Chapter 3 Stoichiometry Calculations with Chemical Formulas and









- Slides: 37

Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations Stoichiometry

Law of Conservation of Mass “We may lay it down as an incontestable axiom that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Upon this principle, the whole art of performing chemical experiments depends. ” --Antoine Lavoisier, 1789 Stoichiometry

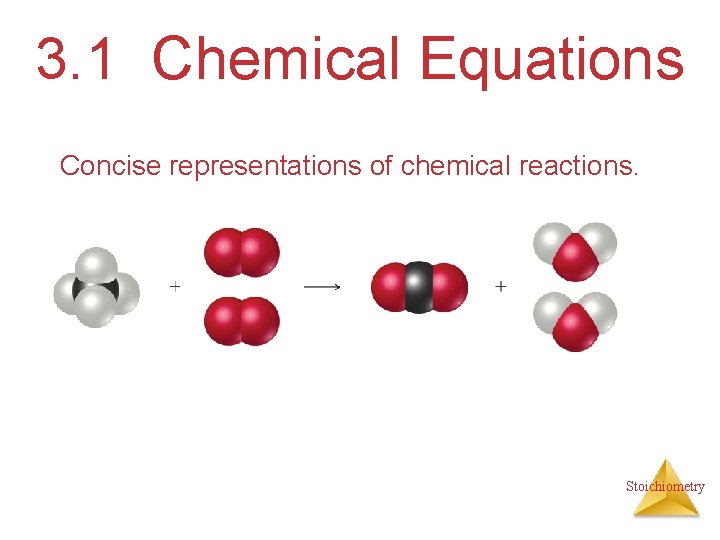
3. 1 Chemical Equations Concise representations of chemical reactions. Stoichiometry

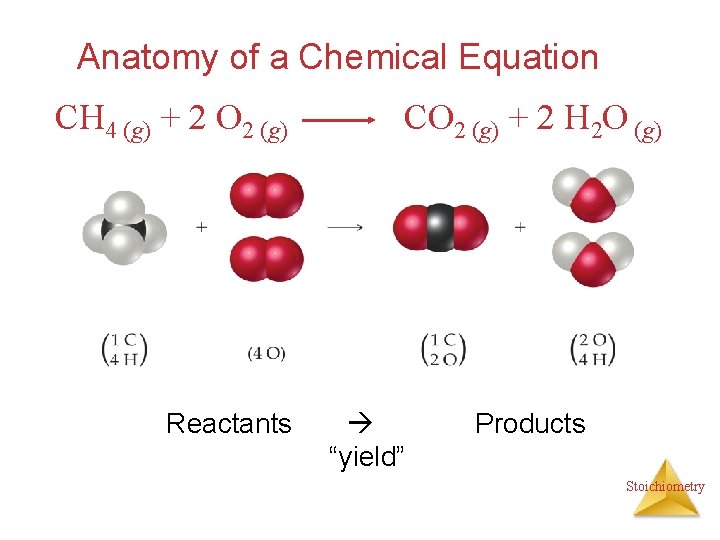
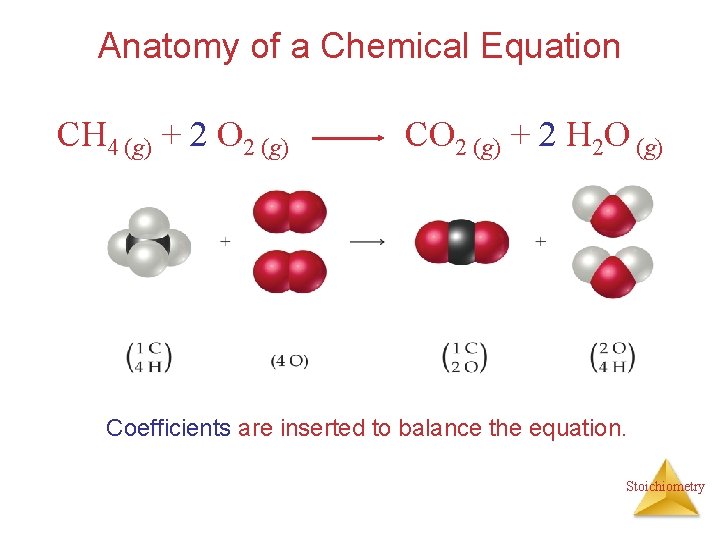
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) Reactants CO 2 (g) + 2 H 2 O (g) “yield” Products Stoichiometry

