CC 512 Crystalline Structure CC 512 CC 512



- Slides: 46


/CC 512/

Crystalline Structure / CC 512 / 신소재과학개론 /CC 512/

Metals crystal structure • There are two main forms of solid substance, characterizing different atoms arrangement in their microstructures: amorphous and crystalline /CC 512/

Amorphous solid • Amorphous solid substance does not possess long-range order of atoms positions. • Some liquids when cooled become more and more viscous and then rigid, retaining random atom characteristic distribution. • This state is called undercooled liquid or amorphous solid. Common glass, most of Polymers, glues and some of Ceramics are amorphous solids. Some of the Metals may be prepared in amorphous solid form by rapid cooling from molten state. /CC 512/

Crystalline solid • Crystalline solid substance is characterized by atoms arranged in a regular pattern, extending in all three dimensions. • The crystalline structure is described in terms of crystal lattice, which is a lattice with atoms or ions attached to the lattice points. The smallest possible part of crystal lattice, determining the structure, is called primitive unit cell. /CC 512/

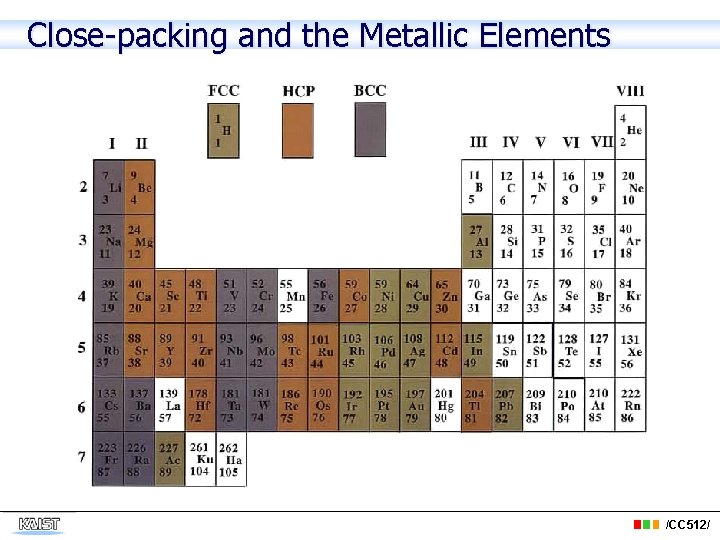
The following elements are common metals: • aluminum(Al), barium(Ba), beryllium (Be), bismuth(Bi), cadmium(Cd), calcium(Ca), cerium(Ce), cesium(Cs), chromium(Cr), cobalt(Co), copper(Cu), gold(Au), indium(In), iridium(Ir), iron(Fe), lead(Pb), lithium(Li), magnesium(Mg), manganese(Mn), mercury(Hg), molybdenum(Mo), nickel(Ni), osmium(Os), palladium(Pd), platinum(Pt), potassium(K), radium(Ra), rhodium(Rh), silver(Ag), sodium(Na), tantalum(Ta), thallium(Tl), thorium(Th), tin(Sn), titanium(Ti), tungsten(W), uranium(U), vanadium(V), • zinc(Zn). /CC 512/

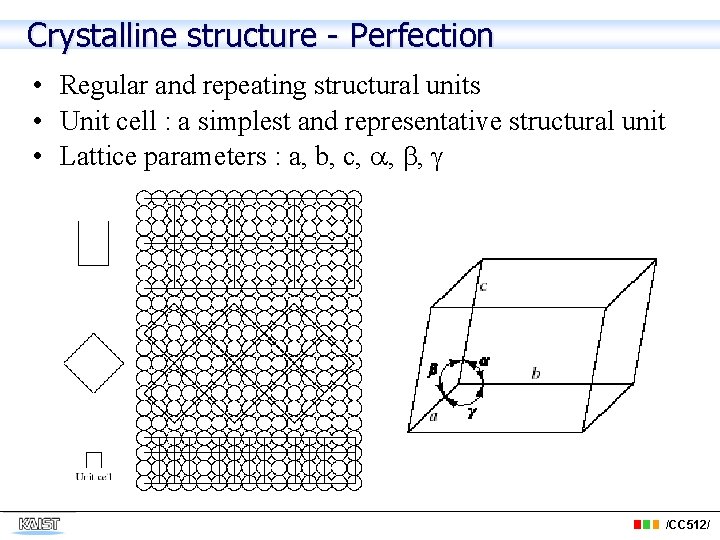
Crystalline structure - Perfection • Regular and repeating structural units • Unit cell : a simplest and representative structural unit • Lattice parameters : a, b, c, , , /CC 512/

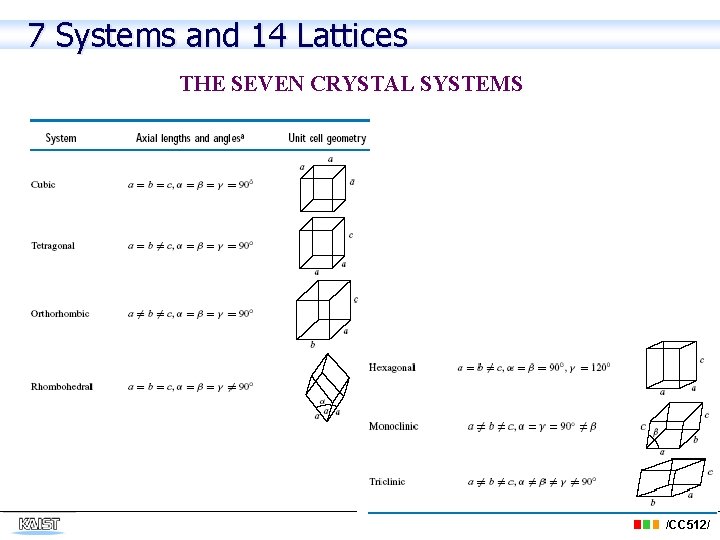
7 Systems and 14 Lattices THE SEVEN CRYSTAL SYSTEMS /CC 512/

