Chapter 3 Stoichiometry Calculations with Chemical Formulas and


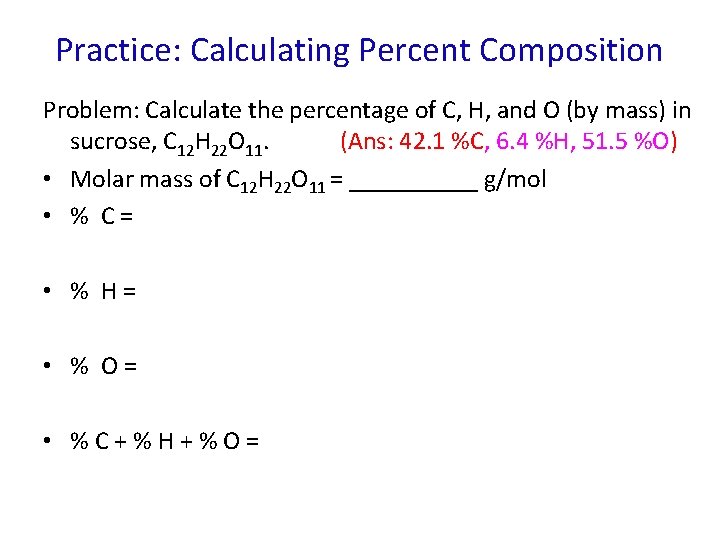
- Slides: 44

Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations

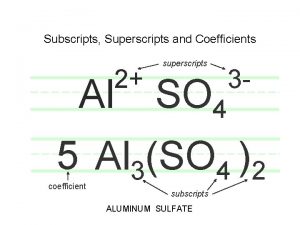
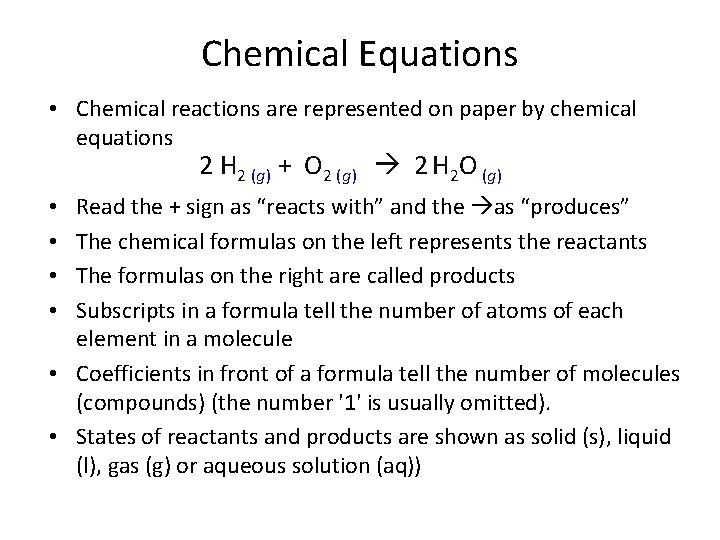
Chemical Equations • Chemical reactions are represented on paper by chemical equations 2 H 2 (g) + O 2 (g) 2 H 2 O (g) Read the + sign as “reacts with” and the as “produces” The chemical formulas on the left represents the reactants The formulas on the right are called products Subscripts in a formula tell the number of atoms of each element in a molecule • Coefficients in front of a formula tell the number of molecules (compounds) (the number '1' is usually omitted). • States of reactants and products are shown as solid (s), liquid (l), gas (g) or aqueous solution (aq)) • •

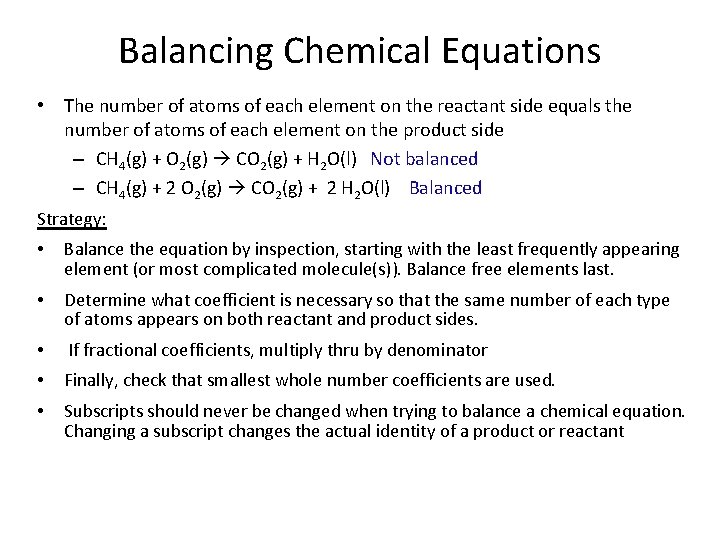
Balancing Chemical Equations • The number of atoms of each element on the reactant side equals the number of atoms of each element on the product side – CH 4(g) + O 2(g) CO 2(g) + H 2 O(l) Not balanced – CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) Balanced Strategy: • Balance the equation by inspection, starting with the least frequently appearing element (or most complicated molecule(s)). Balance free elements last. • Determine what coefficient is necessary so that the same number of each type of atoms appears on both reactant and product sides. • If fractional coefficients, multiply thru by denominator • Finally, check that smallest whole number coefficients are used. • Subscripts should never be changed when trying to balance a chemical equation. Changing a subscript changes the actual identity of a product or reactant

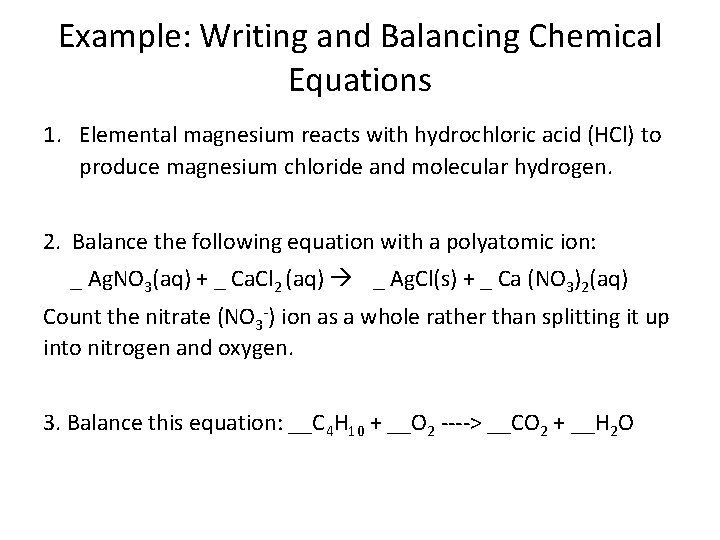
Example: Writing and Balancing Chemical Equations 1. Elemental magnesium reacts with hydrochloric acid (HCl) to produce magnesium chloride and molecular hydrogen. 2. Balance the following equation with a polyatomic ion: _ Ag. NO 3(aq) + _ Ca. Cl 2 (aq) _ Ag. Cl(s) + _ Ca (NO 3)2(aq) Count the nitrate (NO 3 -) ion as a whole rather than splitting it up into nitrogen and oxygen. 3. Balance this equation: __C 4 H 10 + __O 2 ----> __CO 2 + __H 2 O

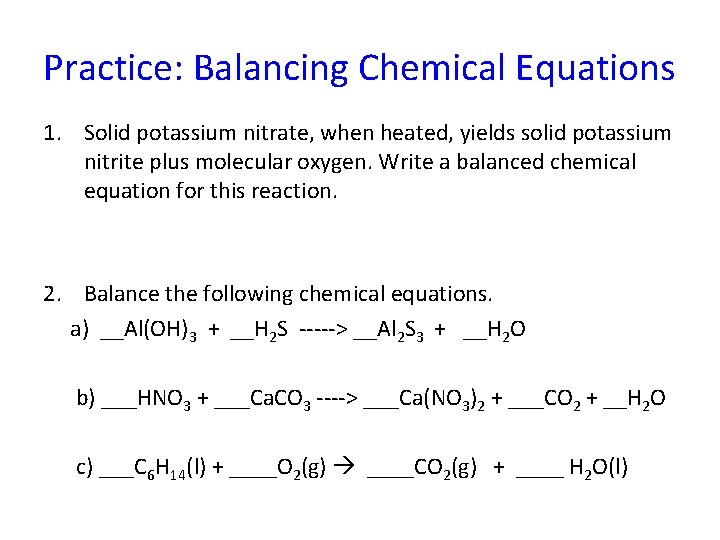
Practice: Balancing Chemical Equations 1. Solid potassium nitrate, when heated, yields solid potassium nitrite plus molecular oxygen. Write a balanced chemical equation for this reaction. 2. Balance the following chemical equations. a) __Al(OH)3 + __H 2 S -----> __Al 2 S 3 + __H 2 O b) ___HNO 3 + ___Ca. CO 3 ----> ___Ca(NO 3)2 + ___CO 2 + __H 2 O c) ___C 6 H 14(l) + ____O 2(g) ____CO 2(g) + ____ H 2 O(l)

Reaction Types • Combination • Decomposition • Combustion A brief summary of these reactions is given in the next four slides. Please refer to your Reaction Handout for Unit I. Work on all the drills.