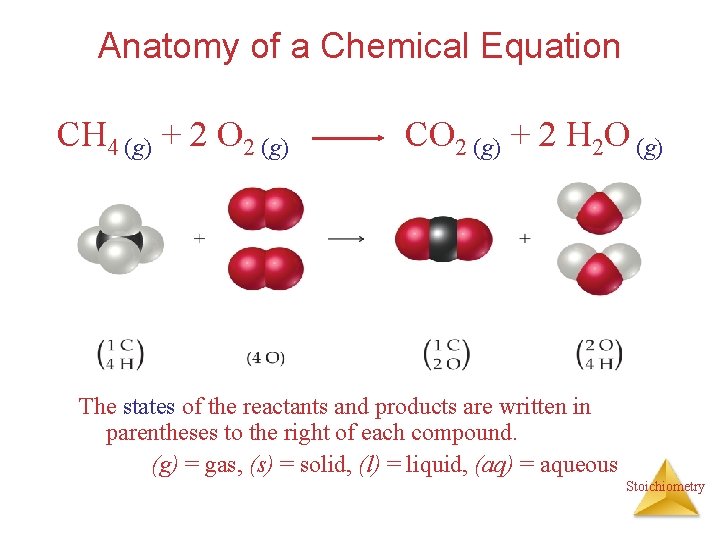
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) The states of the reactants and products are written in parentheses to the right of each compound. (g) = gas, (s) = solid, (l) = liquid, (aq) = aqueous Stoichiometry

Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Coefficients are inserted to balance the equation. Stoichiometry

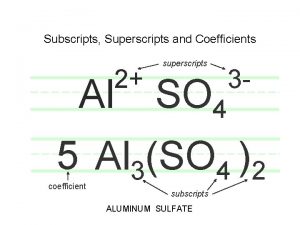
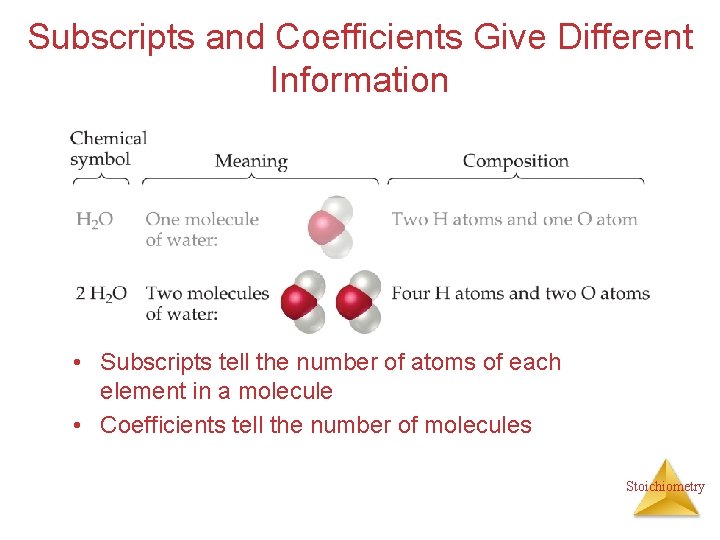
Subscripts and Coefficients Give Different Information • Subscripts tell the number of atoms of each element in a molecule • Coefficients tell the number of molecules Stoichiometry

3. 2 Patterns of Chemical Reactivity (Reaction Types) Stoichiometry

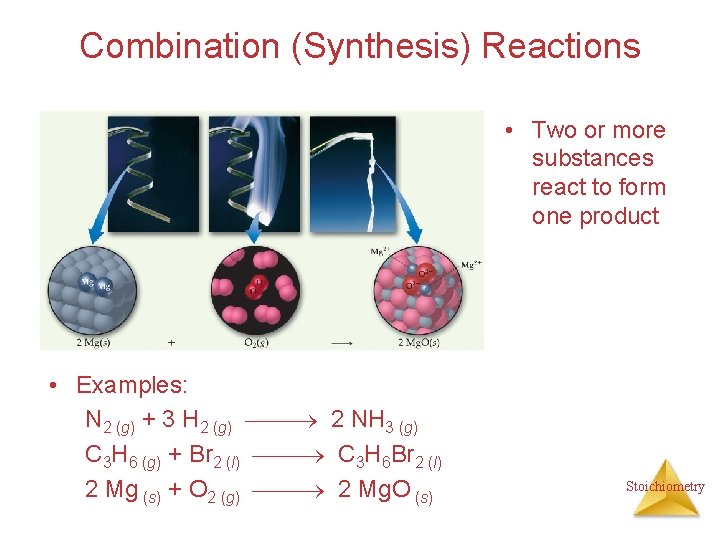
Combination (Synthesis) Reactions • Two or more substances react to form one product • Examples: N 2 (g) + 3 H 2 (g) 2 NH 3 (g) C 3 H 6 (g) + Br 2 (l) C 3 H 6 Br 2 (l) 2 Mg (s) + O 2 (g) 2 Mg. O (s) Stoichiometry

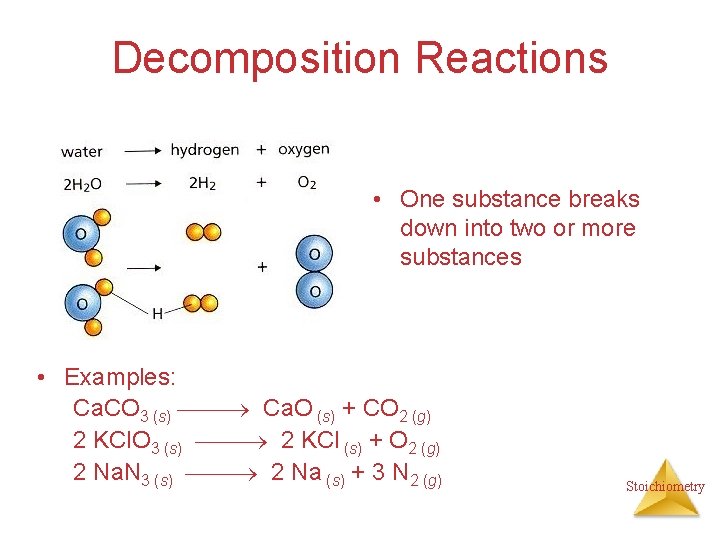
Decomposition Reactions • One substance breaks down into two or more substances • Examples: Ca. CO 3 (s) Ca. O (s) + CO 2 (g) 2 KCl. O 3 (s) 2 KCl (s) + O 2 (g) 2 Na. N 3 (s) 2 Na (s) + 3 N 2 (g) Stoichiometry

