Atomic Mass and the Mole Relative Atomic Mass







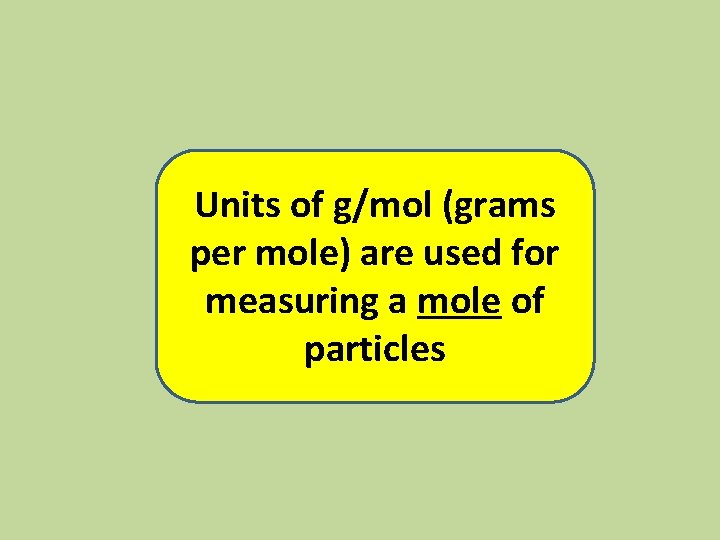

















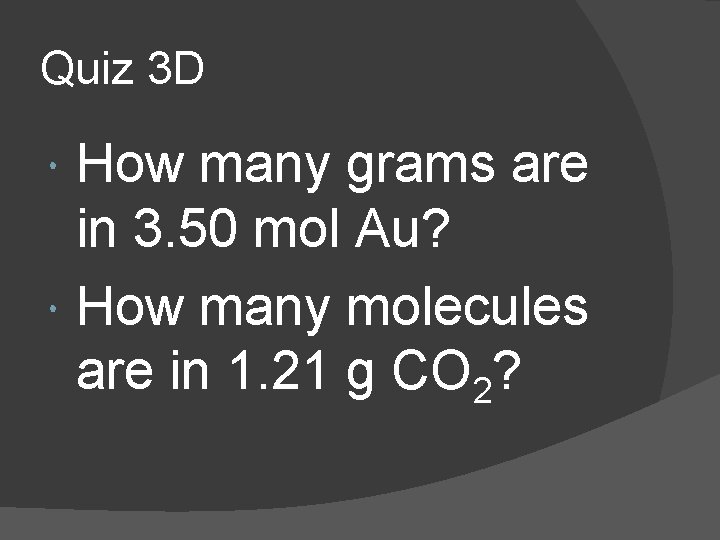
- Slides: 42

Atomic Mass and the Mole

Relative Atomic Mass • Units of grams are TOO LARGE for atoms! • Relative atomic mass – compare to small particles – amu – “atomic mass units”

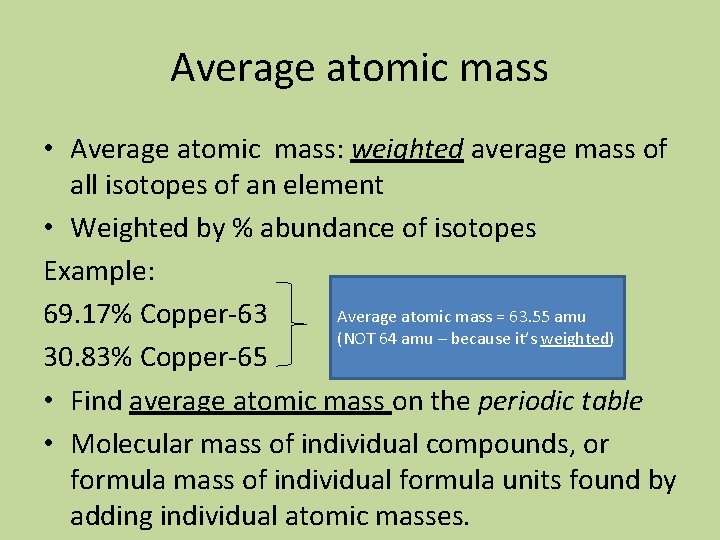
Average atomic mass • Average atomic mass: weighted average mass of all isotopes of an element • Weighted by % abundance of isotopes Example: Average atomic mass = 63. 55 amu 69. 17% Copper-63 (NOT 64 amu – because it’s weighted) 30. 83% Copper-65 • Find average atomic mass on the periodic table • Molecular mass of individual compounds, or formula mass of individual formula units found by adding individual atomic masses.

