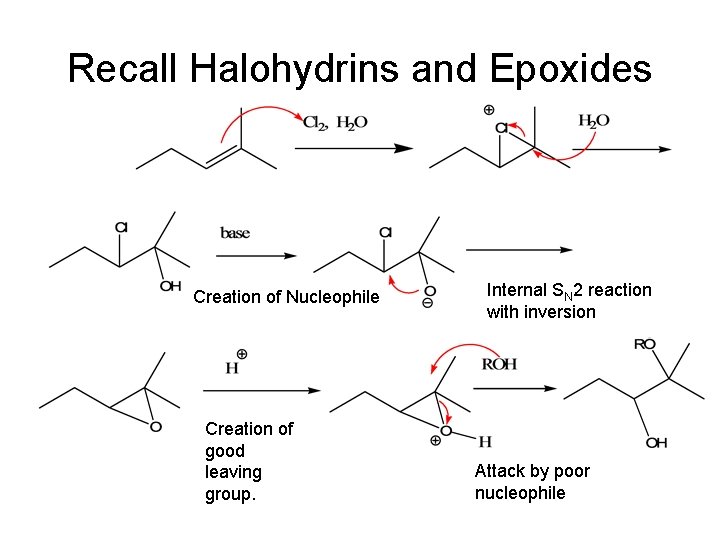
Recall Halohydrins and Epoxides Creation of Nucleophile Creation of good leaving group. Internal SN 2 reaction with inversion Attack by poor nucleophile
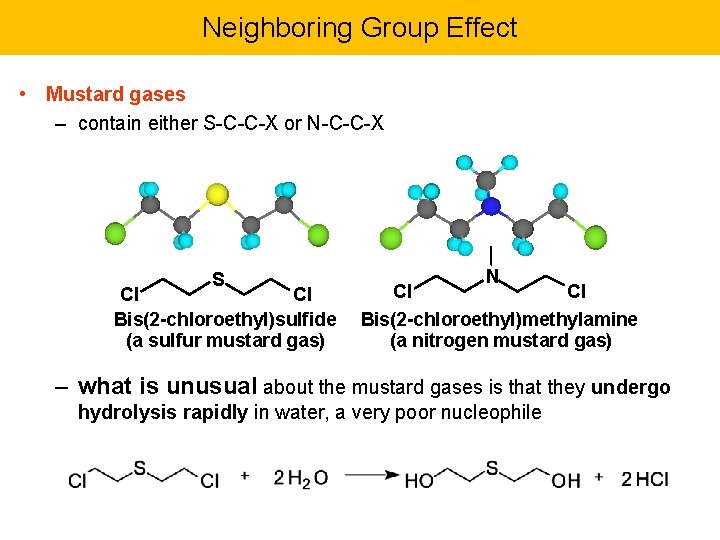
Neighboring Group Effect • Mustard gases – contain either S-C-C-X or N-C-C-X S Cl Cl Bis(2 -chloroethyl)sulfide (a sulfur mustard gas) N Cl Cl Bis(2 -chloroethyl)methylamine (a nitrogen mustard gas) – what is unusual about the mustard gases is that they undergo hydrolysis rapidly in water, a very poor nucleophile
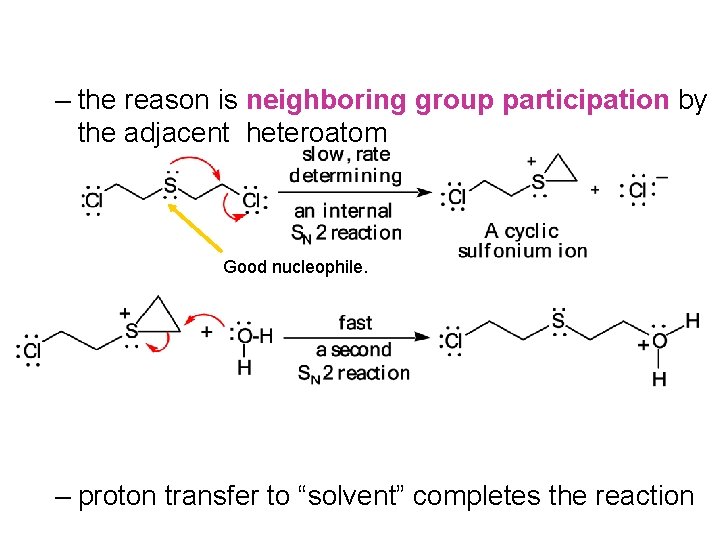
– the reason is neighboring group participation by the adjacent heteroatom Good nucleophile. – proton transfer to “solvent” completes the reaction
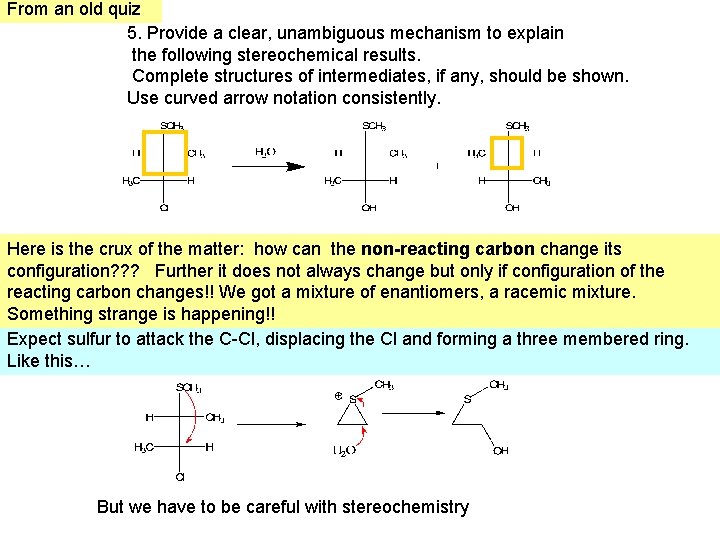
From an old quiz 5. Provide a clear, unambiguous mechanism to explain the following stereochemical results. Complete structures of intermediates, if any, should be shown. Use curved arrow notation consistently. Here is the crux of the matter: how can the non-reacting carbon change its configuration? ? ? Further it does not