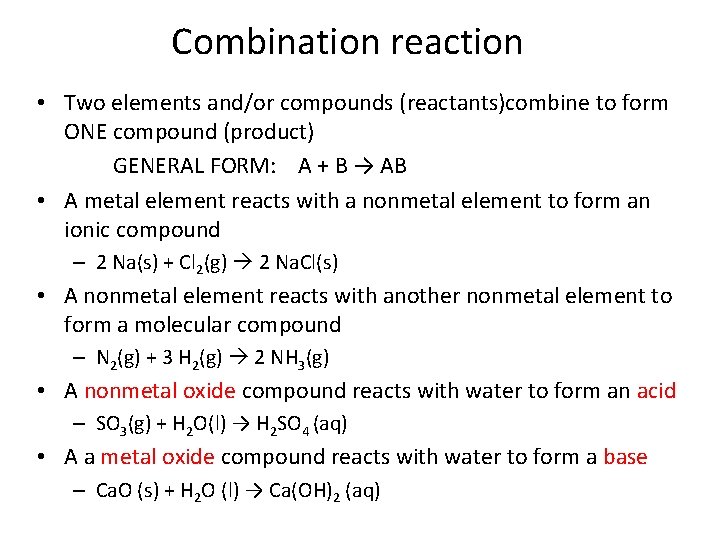
Combination reaction • Two elements and/or compounds (reactants)combine to form ONE compound (product) GENERAL FORM: A + B → AB • A metal element reacts with a nonmetal element to form an ionic compound – 2 Na(s) + Cl 2(g) 2 Na. Cl(s) • A nonmetal element reacts with another nonmetal element to form a molecular compound – N 2(g) + 3 H 2(g) 2 NH 3(g) • A nonmetal oxide compound reacts with water to form an acid – SO 3(g) + H 2 O(l) → H 2 SO 4 (aq) • A a metal oxide compound reacts with water to form a base – Ca. O (s) + H 2 O (l) → Ca(OH)2 (aq)

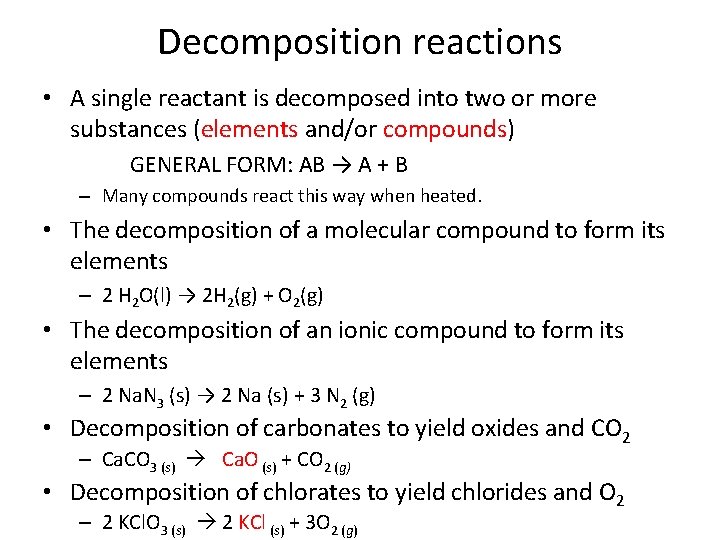
Decomposition reactions • A single reactant is decomposed into two or more substances (elements and/or compounds) GENERAL FORM: AB → A + B – Many compounds react this way when heated. • The decomposition of a molecular compound to form its elements – 2 H 2 O(l) → 2 H 2(g) + O 2(g) • The decomposition of an ionic compound to form its elements – 2 Na. N 3 (s) → 2 Na (s) + 3 N 2 (g) • Decomposition of carbonates to yield oxides and CO 2 – Ca. CO 3 (s) Ca. O (s) + CO 2 (g) • Decomposition of chlorates to yield chlorides and O 2 – 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g)

Combustion reaction • Reactions of substances with oxygen to produce CO 2, H 2 O and heat • General Form: Cx. Hy + O 2 (g) → CO 2 + H 2 O (g) or Cx. Hy. Oz + O 2 (g) → CO 2 (g) + H 2 O (g) • Hydrocarbons react with oxygen in the air to produce CO 2 and H 2 O. – C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) • Combustion of oxygen-containing derivatives of hydrocarbons such as alcohols, sugars also produce CO 2 and H 2 O – 2 C 3 H 7 OH(l) + 9 O 2(g) → 6 CO 2(g) + 8 H 2 O(g) – C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g)

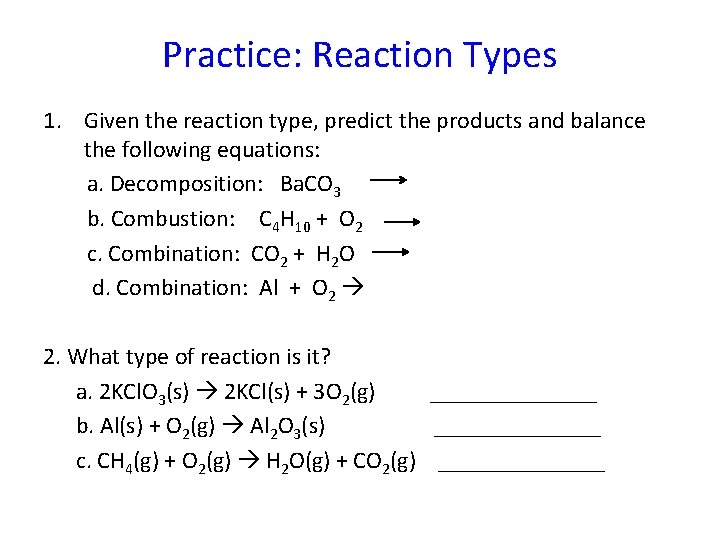
Practice: Reaction Types 1. Given the reaction type, predict the products and balance the following equations: a. Decomposition: Ba. CO 3 b. Combustion: C 4 H 10 + O 2 c. Combination: CO 2 + H 2 O d. Combination: Al + O 2 2. What type of reaction is it? a. 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) _______ b. Al(s) + O 2(g) Al 2 O 3(s) _______ c. CH 4(g) + O 2(g) H 2 O(g) + CO 2(g) _______

Avogadro’s Number and the Mole Dozen = 12 Pair = 2 The Mole (mol): A unit to count numbers of particles The mole (mol) is the amount of a substance that contains as many elementary entities as there atoms in exactly 12. 00 grams of 12 C 1 mol ~ 6. 02 x 1023 = Avogadro’s number is a conversion factor between moles and the number of particles. 11