Units of amu are used for measuring single atoms, molecules, or formula units


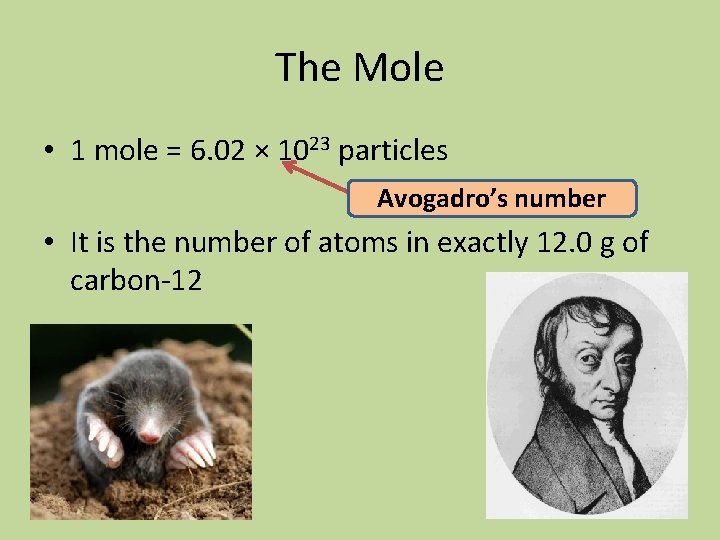
The Mole • 1 mole = 6. 02 × 1023 particles Avogadro’s number • It is the number of atoms in exactly 12. 0 g of carbon-12

What is a mole? • A counting number (like a dozen) – If you have a dozen cookies, how many do you have? – If you have a mole of cookies, how many do you have? – If you have 3. 5 dozen cookies, how many do you have? – If you have 3. 5 moles of cookies, how many do you have?

How big is a mole? • It is HUGE. If every person on earth counted A mole of basketballs out atoms during his or her entire life, would countingfill anfour atombags every the second, it would 3 million years to sizetake of the earth. count out a mole of atoms.

Molar Mass • • Mass of one mole of a substance Units are g/mol Find molar mass on periodic table Molar mass of compounds found by adding individual molar masses
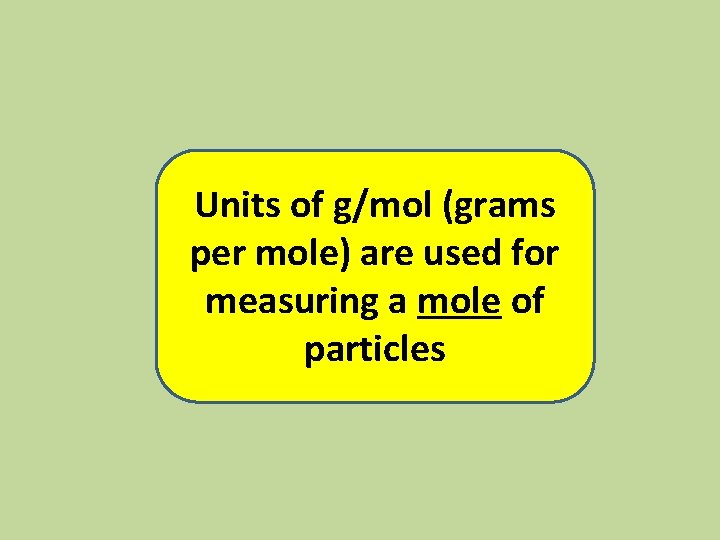
Units of g/mol (grams per mole) are used for measuring a mole of particles

Practice • What is the molar mass of each of the following elements? – Sodium (Na) – Nickel (Ni) – Xenon (Xe)

Practice • What is the molar mass of each of the following compounds? – H 2 O – NH 3 – C 2 H 6 O

REMEMBER: Atomic mass and molar mass are the same number, just different units.

