Chapter 3 Stoichiometry Calculations with Chemical Formulas and






- Slides: 23

Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations

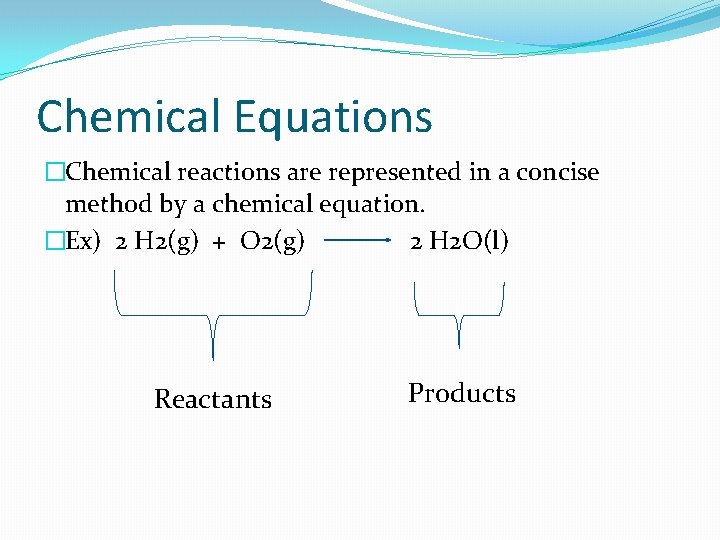
Chemical Equations �Chemical reactions are represented in a concise method by a chemical equation. �Ex) 2 H 2(g) + O 2(g) 2 H 2 O(l) Reactants Products

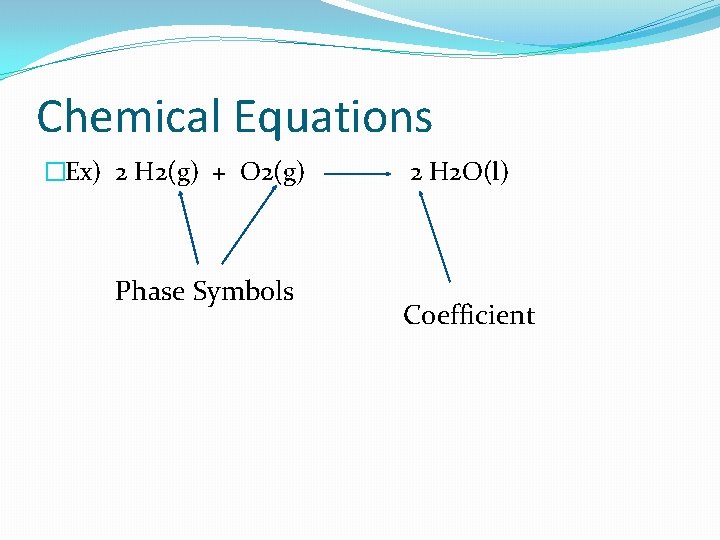
Chemical Equations �Ex) 2 H 2(g) + O 2(g) Phase Symbols 2 H 2 O(l) Coefficient

Balancing an Equation �A subscript in a chemical formula tells us how many of each type of atom are in the compound. �Ex) C 6 H 12 O 6 �Subscripts cannot be altered!!! �Atoms can be created nor destroyed in a chemical reaction. �Thus, we balance a reaction by adding coefficients in front of each substance.

Balancing an Equation �Balance by inspection. �Use a tally sheet. �Start with elements that occur once on each side. �Combustion – do C, then H, then O. �LEP #1.