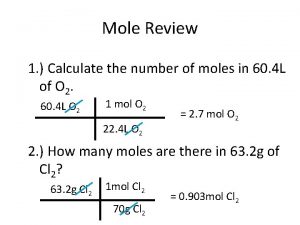
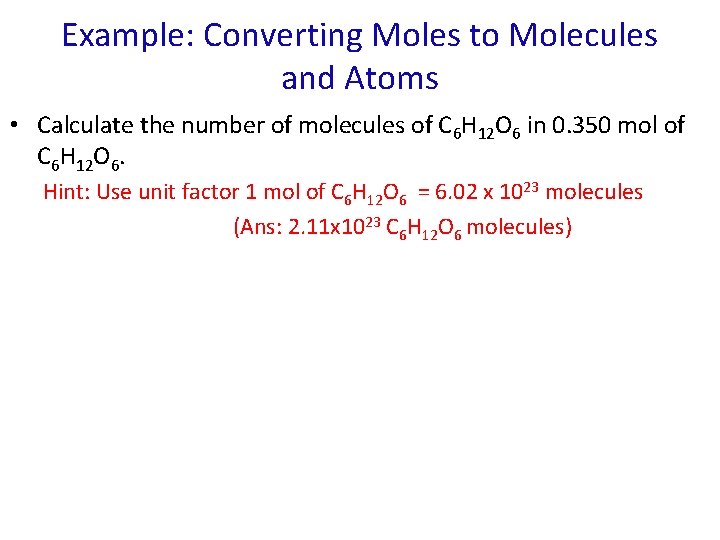
Example: Converting Moles to Molecules and Atoms • Calculate the number of molecules of C 6 H 12 O 6 in 0. 350 mol of C 6 H 12 O 6. Hint: Use unit factor 1 mol of C 6 H 12 O 6 = 6. 02 x 1023 molecules (Ans: 2. 11 x 1023 C 6 H 12 O 6 molecules)

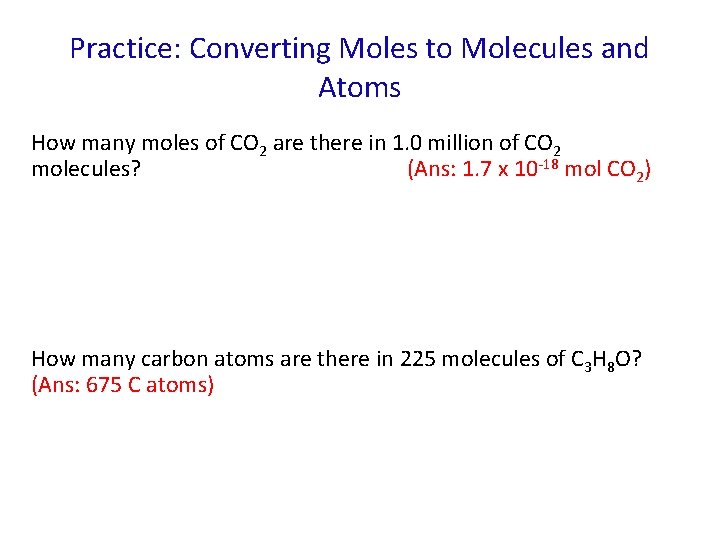
Practice: Converting Moles to Molecules and Atoms How many moles of CO 2 are there in 1. 0 million of CO 2 molecules? (Ans: 1. 7 x 10 -18 mol CO 2) How many carbon atoms are there in 225 molecules of C 3 H 8 O? (Ans: 675 C atoms)

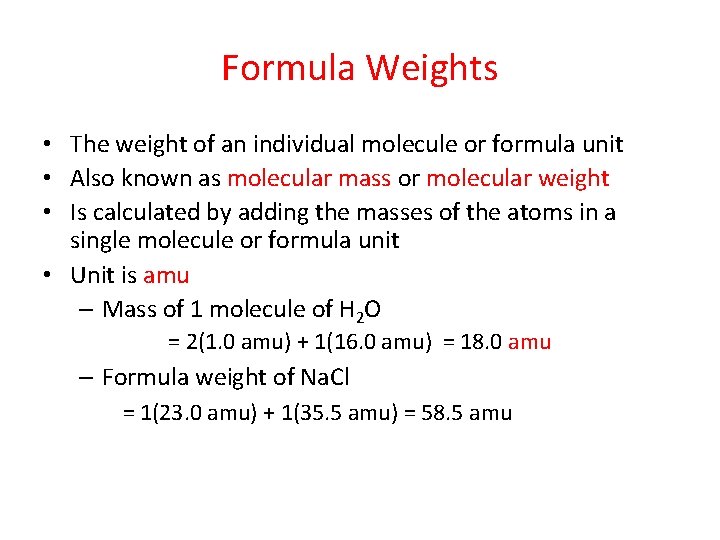
Formula Weights • The weight of an individual molecule or formula unit • Also known as molecular mass or molecular weight • Is calculated by adding the masses of the atoms in a single molecule or formula unit • Unit is amu – Mass of 1 molecule of H 2 O = 2(1. 0 amu) + 1(16. 0 amu) = 18. 0 amu – Formula weight of Na. Cl = 1(23. 0 amu) + 1(35. 5 amu) = 58. 5 amu

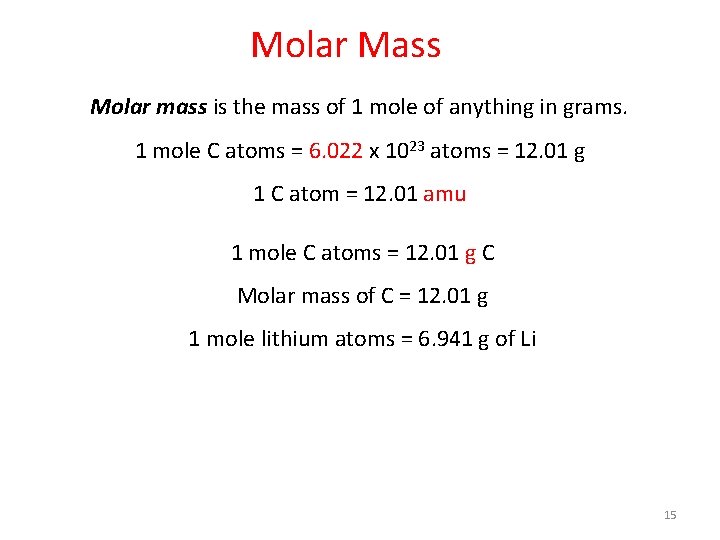
Molar Mass Molar mass is the mass of 1 mole of anything in grams. 1 mole C atoms = 6. 022 x 1023 atoms = 12. 01 g 1 C atom = 12. 01 amu 1 mole C atoms = 12. 01 g C Molar mass of C = 12. 01 g 1 mole lithium atoms = 6. 941 g of Li 15

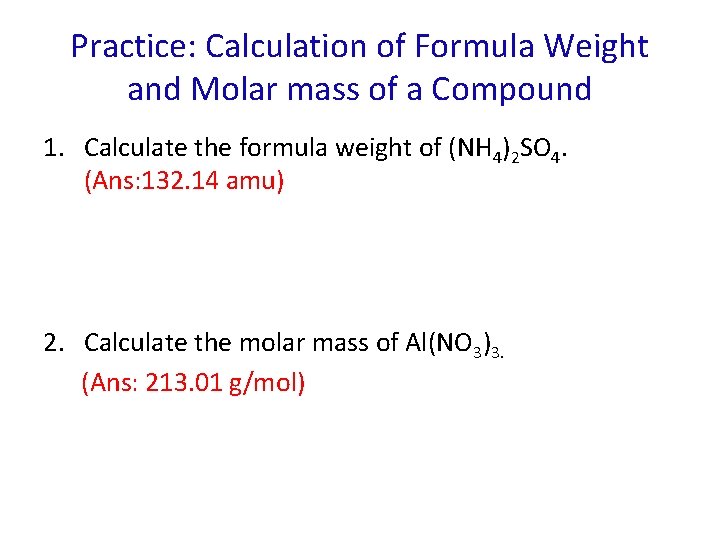
Practice: Calculation of Formula Weight and Molar mass of a Compound 1. Calculate the formula weight of (NH 4)2 SO 4. (Ans: 132. 14 amu) 2. Calculate the molar mass of Al(NO 3)3. (Ans: 213. 01 g/mol)