ATOMS: THE BUILDING BLOCKS OF MATTER

Late 1700 s – Study of reactions led to new ideas Law of conservation of mass: Mass can’t be created or destroyed by ordinary chemical or physical reactions Law of definite proportions: A compound contains the same proportions of mass regardless of sample size Ex. Na. Cl will always be 39. 9% Na and 60. 1% Cl Law of multiple proportions: If two or more compounds of the same two elements exist, then the ratio of the masses are ratios of whole numbers Ex. C and O can combine to form both CO and CO 2

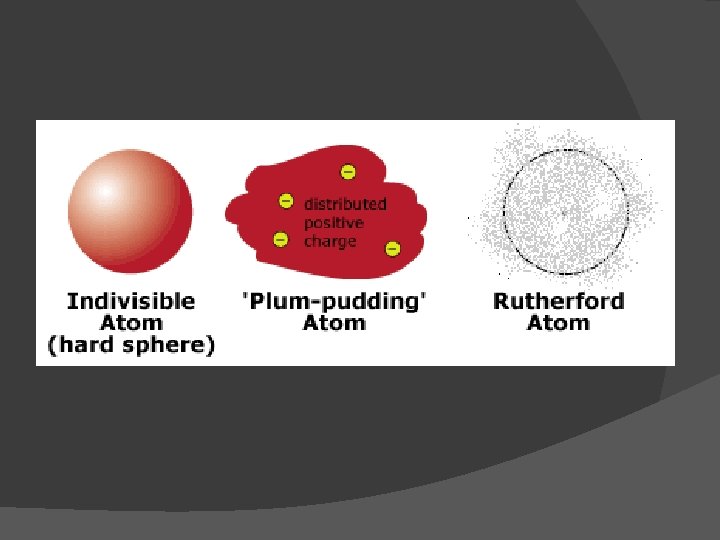
John Dalton Billiard ball model (1803) John Dalton viewed the atom as a small solid sphere.

John Dalton’s Atomic Theory: (Early 1800 s) 1. 2. 3. 4. 5. All matter is made of atoms The same elements have exactly the same atoms. In other words, all atoms of an element are identical Atoms cannot be divided, created, or destroyed Atoms of different elements combine in whole-number ratios to form compounds Atoms are combined, separated, or rearranged in chemical reactions

Modern Atomic Theory (slight changes to Dalton’s theory) 2. Atoms of the same element CAN be different (isotopes and ions) 3. Atoms CAN be divided – protons, neutrons, and electrons – nuclear reactions can split an atom

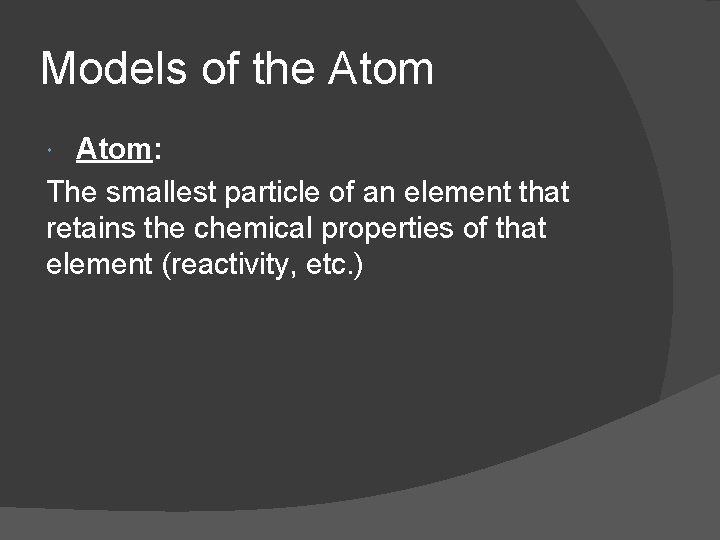
Models of the Atom: The smallest particle of an element that retains the chemical properties of that element (reactivity, etc. )