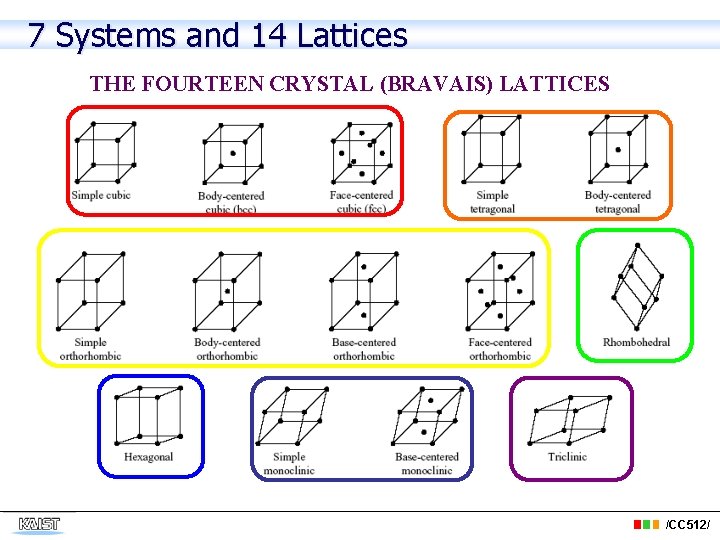
7 Systems and 14 Lattices THE FOURTEEN CRYSTAL (BRAVAIS) LATTICES /CC 512/

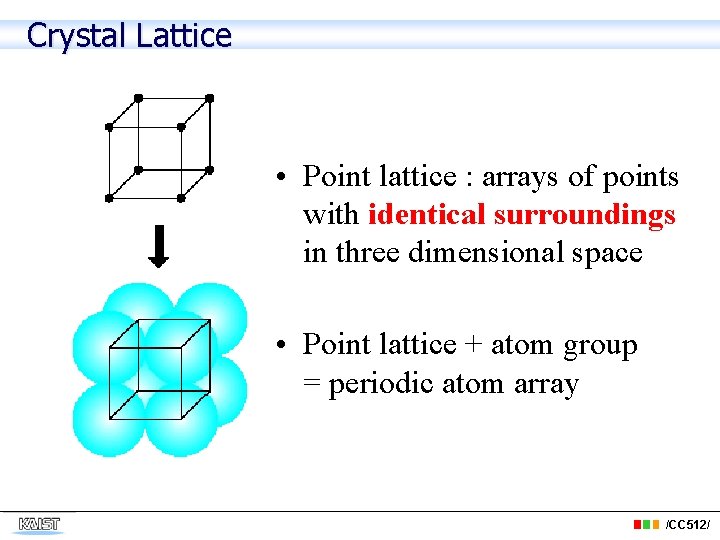
Crystal Lattice • Point lattice : arrays of points with identical surroundings in three dimensional space • Point lattice + atom group = periodic atom array /CC 512/

Crystal Lattice /CC 512/

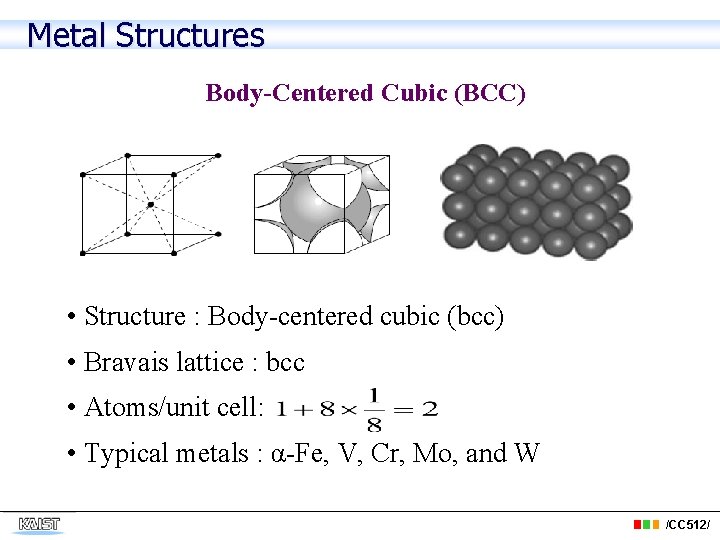
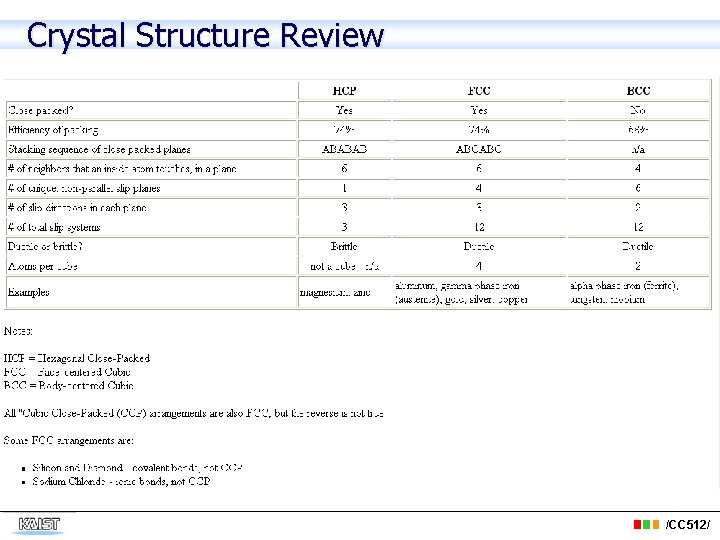
Metal Structures Body-Centered Cubic (BCC) • Structure : Body-centered cubic (bcc) • Bravais lattice : bcc • Atoms/unit cell: • Typical metals : α-Fe, V, Cr, Mo, and W /CC 512/

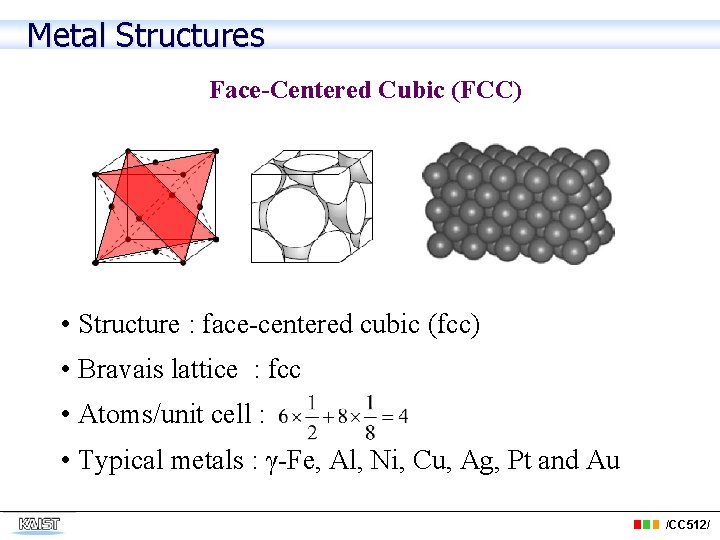
Metal Structures Face-Centered Cubic (FCC) • Structure : face-centered cubic (fcc) • Bravais lattice : fcc • Atoms/unit cell : • Typical metals : γ-Fe, Al, Ni, Cu, Ag, Pt and Au /CC 512/