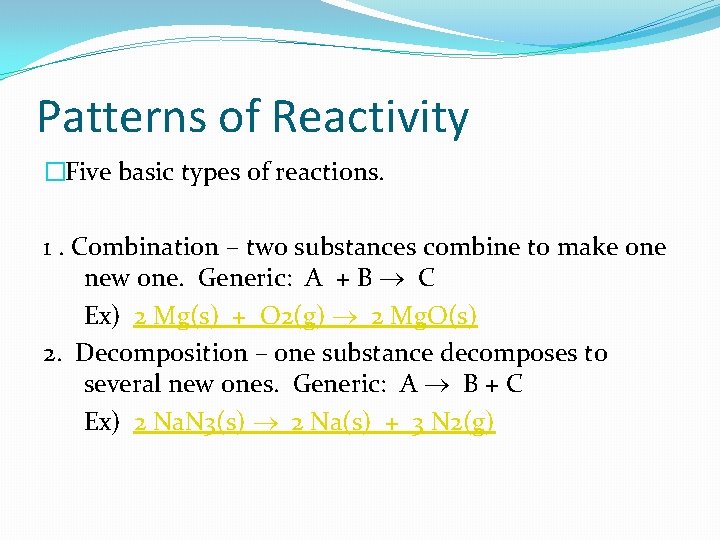
Patterns of Reactivity �Five basic types of reactions. 1. Combination – two substances combine to make one new one. Generic: A + B C Ex) 2 Mg(s) + O 2(g) 2 Mg. O(s) 2. Decomposition – one substance decomposes to several new ones. Generic: A B + C Ex) 2 Na. N 3(s) 2 Na(s) + 3 N 2(g)

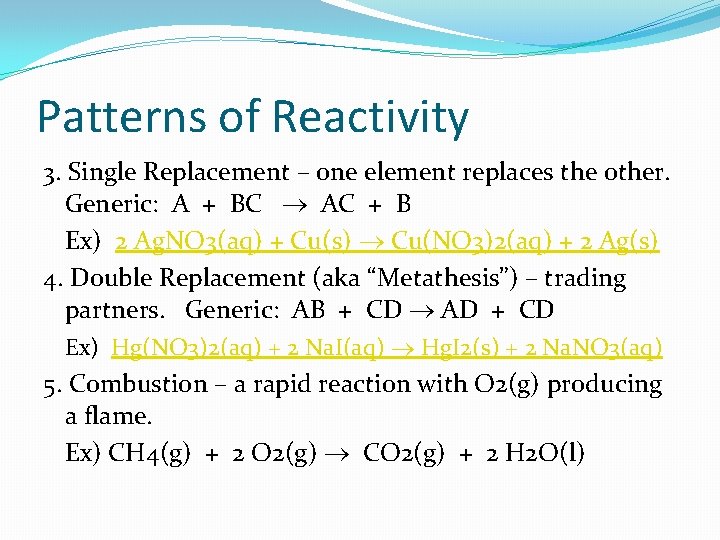
Patterns of Reactivity 3. Single Replacement – one element replaces the other. Generic: A + BC AC + B Ex) 2 Ag. NO 3(aq) + Cu(s) Cu(NO 3)2(aq) + 2 Ag(s) 4. Double Replacement (aka “Metathesis”) – trading partners. Generic: AB + CD AD + CD Ex) Hg(NO 3)2(aq) + 2 Na. I(aq) Hg. I 2(s) + 2 Na. NO 3(aq) 5. Combustion – a rapid reaction with O 2(g) producing a flame. Ex) CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l)

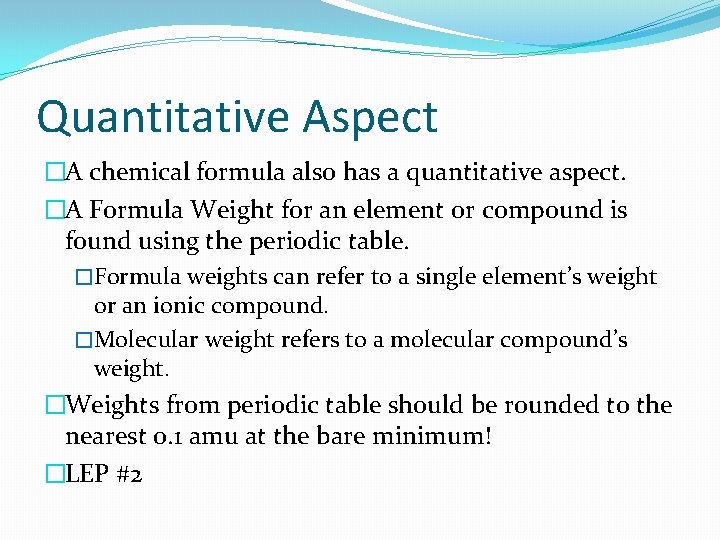
Quantitative Aspect �A chemical formula also has a quantitative aspect. �A Formula Weight for an element or compound is found using the periodic table. �Formula weights can refer to a single element’s weight or an ionic compound. �Molecular weight refers to a molecular compound’s weight. �Weights from periodic table should be rounded to the nearest 0. 1 amu at the bare minimum! �LEP #2