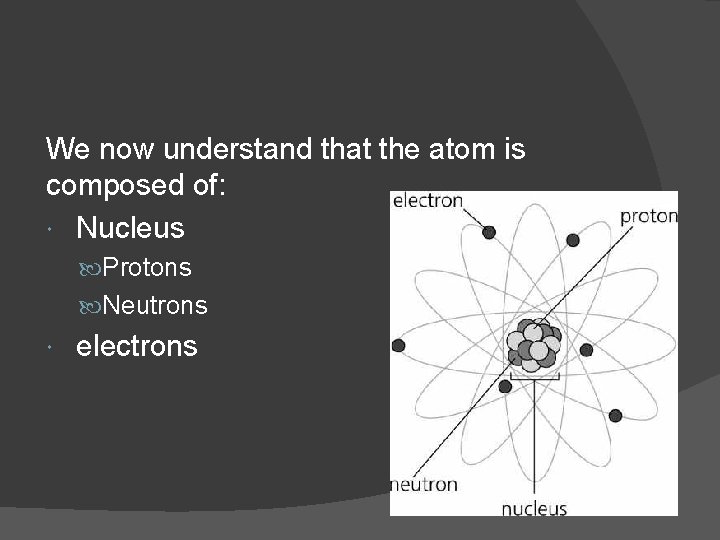
We now understand that the atom is composed of: Nucleus Protons Neutrons electrons

Discovery of the Electron

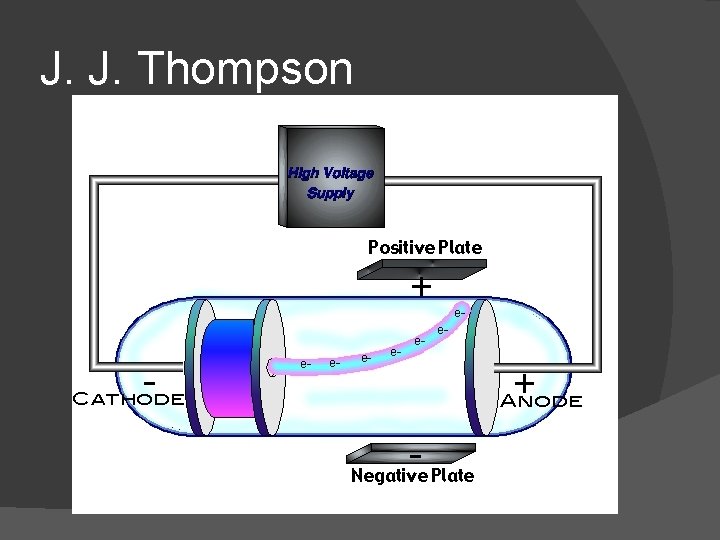
J. J. Thompson

J. J. Thomson Experiment: Cathode ray tube (1897) Discovered electron!!! Measured charge-to-mass ratio of electron Electron is negatively charged