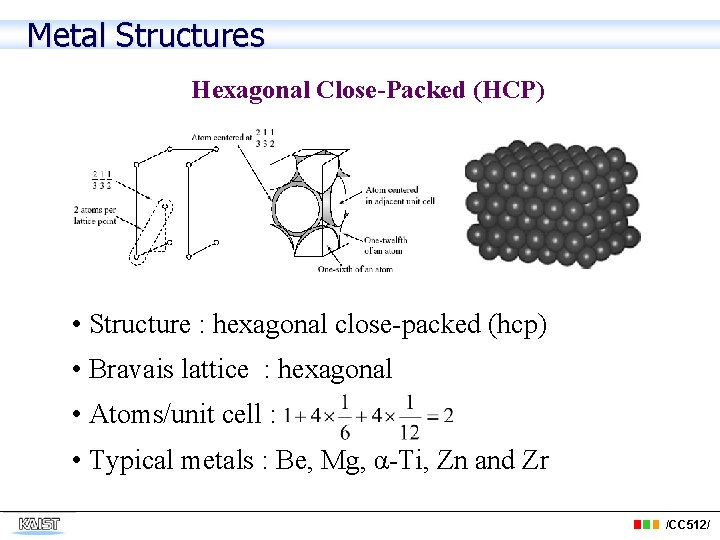
Metal Structures Hexagonal Close-Packed (HCP) • Structure : hexagonal close-packed (hcp) • Bravais lattice : hexagonal • Atoms/unit cell : • Typical metals : Be, Mg, α-Ti, Zn and Zr /CC 512/

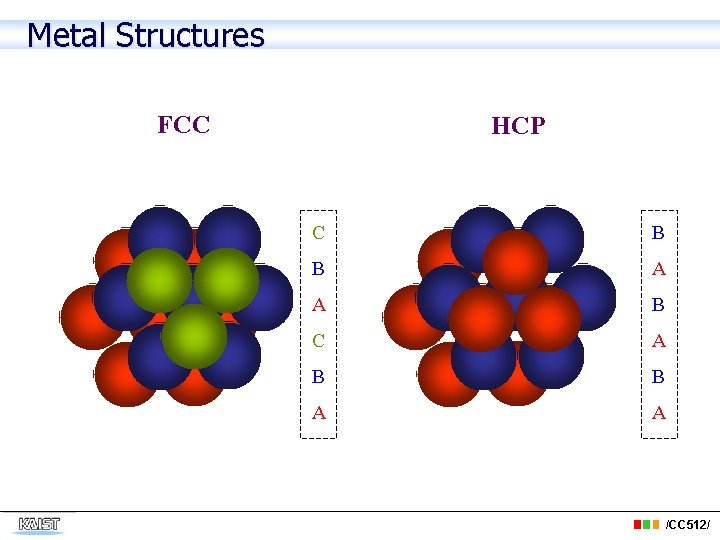
Metal Structures FCC HCP C B B A A B C A B B A A /CC 512/

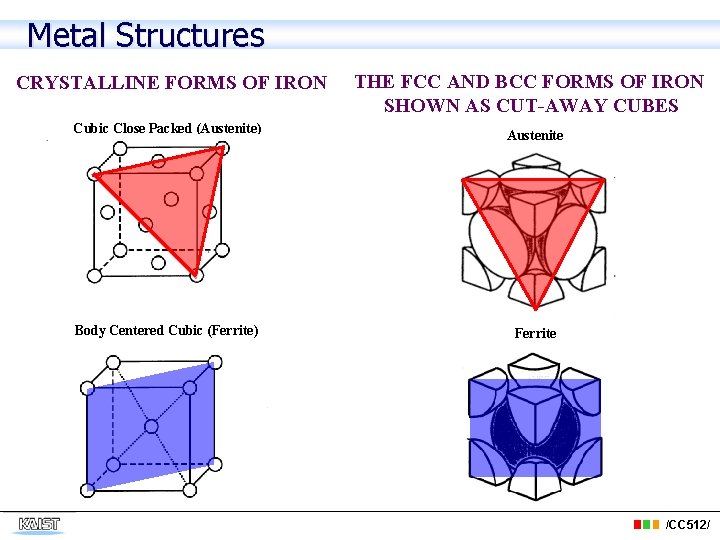
Metal Structures CRYSTALLINE FORMS OF IRON THE FCC AND BCC FORMS OF IRON SHOWN AS CUT-AWAY CUBES Cubic Close Packed (Austenite) Austenite Body Centered Cubic (Ferrite) Ferrite /CC 512/

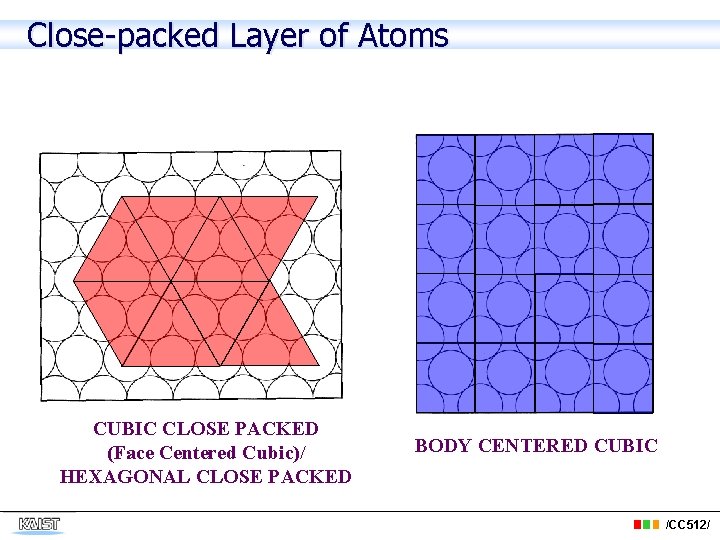
Close-packed Layer of Atoms CUBIC CLOSE PACKED (Face Centered Cubic)/ HEXAGONAL CLOSE PACKED BODY CENTERED CUBIC /CC 512/