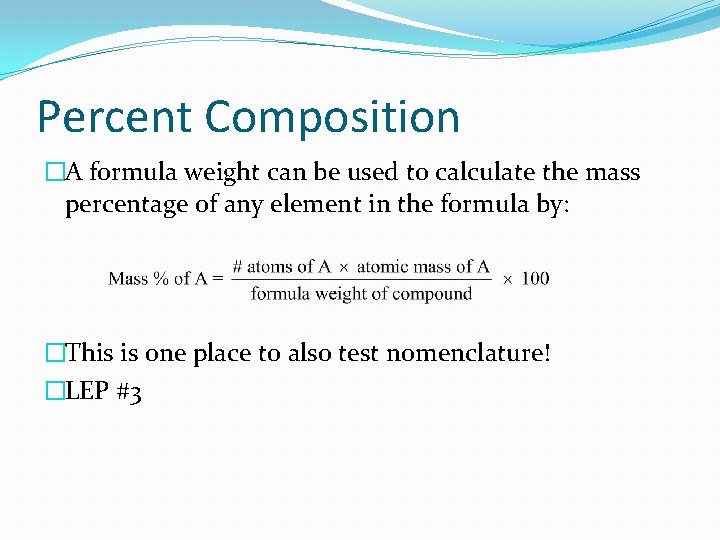
Percent Composition �A formula weight can be used to calculate the mass percentage of any element in the formula by: �This is one place to also test nomenclature! �LEP #3

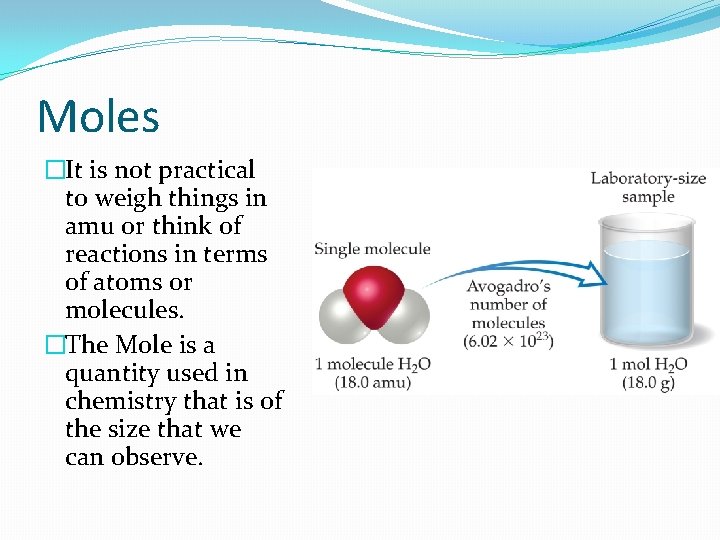
Moles �It is not practical to weigh things in amu or think of reactions in terms of atoms or molecules. �The Mole is a quantity used in chemistry that is of the size that we can observe.