Combustion Reactions • Rapid reactions that produce a flame • Most often involve hydrocarbons reacting with oxygen in the air • Examples: CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) Stoichiometry

3. 3 Formula Weights Stoichiometry

Formula Weight (FW) • Sum of the atomic weights for the atoms in a chemical formula. • For example, the formula weight of calcium chloride, Ca. Cl 2, is + Ca: 1 x (40. 1 amu) Cl: 2 x (35. 3 amu) 111. 1 amu • These are generally reported for ionic compounds. • Because ionic substances, such as Na. Cl, exist as threedimensional arrays of ions (see Fig 2. 23), it is not accurate to speak of molecules of Na. Cl. The term formula unit represents the chemical formula of the substance, i. e. , Na. Cl. (one Na+ + one Cl-) • The formula weight of Na. Cl, Ca. Cl 2, or any ionic substance, is the mass of one formula unit. Stoichiometry

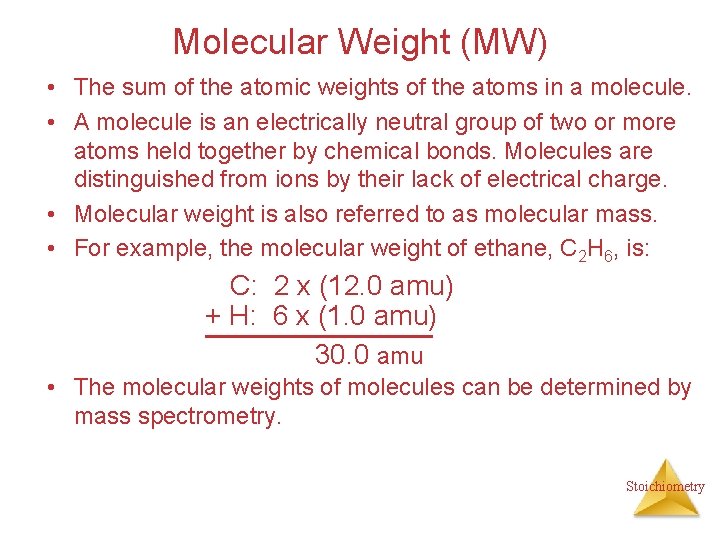
Molecular Weight (MW) • The sum of the atomic weights of the atoms in a molecule. • A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. • Molecular weight is also referred to as molecular mass. • For example, the molecular weight of ethane, C 2 H 6, is: C: 2 x (12. 0 amu) + H: 6 x (1. 0 amu) 30. 0 amu • The molecular weights of molecules can be determined by mass spectrometry. Stoichiometry

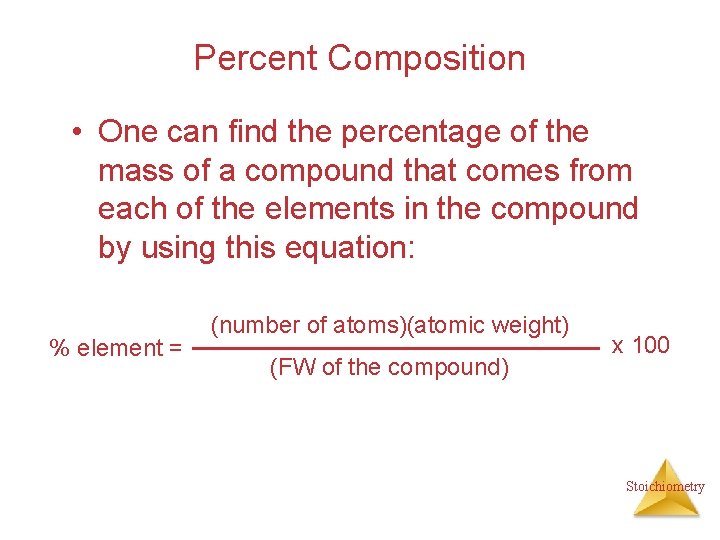
Percent Composition • One can find the percentage of the mass of a compound that comes from each of the elements in the compound by using this equation: % element = (number of atoms)(atomic weight) (FW of the compound) x 100 Stoichiometry