Close-packing and the Metallic Elements /CC 512/

Crystal Structure Review /CC 512/

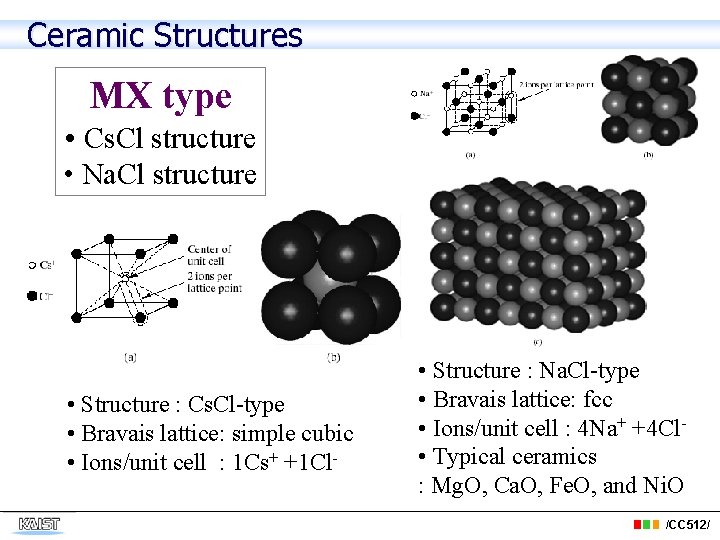
Ceramic Structures MX type • Cs. Cl structure • Na. Cl structure • Structure : Cs. Cl-type • Bravais lattice: simple cubic • Ions/unit cell : 1 Cs+ +1 Cl- • Structure : Na. Cl-type • Bravais lattice: fcc • Ions/unit cell : 4 Na+ +4 Cl • Typical ceramics : Mg. O, Ca. O, Fe. O, and Ni. O /CC 512/

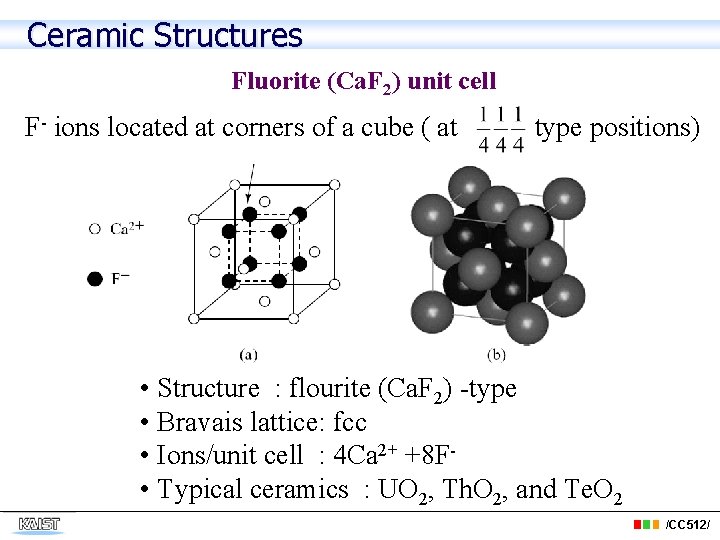
Ceramic Structures Fluorite (Ca. F 2) unit cell F- ions located at corners of a cube ( at type positions) • Structure : flourite (Ca. F 2) -type • Bravais lattice: fcc • Ions/unit cell : 4 Ca 2+ +8 F • Typical ceramics : UO 2, Th. O 2, and Te. O 2 /CC 512/

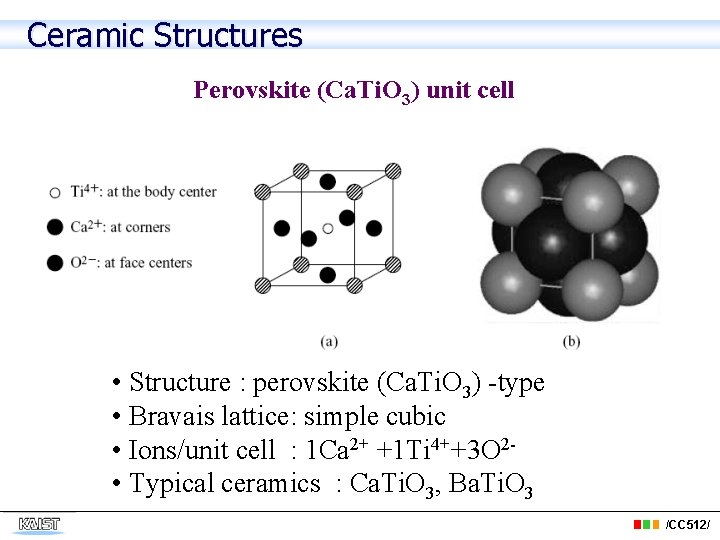
Ceramic Structures Perovskite (Ca. Ti. O 3) unit cell • Structure : perovskite (Ca. Ti. O 3) -type • Bravais lattice: simple cubic • Ions/unit cell : 1 Ca 2+ +1 Ti 4++3 O 2 • Typical ceramics : Ca. Ti. O 3, Ba. Ti. O 3 /CC 512/

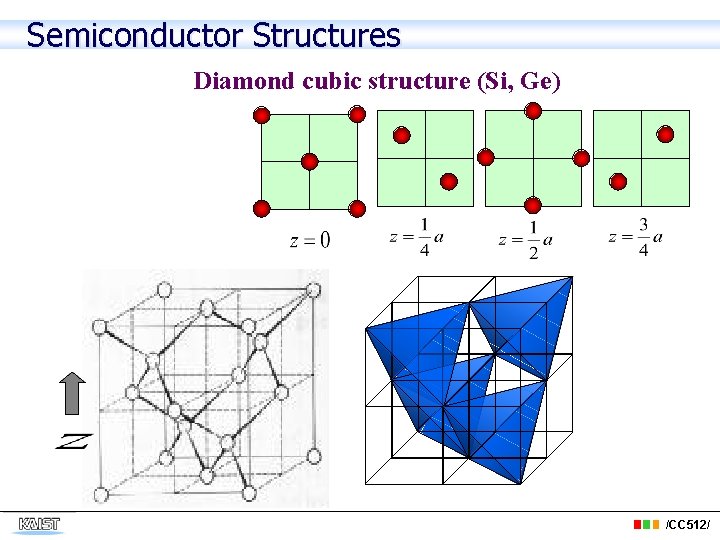
Semiconductor Structures Diamond cubic structure (Si, Ge) /CC 512/