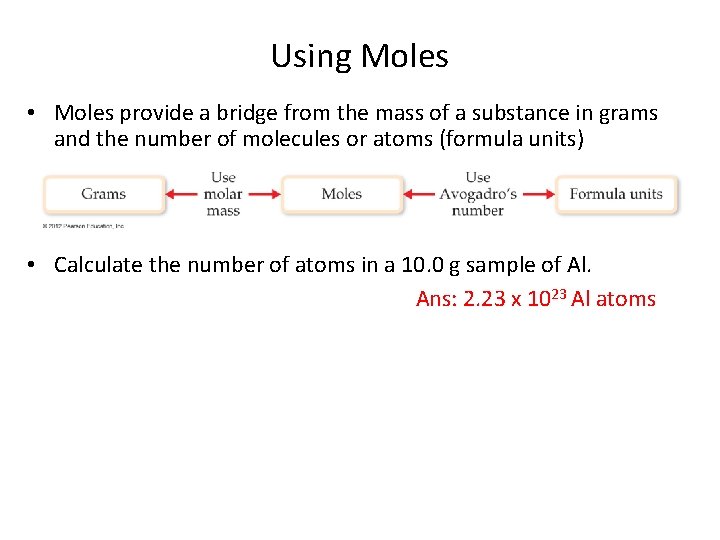
Using Moles • Moles provide a bridge from the mass of a substance in grams and the number of molecules or atoms (formula units) • Calculate the number of atoms in a 10. 0 g sample of Al. Ans: 2. 23 x 1023 Al atoms

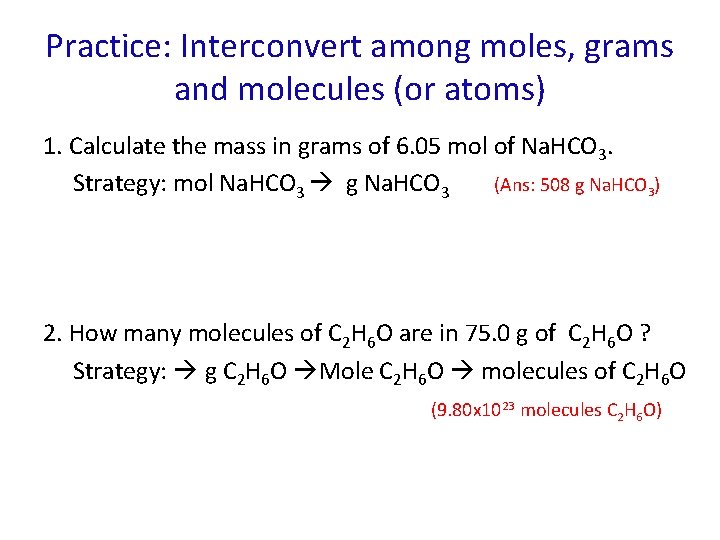
Practice: Interconvert among moles, grams and molecules (or atoms) 1. Calculate the mass in grams of 6. 05 mol of Na. HCO 3. Strategy: mol Na. HCO 3 g Na. HCO 3 (Ans: 508 g Na. HCO 3) 2. How many molecules of C 2 H 6 O are in 75. 0 g of C 2 H 6 O ? Strategy: g C 2 H 6 O Mole C 2 H 6 O molecules of C 2 H 6 O (9. 80 x 1023 molecules C 2 H 6 O)

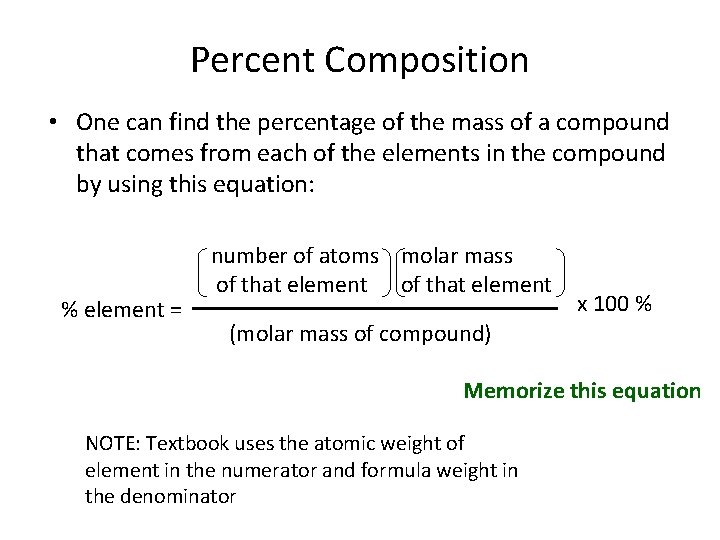
Percent Composition • One can find the percentage of the mass of a compound that comes from each of the elements in the compound by using this equation: % element = number of atoms molar mass of that element x 100 % (molar mass of compound) Memorize this equation NOTE: Textbook uses the atomic weight of element in the numerator and formula weight in the denominator

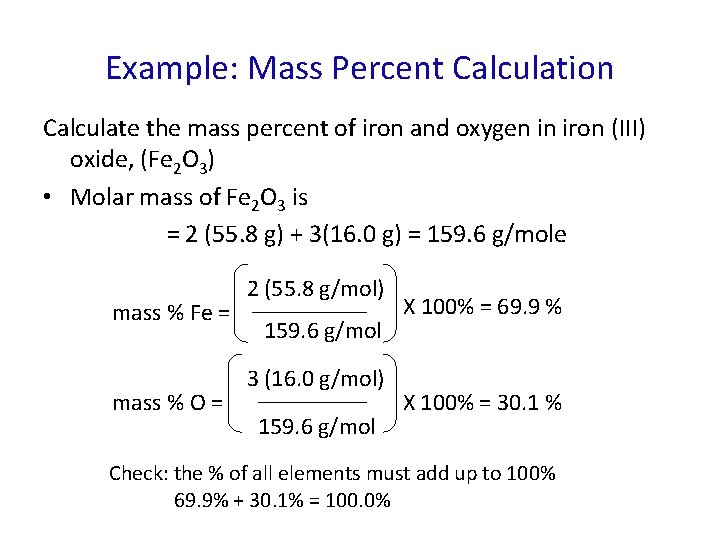
Example: Mass Percent Calculation Calculate the mass percent of iron and oxygen in iron (III) oxide, (Fe 2 O 3) • Molar mass of Fe 2 O 3 is = 2 (55. 8 g) + 3(16. 0 g) = 159. 6 g/mole mass % Fe = mass % O = 2 (55. 8 g/mol) 159. 6 g/mol 3 (16. 0 g/mol) 159. 6 g/mol X 100% = 69. 9 % X 100% = 30. 1 % Check: the % of all elements must add up to 100% 69. 9% + 30. 1% = 100. 0%

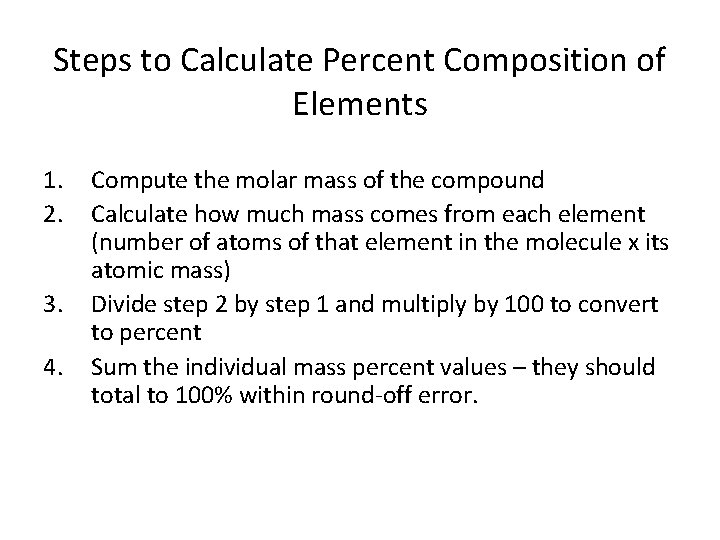
Steps to Calculate Percent Composition of Elements 1. 2. 3. 4. Compute the molar mass of the compound Calculate how much mass comes from each element (number of atoms of that element in the molecule x its atomic mass) Divide step 2 by step 1 and multiply by 100 to convert to percent Sum the individual mass percent values – they should total to 100% within round-off error.
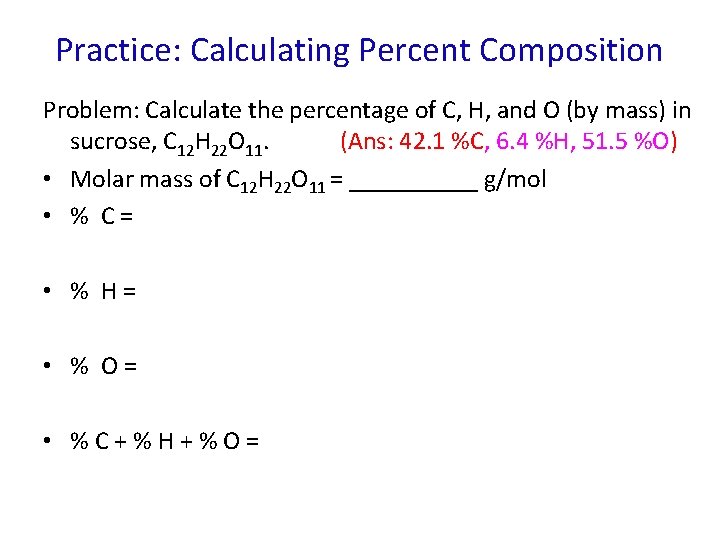
Practice: Calculating Percent Composition Problem: Calculate the percentage of C, H, and O (by mass) in sucrose, C 12 H 22 O 11. (Ans: 42. 1 %C, 6. 4 %H, 51. 5 %O) • Molar mass of C 12 H 22 O 11 = _____ g/mol • % C= • % H= • % O= • %C+%H+%O=