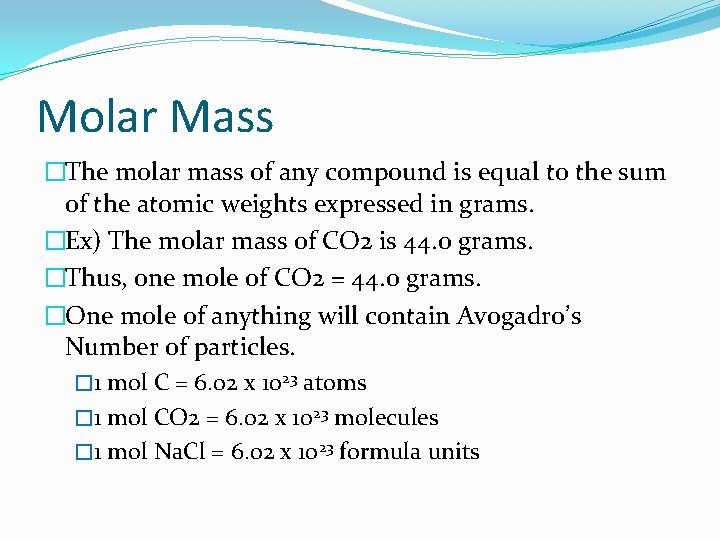
Molar Mass �The molar mass of any compound is equal to the sum of the atomic weights expressed in grams. �Ex) The molar mass of CO 2 is 44. 0 grams. �Thus, one mole of CO 2 = 44. 0 grams. �One mole of anything will contain Avogadro’s Number of particles. � 1 mol C = 6. 02 x 1023 atoms � 1 mol CO 2 = 6. 02 x 1023 molecules � 1 mol Na. Cl = 6. 02 x 1023 formula units

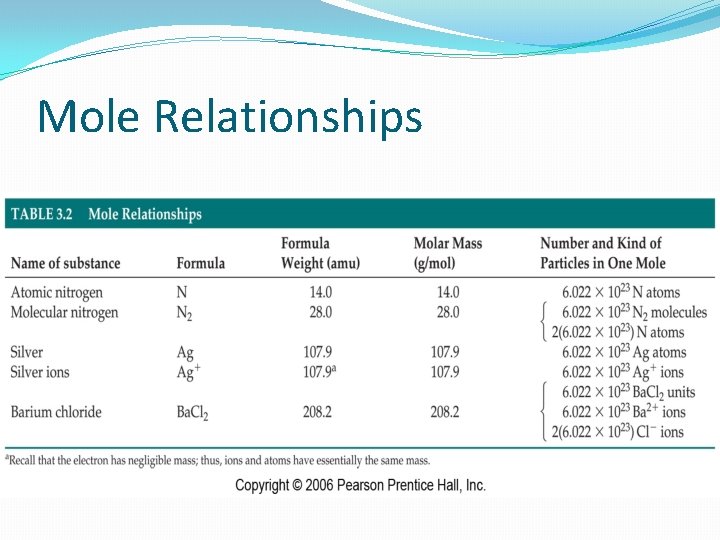
Mole Relationships

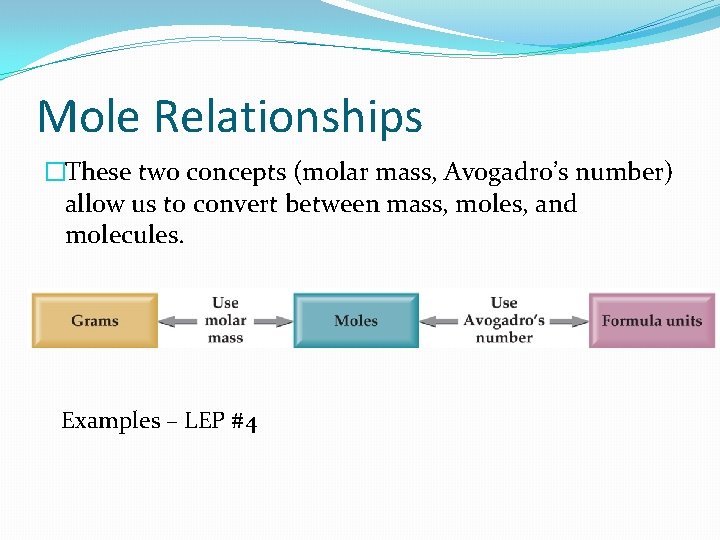
Mole Relationships �These two concepts (molar mass, Avogadro’s number) allow us to convert between mass, moles, and molecules. Examples – LEP #4

Empirical Formulas �We can use moles to find an empirical (simplest) formula from mass percentages by: 1. Assume a 100 gram sample (% grams). 2. Convert grams of each element to moles use the formula weights. 3. Divide each mole amount by the smallest one. 4. Using a multiplier to eliminate fractions like: 0. 25, 0. 33, 0. 50, 0. 67, and 0. 75.