Semiconductor Structures Diamond cubic structure (Si, Ge) /CC 512/

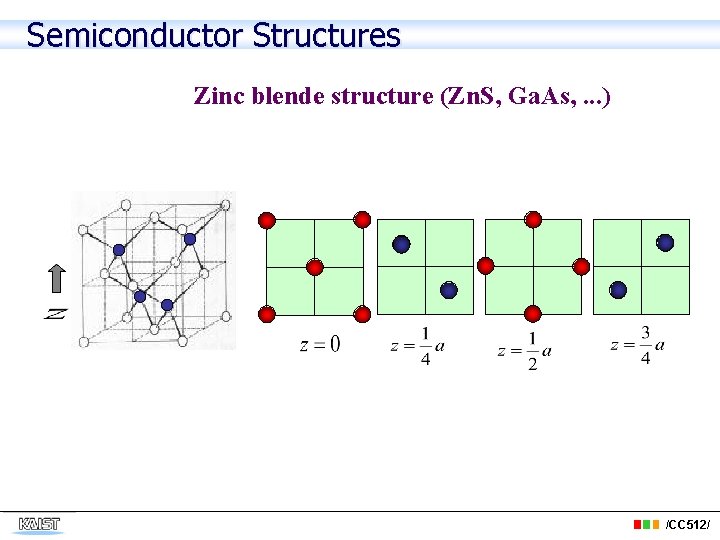
Semiconductor Structures Zinc blende structure (Zn. S, Ga. As, . . . ) /CC 512/

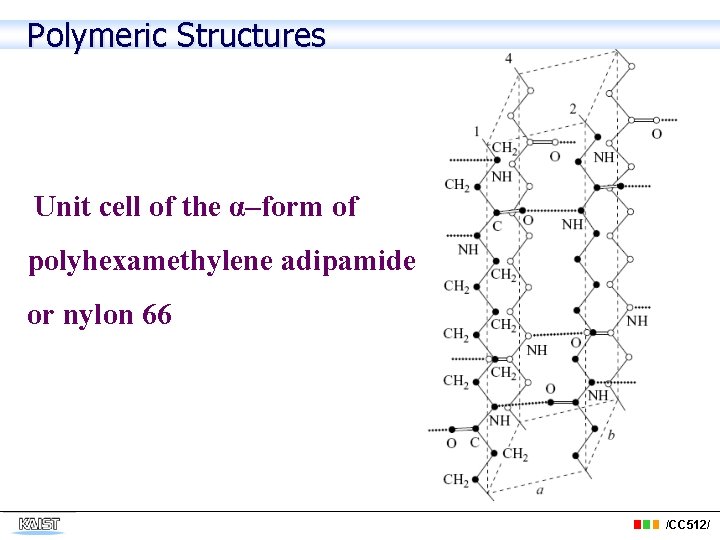
Polymeric Structures Unit cell of the α–form of polyhexamethylene adipamide or nylon 66 /CC 512/

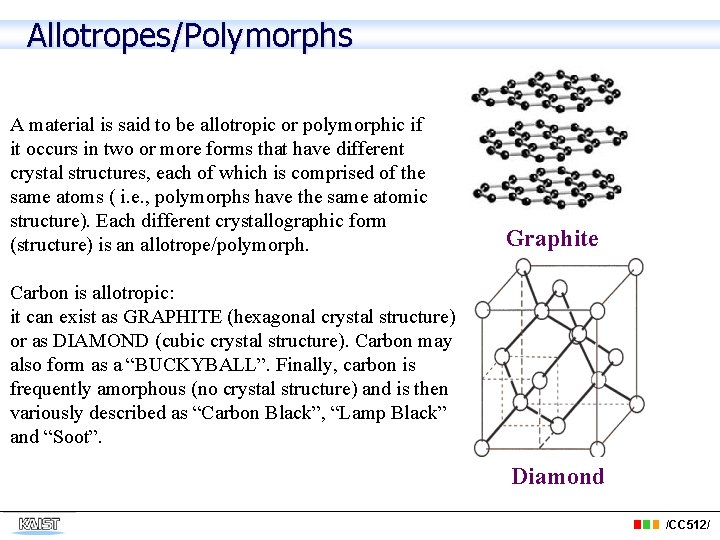
Allotropes/Polymorphs A material is said to be allotropic or polymorphic if it occurs in two or more forms that have different crystal structures, each of which is comprised of the same atoms ( i. e. , polymorphs have the same atomic structure). Each different crystallographic form (structure) is an allotrope/polymorph. Graphite Carbon is allotropic: it can exist as GRAPHITE (hexagonal crystal structure) or as DIAMOND (cubic crystal structure). Carbon may also form as a “BUCKYBALL”. Finally, carbon is frequently amorphous (no crystal structure) and is then variously described as “Carbon Black”, “Lamp Black” and “Soot”. Diamond /CC 512/