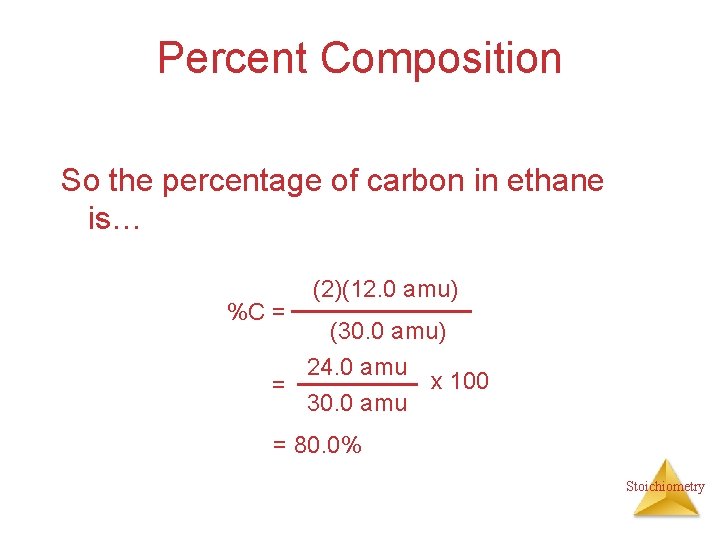
Percent Composition So the percentage of carbon in ethane is… %C = (2)(12. 0 amu) (30. 0 amu) 24. 0 amu x 100 = 30. 0 amu = 80. 0% Stoichiometry

3. 4 Avogadro’s Number and the Mole Stoichiometry

Avogadro’s Number • 6. 022 x 1023 • 1 mole contains as many particles (atoms or molecules)as are in 12 grams of isotopically pure 12 C. Stoichiometry

Molar Mass • By definition, this is the mass of 1 mol of a substance (i. e. , g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry

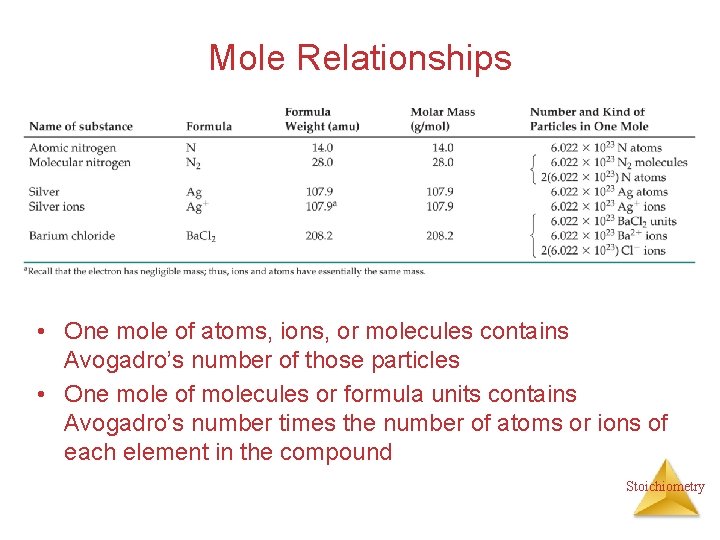
Mole Relationships • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry

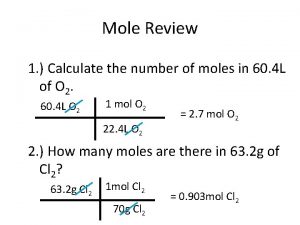
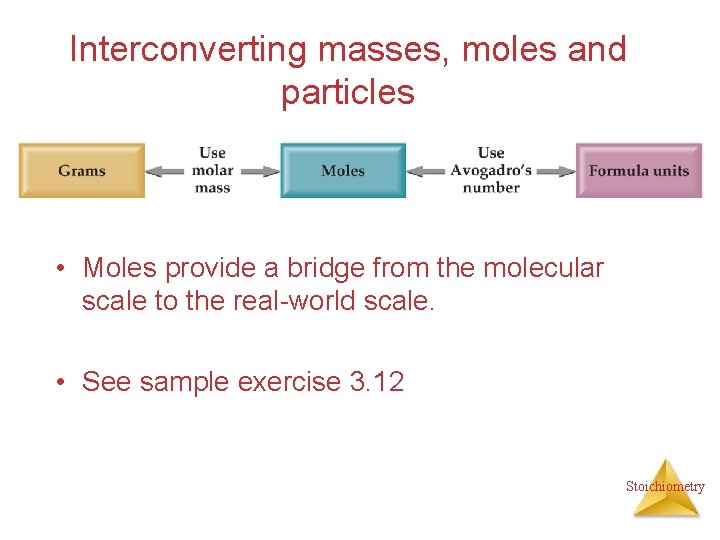
Interconverting masses, moles and particles • Moles provide a bridge from the molecular scale to the real-world scale. • See sample exercise 3. 12 Stoichiometry