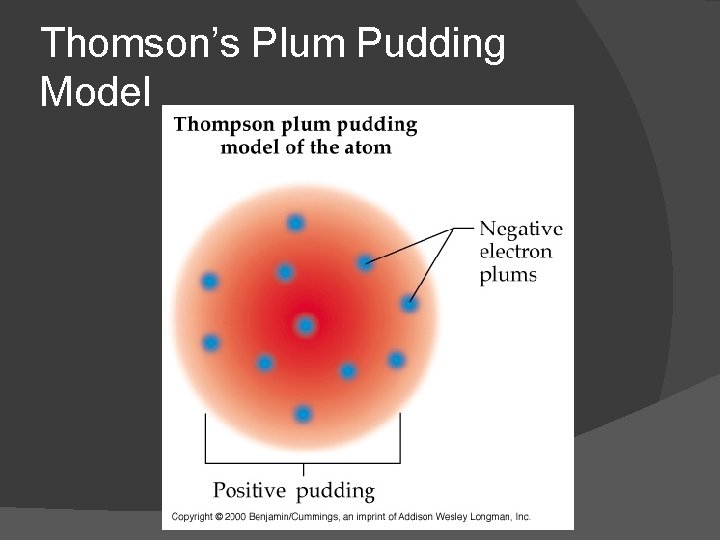
Thomson’s Plum Pudding Model


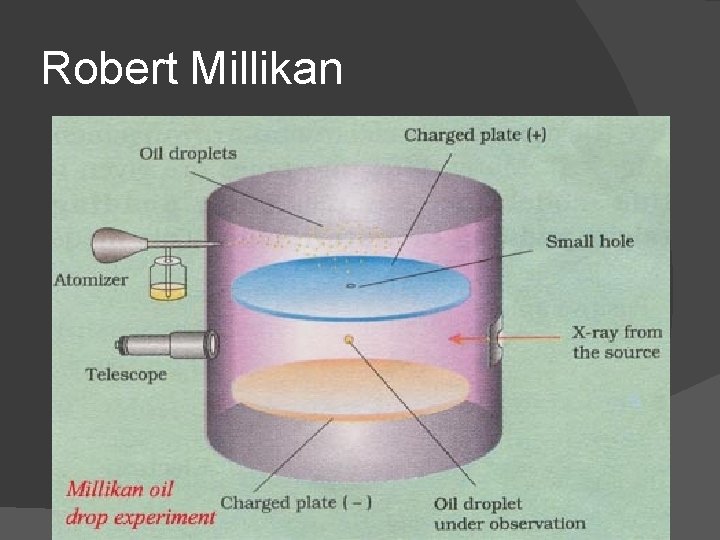
Robert Millikan

Robert Millikan Experiment: Oil drop experiment (1909) Measured charge of electron Both charge of electron and chargeto-mass ratio were used to determine the mass of an electron Mass of electron is 1/1837 mass of hydrogen atom

Discovery of the nucleus

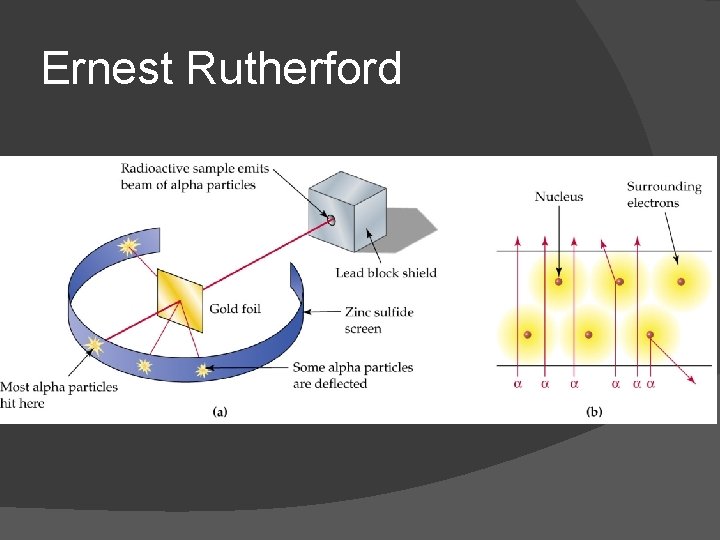
Ernest Rutherford

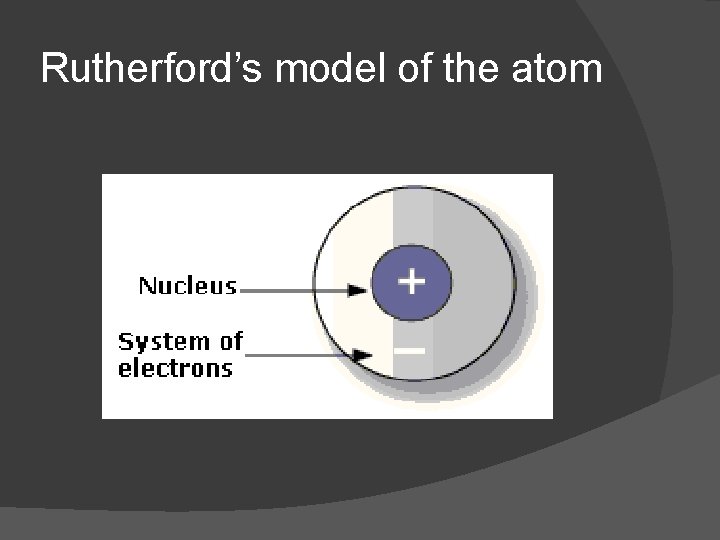
Ernest Rutherford Experiment: Gold foil experiment Discovered nucleus!!!! All mass of an atom is in the nucleus Nucleus is VERY massive