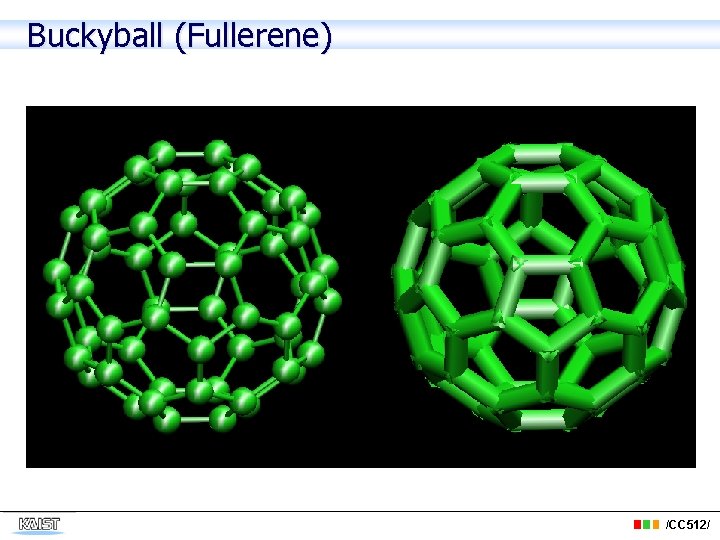
Buckyball (Fullerene) /CC 512/

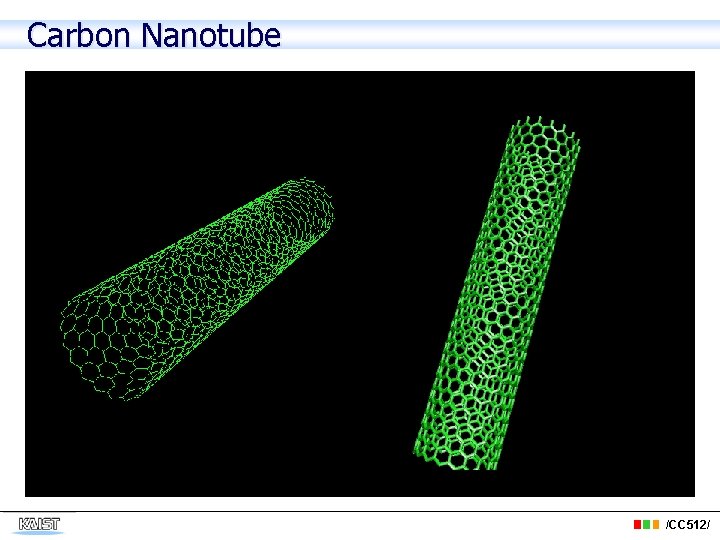
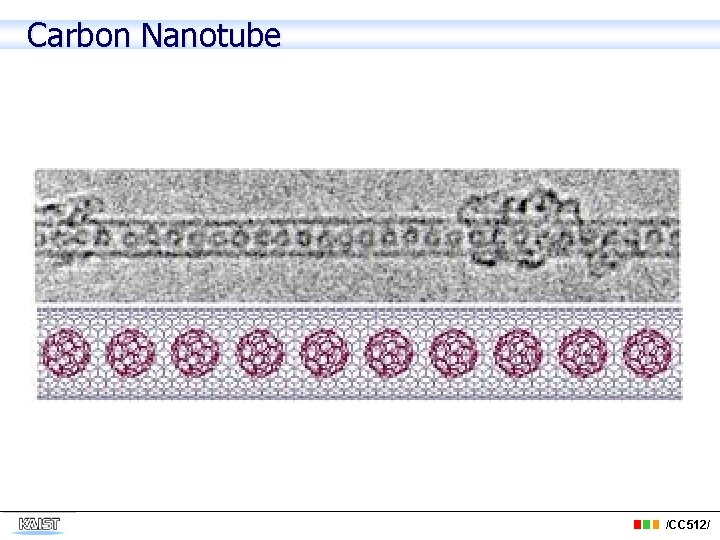
Carbon Nanotube /CC 512/

Carbon Nanotube /CC 512/

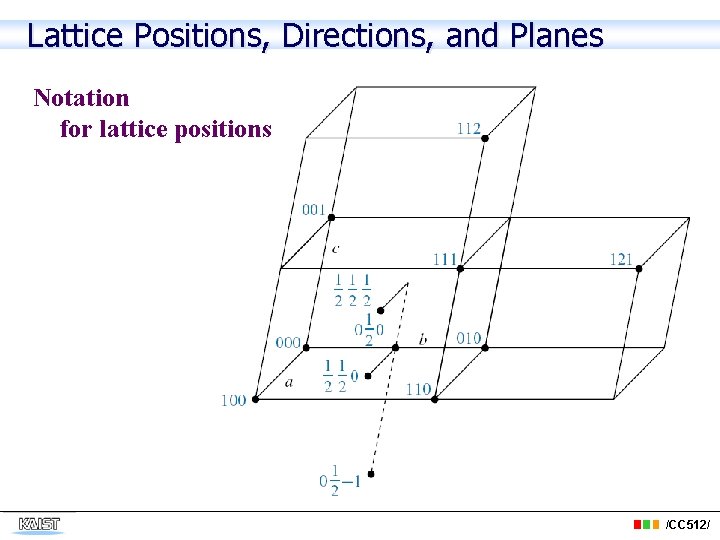
Lattice Positions, Directions, and Planes Notation for lattice positions /CC 512/

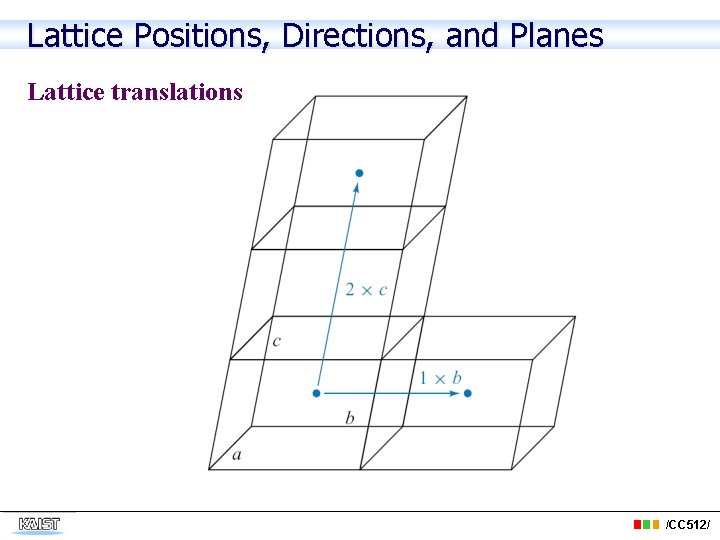
Lattice Positions, Directions, and Planes Lattice translations /CC 512/

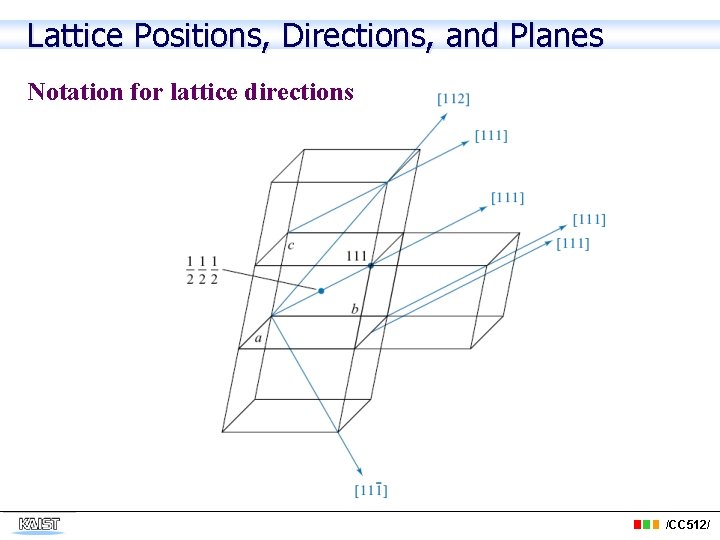
Lattice Positions, Directions, and Planes Notation for lattice directions /CC 512/

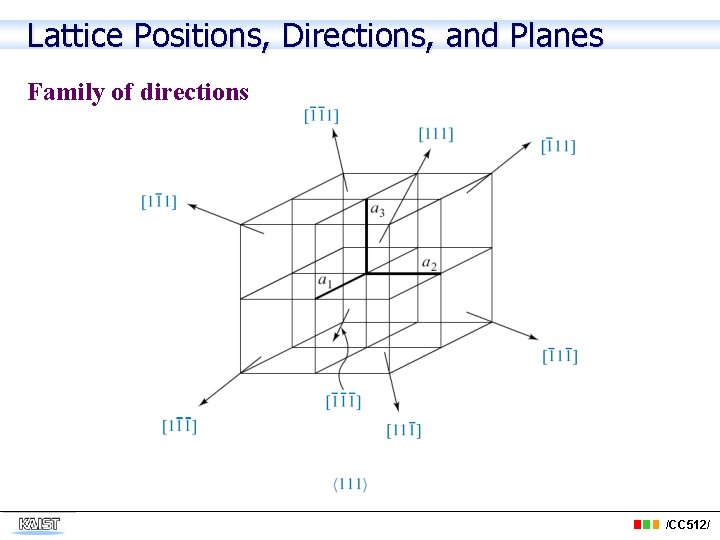
Lattice Positions, Directions, and Planes Family of directions /CC 512/