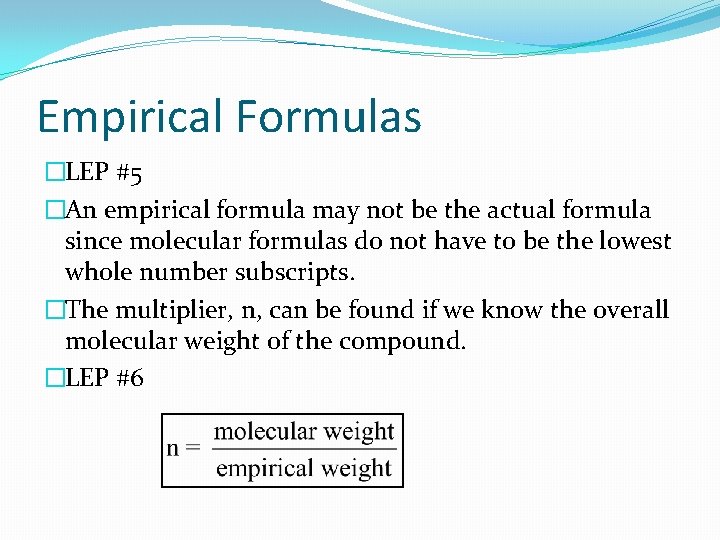
Empirical Formulas �LEP #5 �An empirical formula may not be the actual formula since molecular formulas do not have to be the lowest whole number subscripts. �The multiplier, n, can be found if we know the overall molecular weight of the compound. �LEP #6

Interpreting a Reaction �A simple reaction like: N 2(g) + 3 H 2(g) 2 NH 3(g), can be interpreted on many levels. �Molecular Level: one molecule of N 2 plus three molecules of H 2 react to form two molecules of NH 3

Interpreting a Reaction �For this reaction, we can establish that: 1 molecule N 2 = 3 molecules H 2 1 molecule N 2 = 2 molecules NH 3 3 molecules H 2 = 2 molecules NH 3 LEP #7

Interpreting a Reaction �The molecular level is really not practical as we cannot do reaction on this scale. �Rather, we can do them on a mole scale. �Thus: one mole of N 2 plus three moles of H 2 react to produce two moles of NH 3. �This means our relations can be shortened to moles. LEP #7

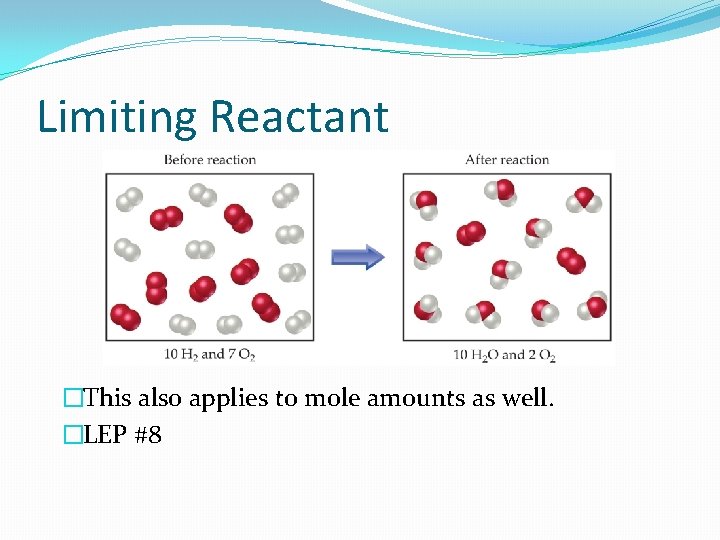
Limiting Reactant �If given amounts of both reactants, we may run out of one of them first. This reactant limits how much can be made. �Analogy: Putting together a bicycle – parts on hand are 200 frames and 350 wheels. How many bicycles can you make? �Ex) 2 H 2 + O 2 2 H 2 O �Suppose a vessel contained 10 molecules of H 2 and 7 molecules of O 2. How many water molecules are possible?