3. 5 Empirical Formulas from Analyses Stoichiometry

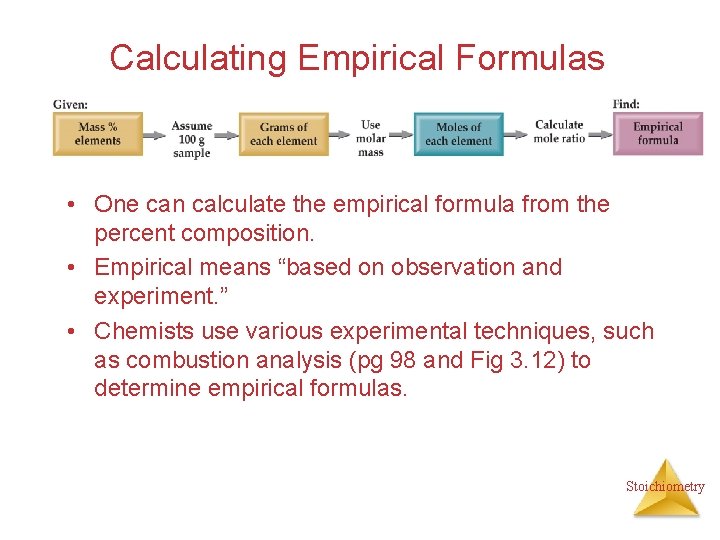
Calculating Empirical Formulas • One can calculate the empirical formula from the percent composition. • Empirical means “based on observation and experiment. ” • Chemists use various experimental techniques, such as combustion analysis (pg 98 and Fig 3. 12) to determine empirical formulas. Stoichiometry

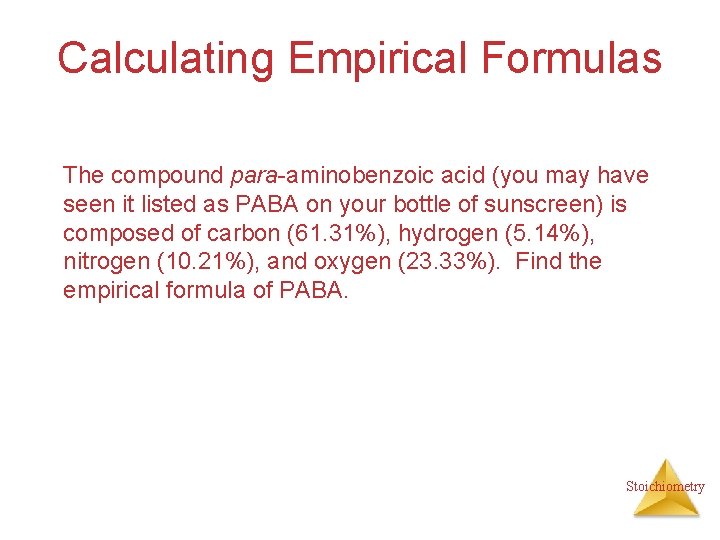
Calculating Empirical Formulas The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), and oxygen (23. 33%). Find the empirical formula of PABA. Stoichiometry

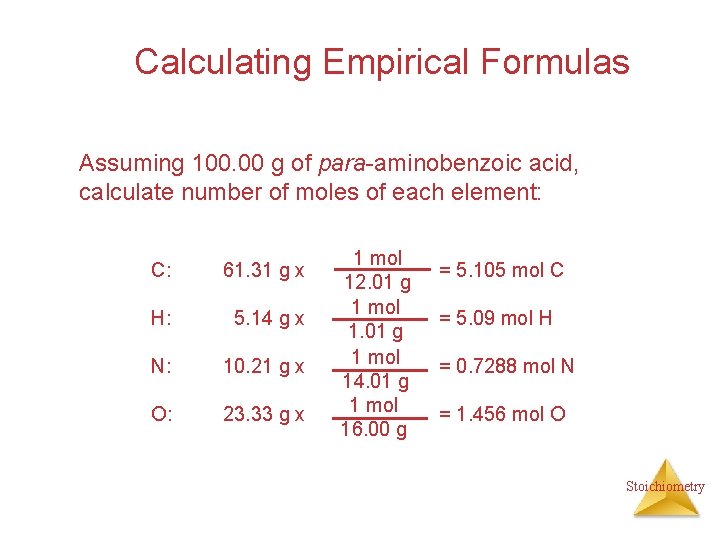
Calculating Empirical Formulas Assuming 100. 00 g of para-aminobenzoic acid, calculate number of moles of each element: C: 61. 31 g x H: 5. 14 g x N: 10. 21 g x O: 23. 33 g x 1 mol 12. 01 g 1 mol 14. 01 g 1 mol 16. 00 g = 5. 105 mol C = 5. 09 mol H = 0. 7288 mol N = 1. 456 mol O Stoichiometry

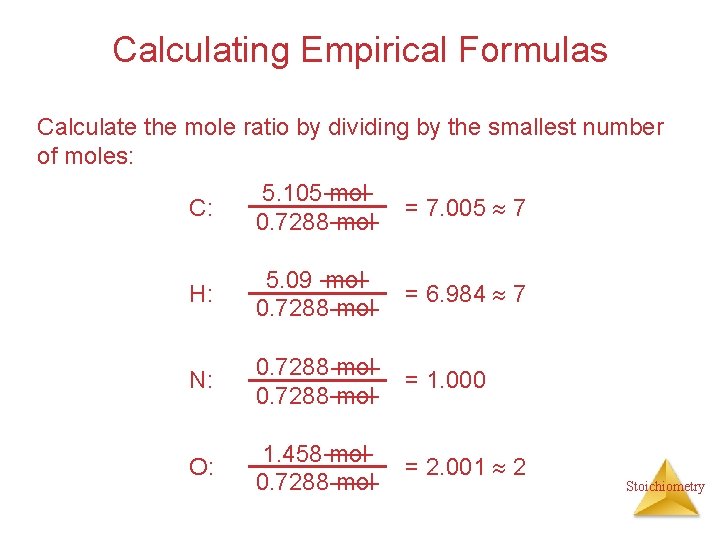
Calculating Empirical Formulas Calculate the mole ratio by dividing by the smallest number of moles: C: 5. 105 mol 0. 7288 mol = 7. 005 7 H: 5. 09 mol 0. 7288 mol = 6. 984 7 N: 0. 7288 mol = 1. 000 O: 1. 458 mol 0. 7288 mol = 2. 001 2 Stoichiometry