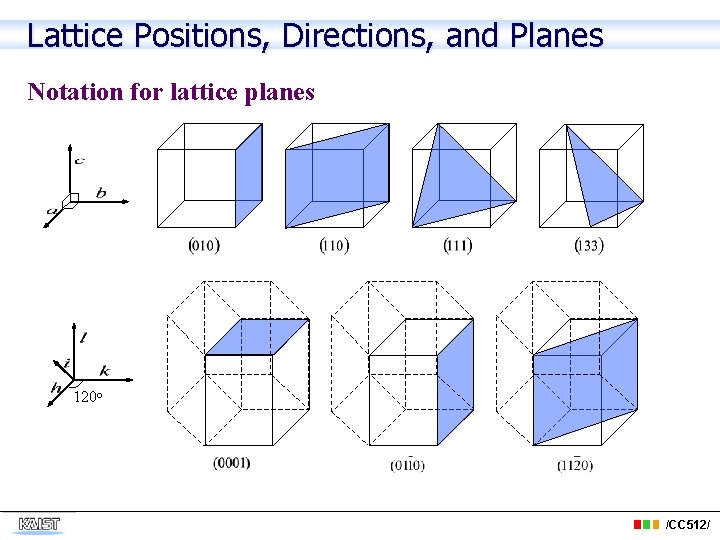
Lattice Positions, Directions, and Planes Notation for lattice planes 120 o /CC 512/

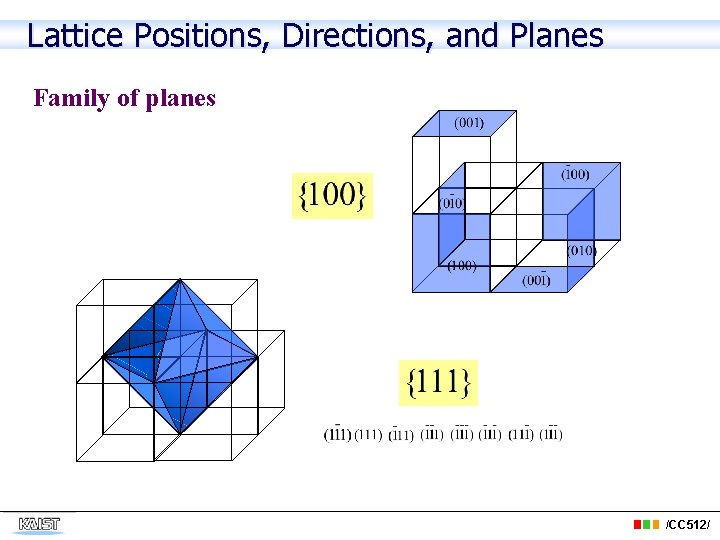
Lattice Positions, Directions, and Planes Family of planes /CC 512/

Single Crystalline / Polycrystalline /CC 512/

X-Ray Diffraction • How to identify unknown crystalline materials? • How to determine crystal structure? § X-ray Diffraction § Transmission electron diffraction by TEM /CC 512/

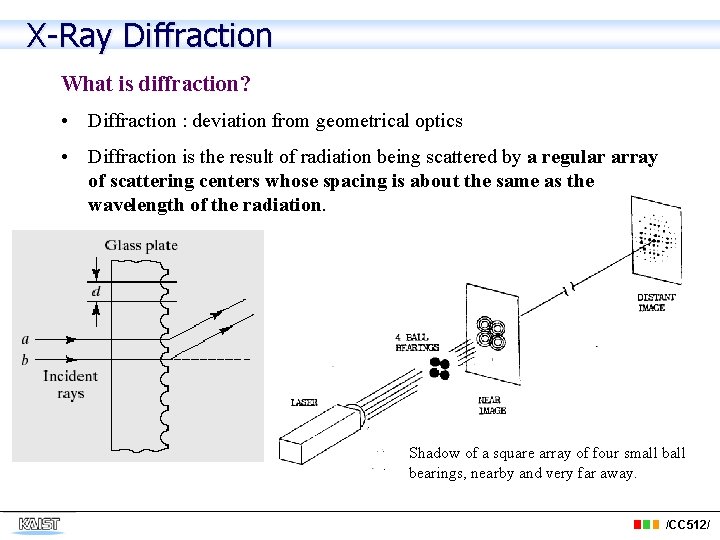
X-Ray Diffraction What is diffraction? • Diffraction : deviation from geometrical optics • Diffraction is the result of radiation being scattered by a regular array of scattering centers whose spacing is about the same as the wavelength of the radiation. Shadow of a square array of four small bearings, nearby and very far away. /CC 512/

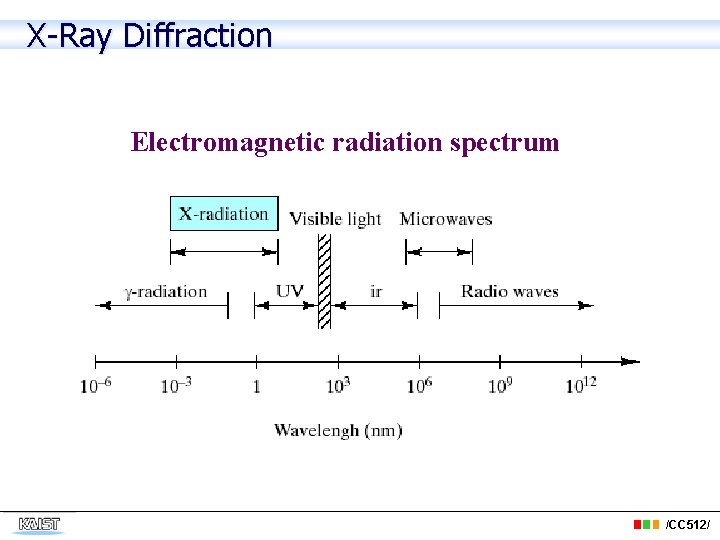
X-Ray Diffraction Electromagnetic radiation spectrum /CC 512/