always change but only if configuration of the reacting carbon changes!! We got a mixture of enantiomers, a racemic mixture. Something strange is happening!! Expect sulfur to attack the C-Cl, displacing the Cl and forming a three membered ring. Like this… But we have to be careful with stereochemistry
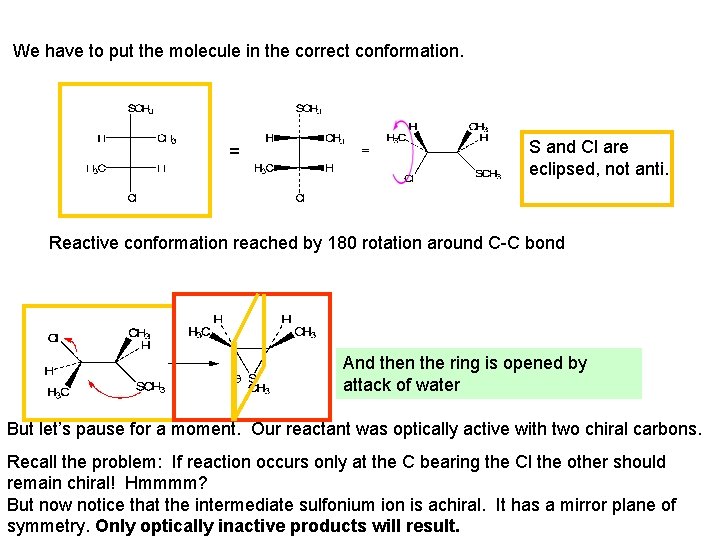
We have to put the molecule in the correct conformation. = S and Cl are eclipsed, not anti. Reactive conformation reached by 180 rotation around C-C bond And then the ring is opened by attack of water But let’s pause for a moment. Our reactant was optically active with two chiral carbons. Recall the problem: If reaction occurs only at the C bearing the Cl the other should remain chiral! Hmmmm? But now notice that the intermediate sulfonium ion is achiral. It has a mirror plane of symmetry. Only optically inactive products will result.
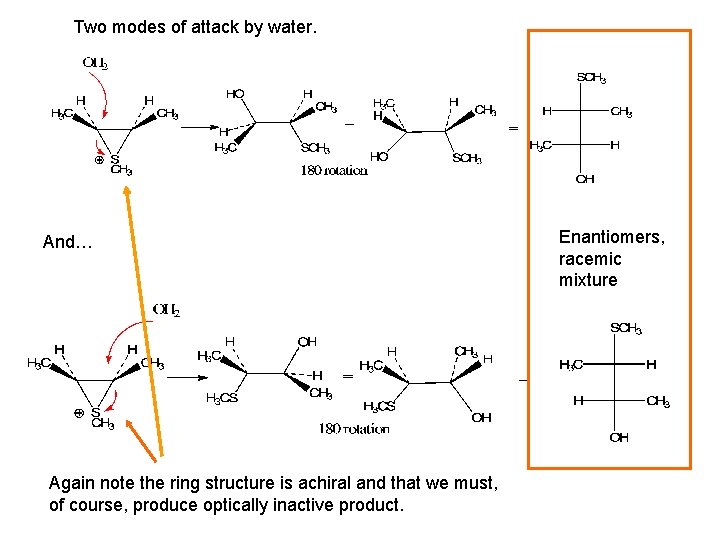
Two modes of attack by water. And… Again note the ring structure is achiral and that we must, of course, produce optically inactive product. Enantiomers, racemic mixture
- Slides: 54