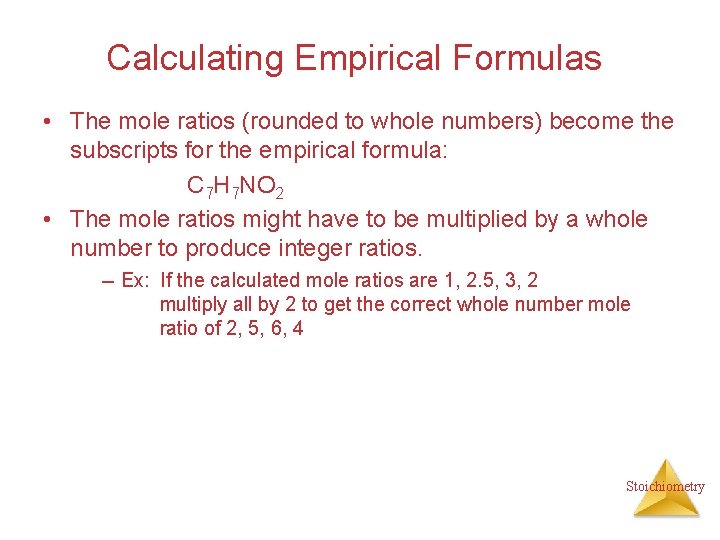
Calculating Empirical Formulas • The mole ratios (rounded to whole numbers) become the subscripts for the empirical formula: C 7 H 7 NO 2 • The mole ratios might have to be multiplied by a whole number to produce integer ratios. -- Ex: If the calculated mole ratios are 1, 2. 5, 3, 2 multiply all by 2 to get the correct whole number mole ratio of 2, 5, 6, 4 Stoichiometry

3. 6 Quantititave Information from Balanced Equations Stoichiometry

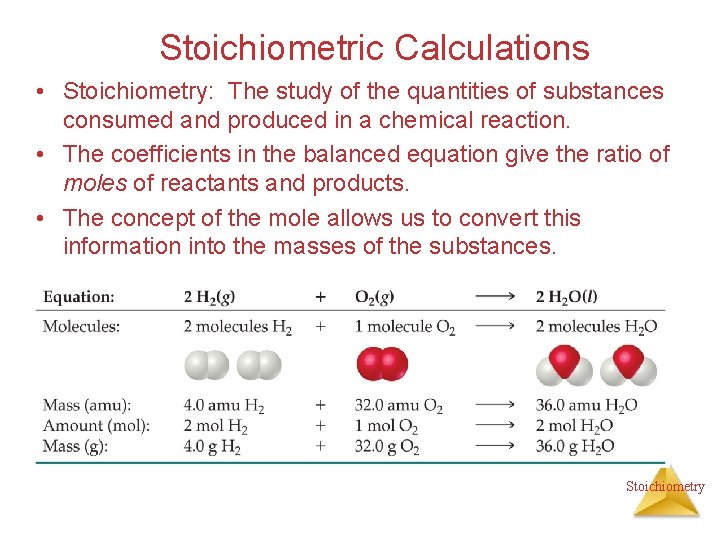
Stoichiometric Calculations • Stoichiometry: The study of the quantities of substances consumed and produced in a chemical reaction. • The coefficients in the balanced equation give the ratio of moles of reactants and products. • The concept of the mole allows us to convert this information into the masses of the substances. Stoichiometry

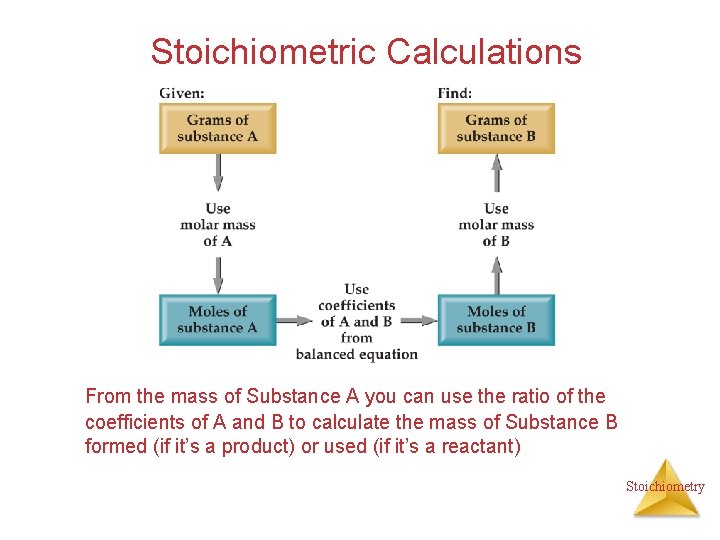
Stoichiometric Calculations From the mass of Substance A you can use the ratio of the coefficients of A and B to calculate the mass of Substance B formed (if it’s a product) or used (if it’s a reactant) Stoichiometry