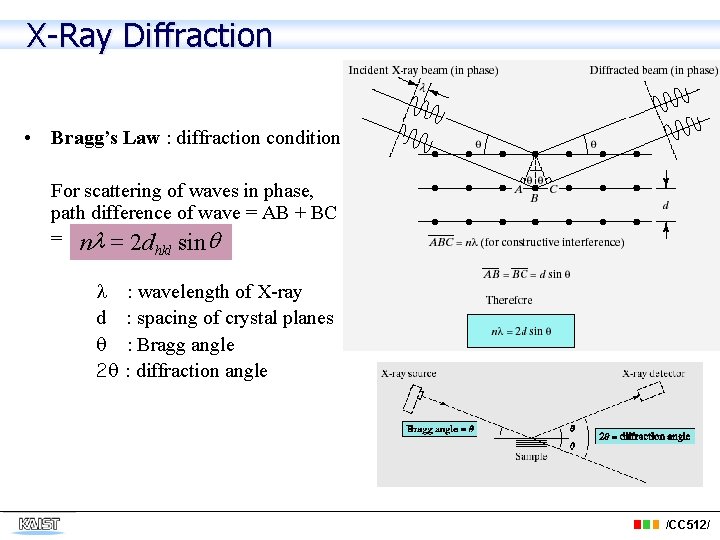
X-Ray Diffraction • Bragg’s Law : diffraction condition For scattering of waves in phase, path difference of wave = AB + BC = nl = 2 d hkl sin d 2 : wavelength of X-ray : spacing of crystal planes : Bragg angle : diffraction angle /CC 512/

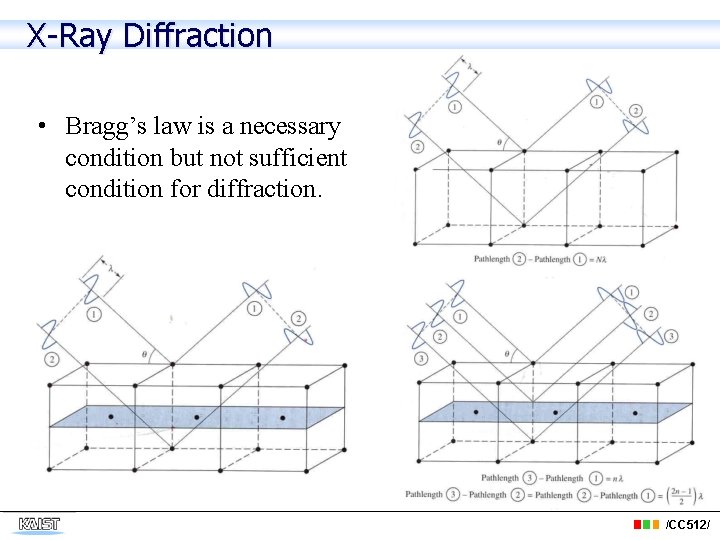
X-Ray Diffraction • Bragg’s law is a necessary condition but not sufficient condition for diffraction. /CC 512/

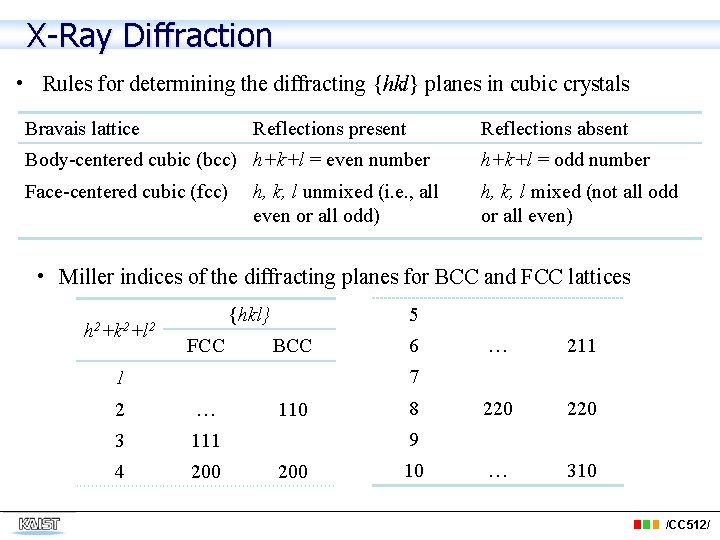
X-Ray Diffraction • Rules for determining the diffracting {hkl} planes in cubic crystals Bravais lattice Reflections present Reflections absent Body-centered cubic (bcc) h+k+l = even number h+k+l = odd number Face-centered cubic (fcc) h, k, l mixed (not all odd or all even) h, k, l unmixed (i. e. , all even or all odd) • Miller indices of the diffracting planes for BCC and FCC lattices h 2+k 2+l 2 5 {hkl} FCC BCC 6 … 211 220 … 310 7 1 2 … 3 111 4 200 110 8 9 200 10 /CC 512/

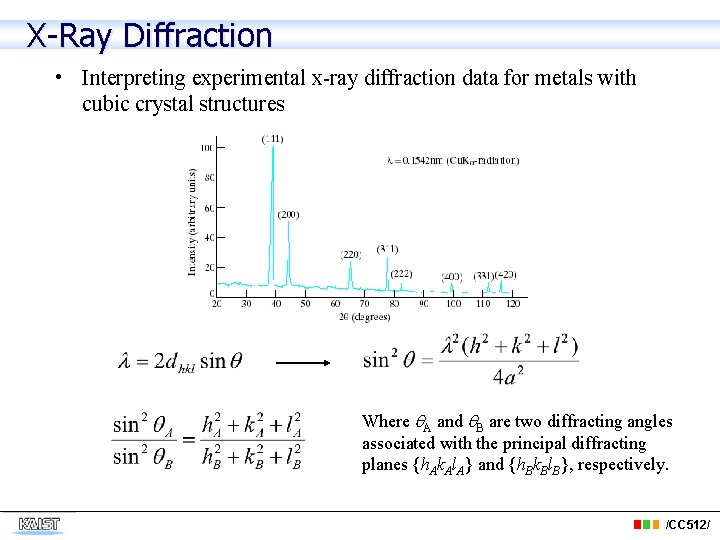
X-Ray Diffraction • Interpreting experimental x-ray diffraction data for metals with cubic crystal structures Where A and B are two diffracting angles associated with the principal diffracting planes {h. Ak. Al. A} and {h. Bk. Bl. B}, respectively. /CC 512/

X-Ray Diffraction ex) An x-ray diffractometer recorder chart for an element which has either the BCC or the FCC crystal structure shows diffraction peaks at the following 2 angles: 40, 58, 86. 8, 100. 4, and 114. 7. The wavelength of the incoming x-ray used was 0. 154 nm. (a) Determine the cubic structure of the element. (b) Determine the lattice constant of the element. (c) Identify the element. /CC 512/