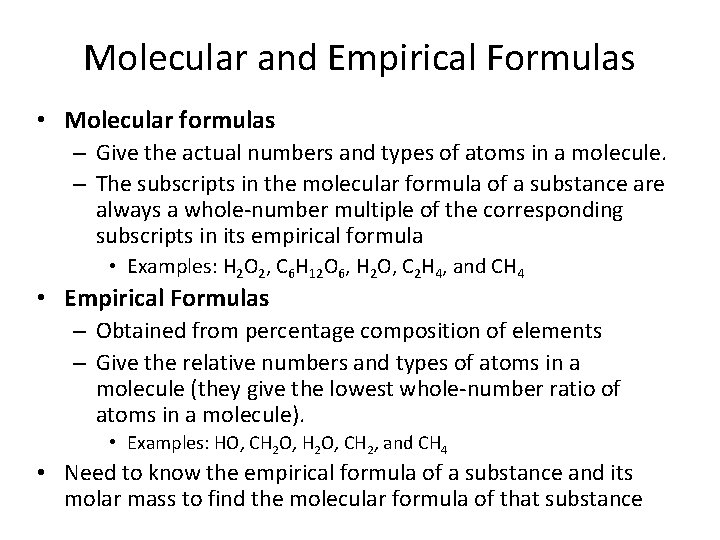
Molecular and Empirical Formulas • Molecular formulas – Give the actual numbers and types of atoms in a molecule. – The subscripts in the molecular formula of a substance are always a whole-number multiple of the corresponding subscripts in its empirical formula • Examples: H 2 O 2, C 6 H 12 O 6, H 2 O, C 2 H 4, and CH 4 • Empirical Formulas – Obtained from percentage composition of elements – Give the relative numbers and types of atoms in a molecule (they give the lowest whole-number ratio of atoms in a molecule). • Examples: HO, CH 2 O, CH 2, and CH 4 • Need to know the empirical formula of a substance and its molar mass to find the molecular formula of that substance

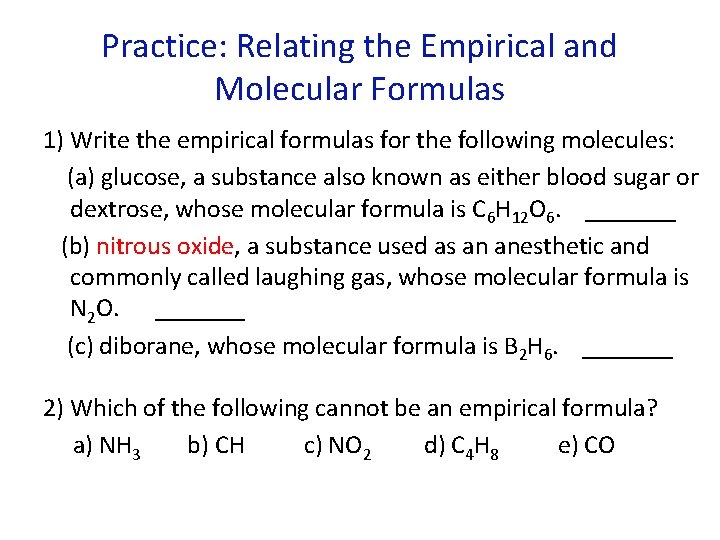
Practice: Relating the Empirical and Molecular Formulas 1) Write the empirical formulas for the following molecules: (a) glucose, a substance also known as either blood sugar or dextrose, whose molecular formula is C 6 H 12 O 6. _______ (b) nitrous oxide, a substance used as an anesthetic and commonly called laughing gas, whose molecular formula is N 2 O. _______ (c) diborane, whose molecular formula is B 2 H 6. _______ 2) Which of the following cannot be an empirical formula? a) NH 3 b) CH c) NO 2 d) C 4 H 8 e) CO

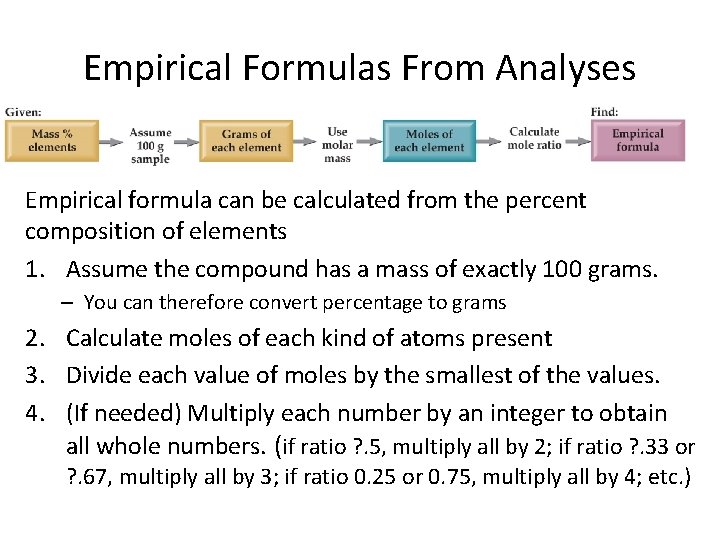
Empirical Formulas From Analyses Empirical formula can be calculated from the percent composition of elements 1. Assume the compound has a mass of exactly 100 grams. – You can therefore convert percentage to grams 2. Calculate moles of each kind of atoms present 3. Divide each value of moles by the smallest of the values. 4. (If needed) Multiply each number by an integer to obtain all whole numbers. (if ratio ? . 5, multiply all by 2; if ratio ? . 33 or ? . 67, multiply all by 3; if ratio 0. 25 or 0. 75, multiply all by 4; etc. )

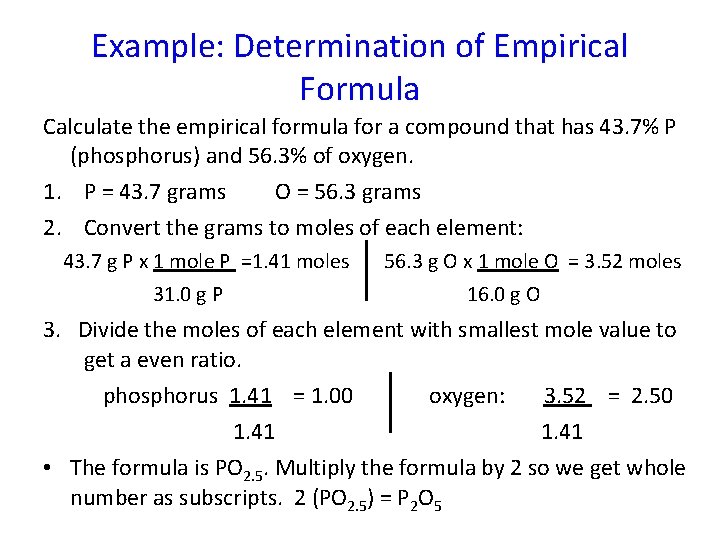
Example: Determination of Empirical Formula Calculate the empirical formula for a compound that has 43. 7% P (phosphorus) and 56. 3% of oxygen. 1. P = 43. 7 grams O = 56. 3 grams 2. Convert the grams to moles of each element: 43. 7 g P x 1 mole P =1. 41 moles 31. 0 g P 56. 3 g O x 1 mole O = 3. 52 moles 16. 0 g O 3. Divide the moles of each element with smallest mole value to get a even ratio. phosphorus 1. 41 = 1. 00 oxygen: 3. 52 = 2. 50 1. 41 • The formula is PO 2. 5. Multiply the formula by 2 so we get whole number as subscripts. 2 (PO 2. 5) = P 2 O 5

Practice: Determination of Empirical Formula A sample of a compound contains 30. 46% nitrogen and 69. 54% oxygen by mass, as determined by a mass spectrometer. What is the empirical formula of the compound?