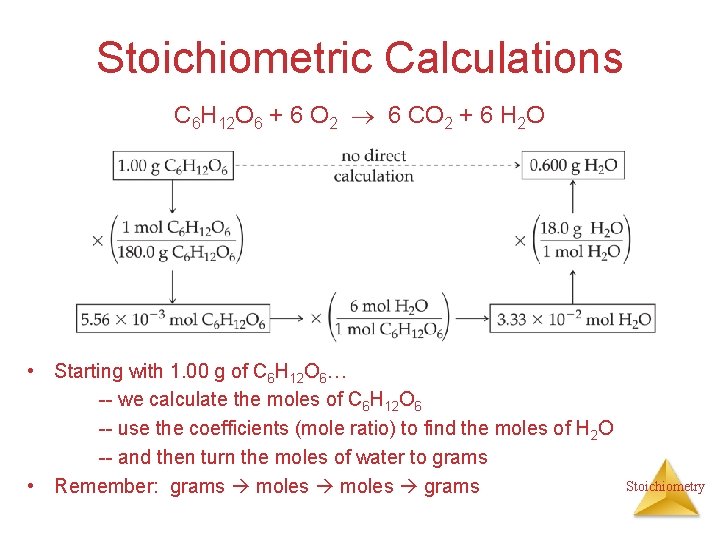
Stoichiometric Calculations C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O • Starting with 1. 00 g of C 6 H 12 O 6… -- we calculate the moles of C 6 H 12 O 6 -- use the coefficients (mole ratio) to find the moles of H 2 O -- and then turn the moles of water to grams • Remember: grams moles grams Stoichiometry

3. 7 Limiting Reactants Stoichiometry

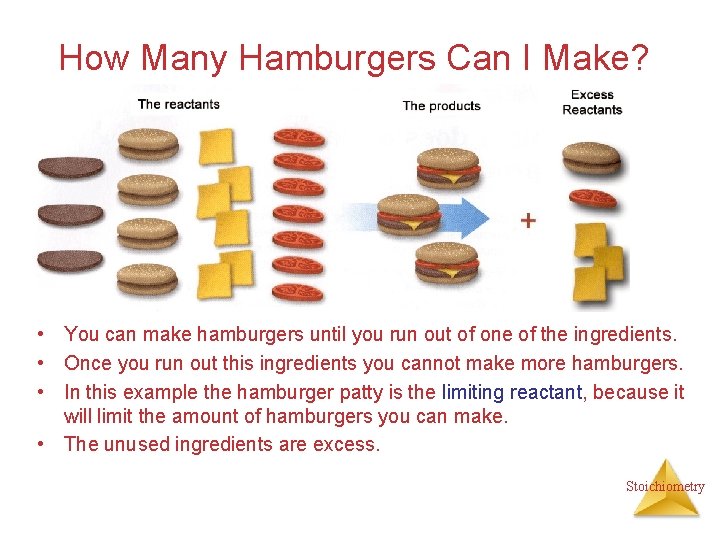
How Many Hamburgers Can I Make? • You can make hamburgers until you run out of one of the ingredients. • Once you run out this ingredients you cannot make more hamburgers. • In this example the hamburger patty is the limiting reactant, because it will limit the amount of hamburgers you can make. • The unused ingredients are excess. Stoichiometry

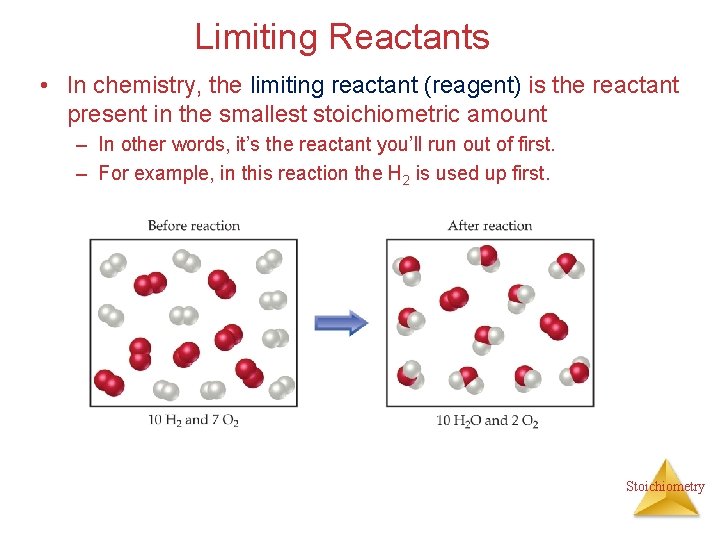
Limiting Reactants • In chemistry, the limiting reactant (reagent) is the reactant present in the smallest stoichiometric amount – In other words, it’s the reactant you’ll run out of first. – For example, in this reaction the H 2 is used up first. Stoichiometry