Limiting Reactant �This also applies to mole amounts as well. �LEP #8

Stoichiometry �Pronounced: stoy-key-OM-uh-tree. �Relating quantities in chemical reactions – in particular – masses. �Cannot use mole-to-mole ratios to convert mass of one substance to mass of another by one single step. �A mass-to-mass conversion must be done in three steps.

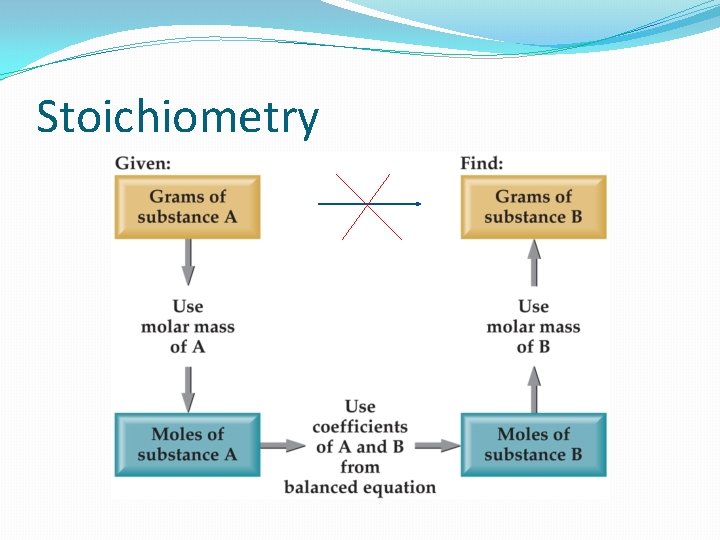
Stoichiometry

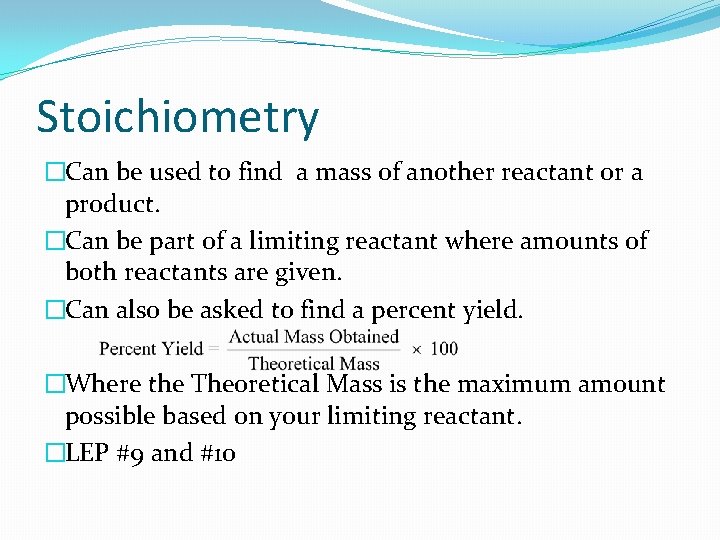
Stoichiometry �Can be used to find a mass of another reactant or a product. �Can be part of a limiting reactant where amounts of both reactants are given. �Can also be asked to find a percent yield. �Where the Theoretical Mass is the maximum amount possible based on your limiting reactant. �LEP #9 and #10
 Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Stoichiometric calculations
Stoichiometric calculations Tribromine octoxide formula
Tribromine octoxide formula Structural steel connection calculations calculations
Structural steel connection calculations calculations Chapter 9 chemical names and formulas
Chapter 9 chemical names and formulas Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas A chemical formula shows the types and
A chemical formula shows the types and Dosage calculation formulas
Dosage calculation formulas Dc shunt motor equations
Dc shunt motor equations Stoichiometry formulas
Stoichiometry formulas Stoichiometry formulas
Stoichiometry formulas How to find the moles
How to find the moles Mole ratio examples
Mole ratio examples Stoichiometry formulas
Stoichiometry formulas Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Stoichiometry refers to
Stoichiometry refers to Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chapter 11 stoichiometry answer key
Chapter 11 stoichiometry answer key