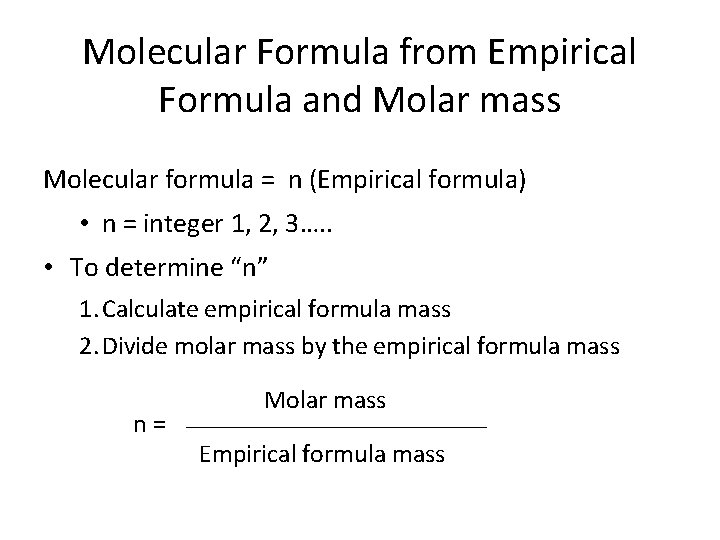
Molecular Formula from Empirical Formula and Molar mass Molecular formula = n (Empirical formula) • n = integer 1, 2, 3…. . • To determine “n” 1. Calculate empirical formula mass 2. Divide molar mass by the empirical formula mass n= Molar mass Empirical formula mass

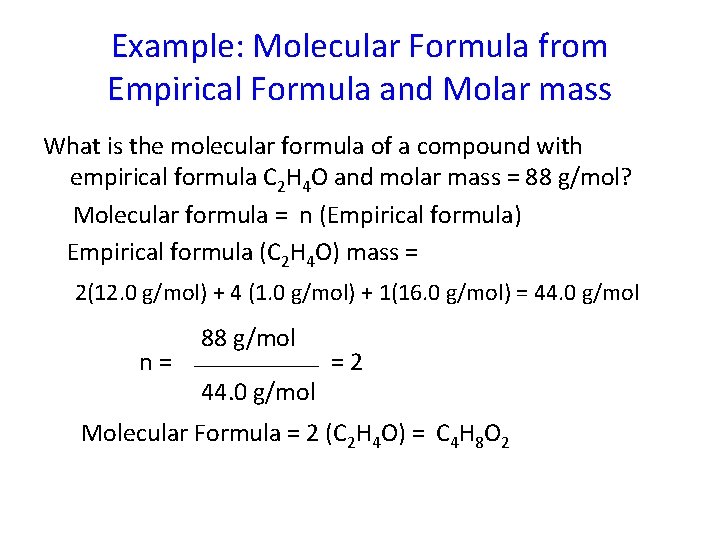
Example: Molecular Formula from Empirical Formula and Molar mass What is the molecular formula of a compound with empirical formula C 2 H 4 O and molar mass = 88 g/mol? Molecular formula = n (Empirical formula) Empirical formula (C 2 H 4 O) mass = 2(12. 0 g/mol) + 4 (1. 0 g/mol) + 1(16. 0 g/mol) = 44. 0 g/mol n= 88 g/mol 44. 0 g/mol =2 Molecular Formula = 2 (C 2 H 4 O) = C 4 H 8 O 2

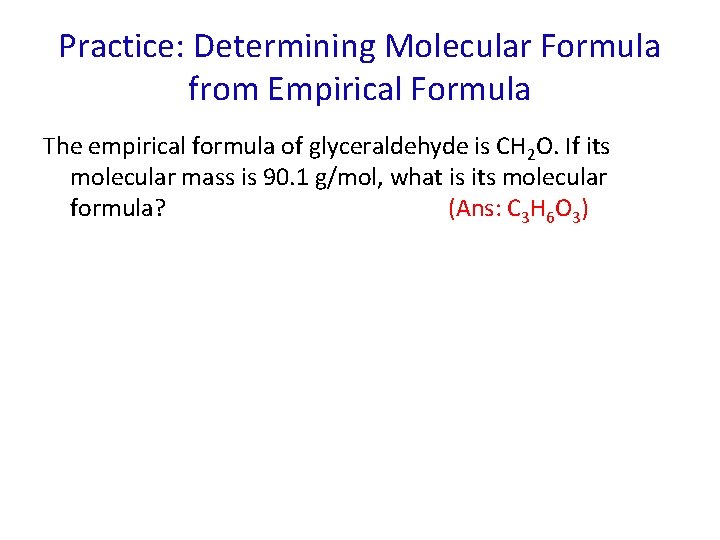
Practice: Determining Molecular Formula from Empirical Formula The empirical formula of glyceraldehyde is CH 2 O. If its molecular mass is 90. 1 g/mol, what is its molecular formula? (Ans: C 3 H 6 O 3)

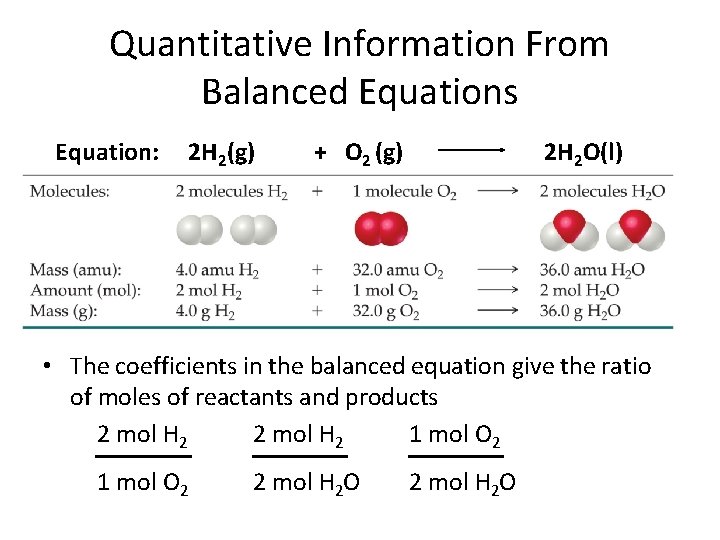
Quantitative Information From Balanced Equations Equation: 2 H 2(g) + O 2 (g) 2 H 2 O(l) • The coefficients in the balanced equation give the ratio of moles of reactants and products 2 mol H 2 1 mol O 2 2 mol H 2 O

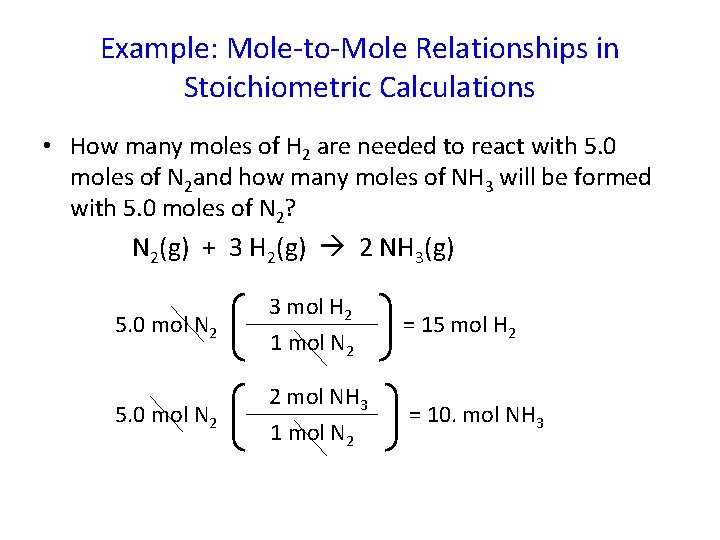
Example: Mole-to-Mole Relationships in Stoichiometric Calculations • How many moles of H 2 are needed to react with 5. 0 moles of N 2 and how many moles of NH 3 will be formed with 5. 0 moles of N 2? N 2(g) + 3 H 2(g) 2 NH 3(g) 5. 0 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 1 mol N 2 = 15 mol H 2 = 10. mol NH 3