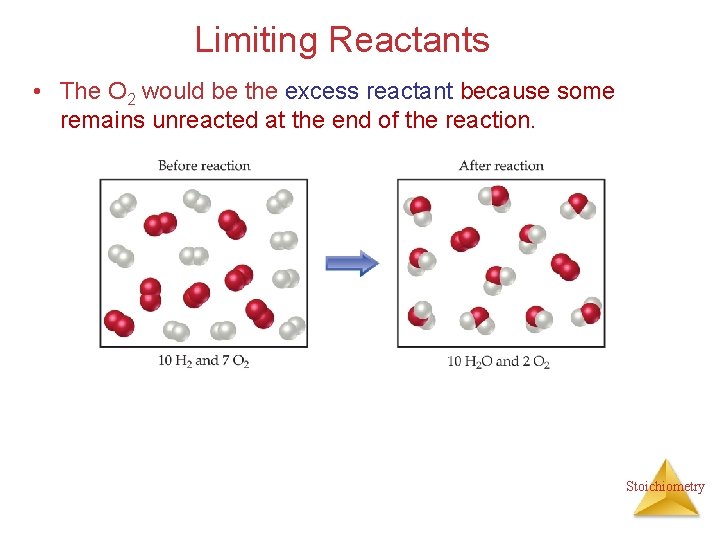
Limiting Reactants • The O 2 would be the excess reactant because some remains unreacted at the end of the reaction. Stoichiometry

Calculating the Amount of Product Formed from a Limiting Reactant • See sample exercise 3. 18 • Assuming one reactant is completely consumed, calculate how much of the second reactant is needed. By comparing the calculated amount with the available amount, we can determine which is limiting. • Use the limiting reactant to determine the quantity of product possible. Stoichiometry

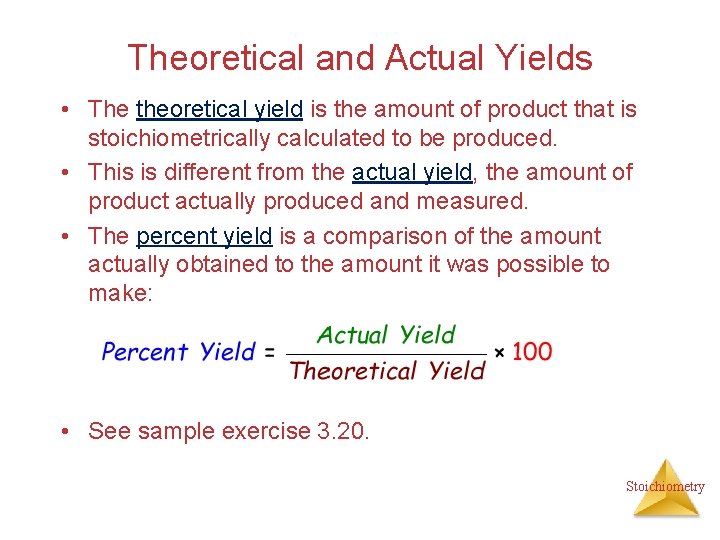
Theoretical and Actual Yields • The theoretical yield is the amount of product that is stoichiometrically calculated to be produced. • This is different from the actual yield, the amount of product actually produced and measured. • The percent yield is a comparison of the amount actually obtained to the amount it was possible to make: • See sample exercise 3. 20. Stoichiometry
 Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Stoichiometry and stoichiometric calculations
Stoichiometry and stoichiometric calculations Dichlorine octoxide formula
Dichlorine octoxide formula Types of connections in steel structures
Types of connections in steel structures Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas A chemical formula shows
A chemical formula shows Parenteral medication formula
Parenteral medication formula Generator calculations formulas
Generator calculations formulas Stoichiometry formulas
Stoichiometry formulas Stoichiometry formulas
Stoichiometry formulas Calculate molar mass
Calculate molar mass Calculate moles
Calculate moles Stoichiometry formulas
Stoichiometry formulas Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry In a chemical reaction, stoichiometry refers to:
In a chemical reaction, stoichiometry refers to: Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chapter 11 stoichiometry study guide answer key
Chapter 11 stoichiometry study guide answer key Writing and naming chemical formulas
Writing and naming chemical formulas Counting atoms worksheet answer key
Counting atoms worksheet answer key Formula of mol
Formula of mol Covalent covalent bond
Covalent covalent bond Ag3po4
Ag3po4 Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Conversions and calculations chapter 6
Conversions and calculations chapter 6 Percent error example
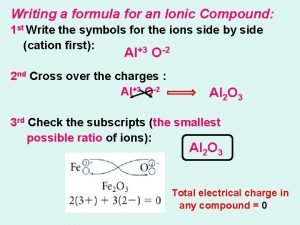
Percent error example Criss cross method writing formulas
Criss cross method writing formulas Subscript chemical formula
Subscript chemical formula How to write an ionic compound formula
How to write an ionic compound formula Formula in chemistry
Formula in chemistry Criss-cross method definition chemistry
Criss-cross method definition chemistry