Practice: Mole-to-Mole Relationships in Stoichiometric Calculations Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 Mg(s) + N 2(g) Mg 3 N 2 (s) The reaction of 3. 5 moles of Mg will produce how many moles of Mg 3 N 2? (ans: 1. 2 mol Mg 3 N 2) The reaction of 6. 25 mole of Mg will require how many moles of N 2? (Ans: 2. 08 mol N 2)

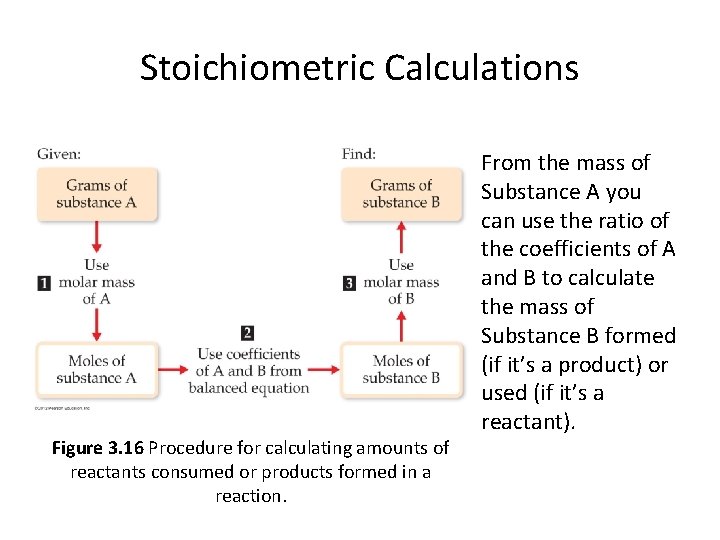
Stoichiometric Calculations Figure 3. 16 Procedure for calculating amounts of reactants consumed or products formed in a reaction. From the mass of Substance A you can use the ratio of the coefficients of A and B to calculate the mass of Substance B formed (if it’s a product) or used (if it’s a reactant).

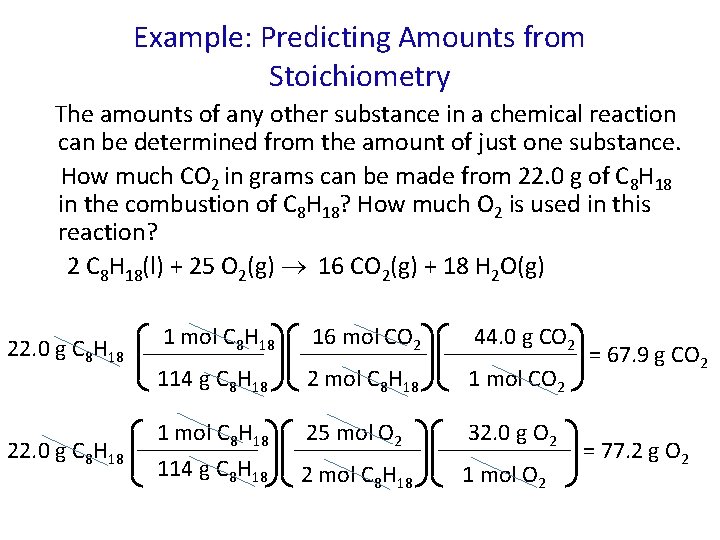
Example: Predicting Amounts from Stoichiometry The amounts of any other substance in a chemical reaction can be determined from the amount of just one substance. How much CO 2 in grams can be made from 22. 0 g of C 8 H 18 in the combustion of C 8 H 18? How much O 2 is used in this reaction? 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) 22. 0 g C 8 H 18 1 mol C 8 H 18 16 mol CO 2 44. 0 g CO 2 114 g C 8 H 18 2 mol C 8 H 18 1 mol CO 2 1 mol C 8 H 18 114 g C 8 H 18 25 mol O 2 32. 0 g O 2 2 mol C 8 H 18 1 mol O 2 = 67. 9 g CO 2 = 77. 2 g O 2

Practice: Stoichiometric Calculations Small amounts of O 2 is prepared in the laboratory using KCl. O 3. 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g). How many grams of O 2 can be prepared from 4. 50 g of KCl. O 3? (Ans: 1. 76 g O 2)

Limiting reagent • The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed – reactant that is completely consumed in the reaction – Makes the least amount of product in a chemical reaction – The reaction stops when the limiting reactant is completely consumed • Any remaining reactants are considered "excess reactants". • The amount of product formed is determined by the "limiting reactant".

Limiting Reagent Analogy Let us assume that you had 12 slices of bread and 7 slices of ham. How many sandwiches you could have possibly made with one ham slice in each sandwich (the theoretical yield) from the available amounts of bread and ham? If you tried to make that many sandwiches, which of the two materials, bread or ham, would have been used up completely (the limiting ingredient)?

Solving a Stoichiometry Problems Involving Limiting Reagent When the amounts of two or more reactants are given, it is important that we know which of them is the limiting reagent, because it is the one that determine how much product will be formed. Identify the limiting reagent 1. – – Convert masses of each reactant to grams of the required product. The reagent that produce the lesser amount of the product is the limiting reagent 2. The amount of the product formed from the limiting reagent is called as the “theoretical yield” of that reaction.

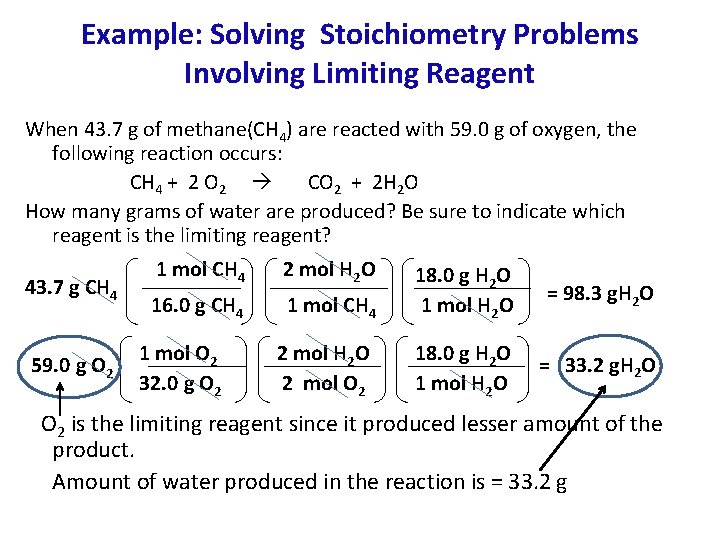
Example: Solving Stoichiometry Problems Involving Limiting Reagent When 43. 7 g of methane(CH 4) are reacted with 59. 0 g of oxygen, the following reaction occurs: CH 4 + 2 O 2 CO 2 + 2 H 2 O How many grams of water are produced? Be sure to indicate which reagent is the limiting reagent? 43. 7 g CH 4 59. 0 g O 2 1 mol CH 4 2 mol H 2 O 16. 0 g CH 4 1 mol O 2 32. 0 g O 2 2 mol H 2 O 2 mol O 2 18. 0 g H 2 O 1 mol H 2 O = 98. 3 g. H 2 O 18. 0 g H 2 O 1 mol H 2 O = 33. 2 g. H 2 O O 2 is the limiting reagent since it produced lesser amount of the product. Amount of water produced in the reaction is = 33. 2 g

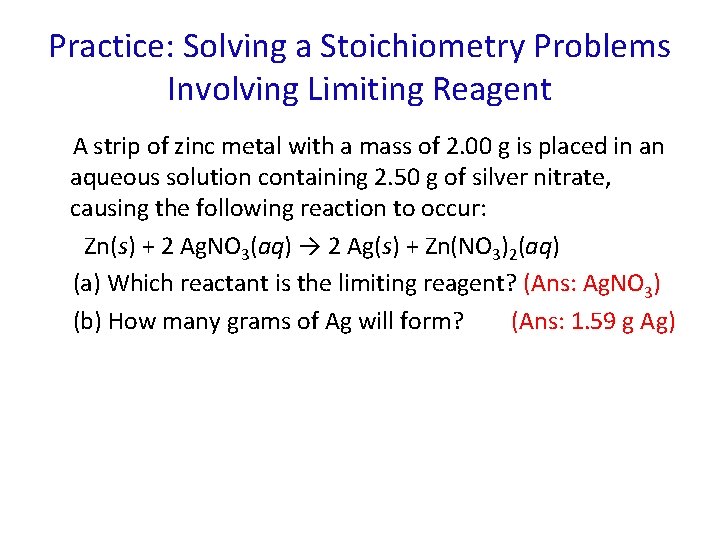
Practice: Solving a Stoichiometry Problems Involving Limiting Reagent A strip of zinc metal with a mass of 2. 00 g is placed in an aqueous solution containing 2. 50 g of silver nitrate, causing the following reaction to occur: Zn(s) + 2 Ag. NO 3(aq) → 2 Ag(s) + Zn(NO 3)2(aq) (a) Which reactant is the limiting reagent? (Ans: Ag. NO 3) (b) How many grams of Ag will form? (Ans: 1. 59 g Ag)

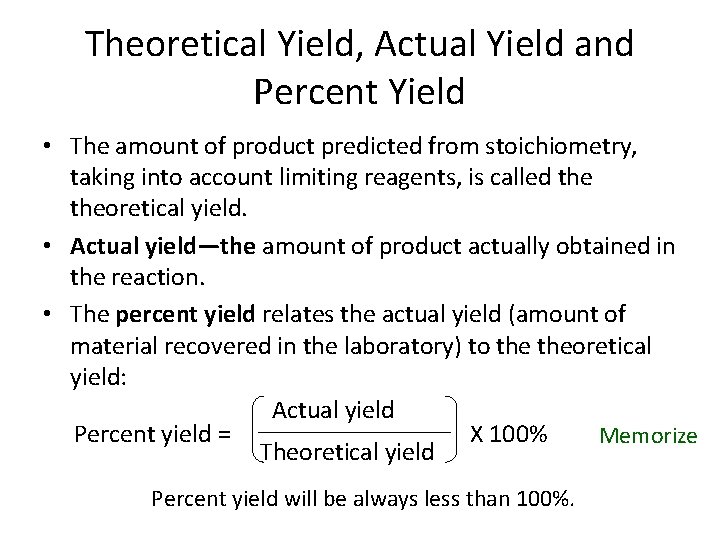
Theoretical Yield, Actual Yield and Percent Yield • The amount of product predicted from stoichiometry, taking into account limiting reagents, is called theoretical yield. • Actual yield—the amount of product actually obtained in the reaction. • The percent yield relates the actual yield (amount of material recovered in the laboratory) to theoretical yield: Actual yield Percent yield = X 100% Memorize Theoretical yield Percent yield will be always less than 100%.

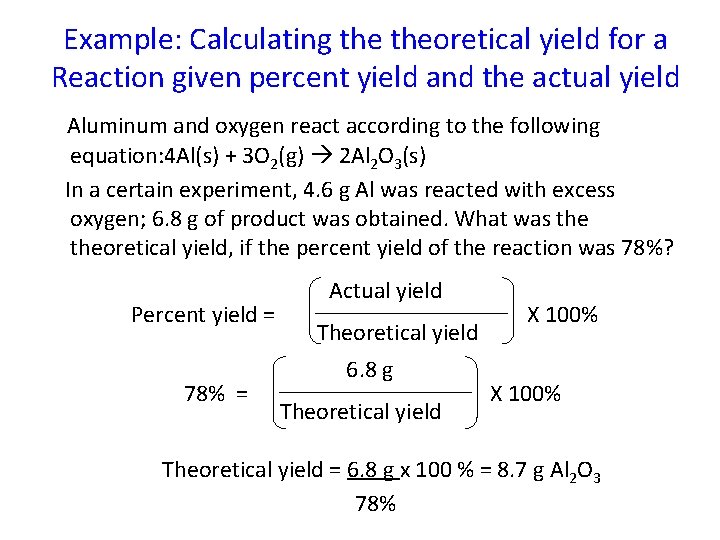
Example: Calculating theoretical yield for a Reaction given percent yield and the actual yield Aluminum and oxygen react according to the following equation: 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) In a certain experiment, 4. 6 g Al was reacted with excess oxygen; 6. 8 g of product was obtained. What was theoretical yield, if the percent yield of the reaction was 78%? Percent yield = 78% = Actual yield Theoretical yield 6. 8 g Theoretical yield X 100% Theoretical yield = 6. 8 g x 100 % = 8. 7 g Al 2 O 3 78%

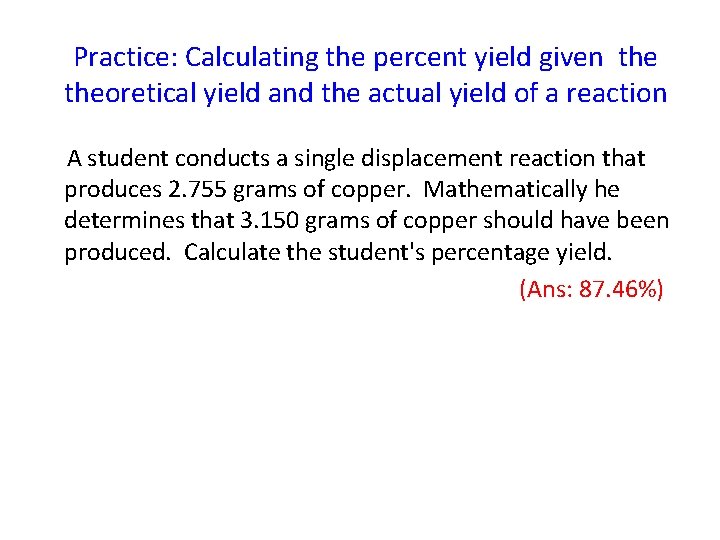
Practice: Calculating the percent yield given theoretical yield and the actual yield of a reaction A student conducts a single displacement reaction that produces 2. 755 grams of copper. Mathematically he determines that 3. 150 grams of copper should have been produced. Calculate the student's percentage yield. (Ans: 87. 46%)
 Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test 7-1 practice problems chemistry answers
7-1 practice problems chemistry answers Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Chemical names and formulas chapter 9
Chemical names and formulas chapter 9 Types of connections in steel structures
Types of connections in steel structures Writing formulas criss-cross method worksheet
Writing formulas criss-cross method worksheet Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas A chemical formula shows the
A chemical formula shows the Parenteral medication formula
Parenteral medication formula Dc shunt motor equations
Dc shunt motor equations Stoichiometry formulas
Stoichiometry formulas Stoichiometry equation
Stoichiometry equation Moles formula
Moles formula Mole ratio examples
Mole ratio examples Stoichiometry formulas
Stoichiometry formulas Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry In a chemical reaction, stoichiometry refers to:
In a chemical reaction, stoichiometry refers to: Chemical accounting: stoichiometry
Chemical accounting: stoichiometry Chemical accounting: stoichiometry
Chemical accounting: stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chapter 11 assessment chemistry
Chapter 11 assessment chemistry In an ionic compound the chemical formula represents
In an ionic compound the chemical formula represents Symbols and formulas
Symbols and formulas What is mole
What is mole Covalent compound formula
Covalent compound formula Rules for naming ionic compounds
Rules for naming ionic compounds Are kc and kp equal
Are kc and kp equal Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Conversions and calculations chapter 6
Conversions and calculations chapter 6 Measurements and calculations chapter 2 test
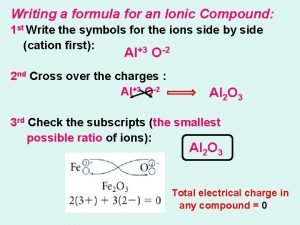
Measurements and calculations chapter 2 test Writing formulas (criss-cross method)
Writing formulas (criss-cross method) Coefficients and subscripts
Coefficients and subscripts Chemical formulas
Chemical formulas Chemical formulas
Chemical formulas Crisscross rule
Crisscross rule Chemical formulas list for class 10
Chemical formulas list for class 10 Rb3p compound name
Rb3p compound name Chemical formulas quiz
Chemical formulas quiz Counting atoms in chemical formulas
Counting atoms in chemical formulas Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Modern chemistry chapter 9 stoichiometry test b answers
Modern chemistry chapter 9 stoichiometry test b answers Chapter 11 stoichiometry
Chapter 11 stoichiometry