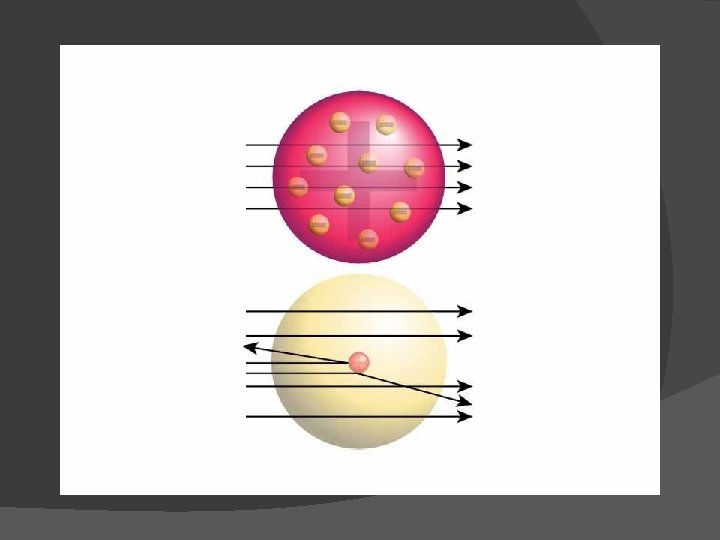
Rutherford’s model of the atom


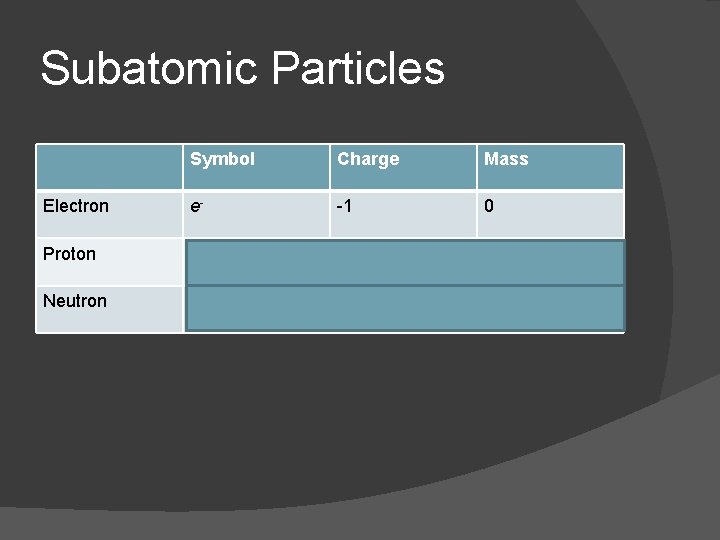
Subatomic Particles Symbol Charge Mass Electron e- -1 0 Proton p+ +1 1 Neutron n 0 0 1

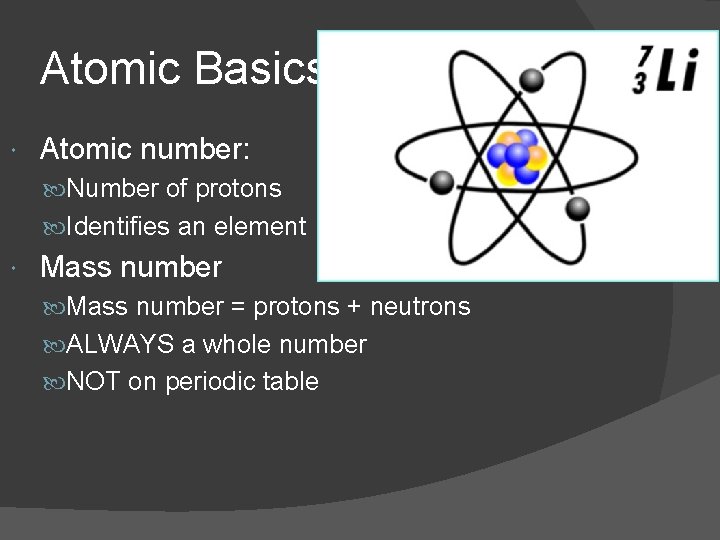
Atomic Basics Atomic number: Number of protons Identifies an element Mass number = protons + neutrons ALWAYS a whole number NOT on periodic table

A proton and neutron have about the same mass. An electron is about 1/2000 the mass of a proton A neutral atom has the same number of electrons as it has protons Nuclei are held together by nuclear forces The electrons determine the size of the atom Electrons move so fast in such a tiny area they make the atom seem solid (like a moving fan blade)

Isotopes Atoms of the same element with different masses. In other words, atoms with the same number of protons but different number of neutrons

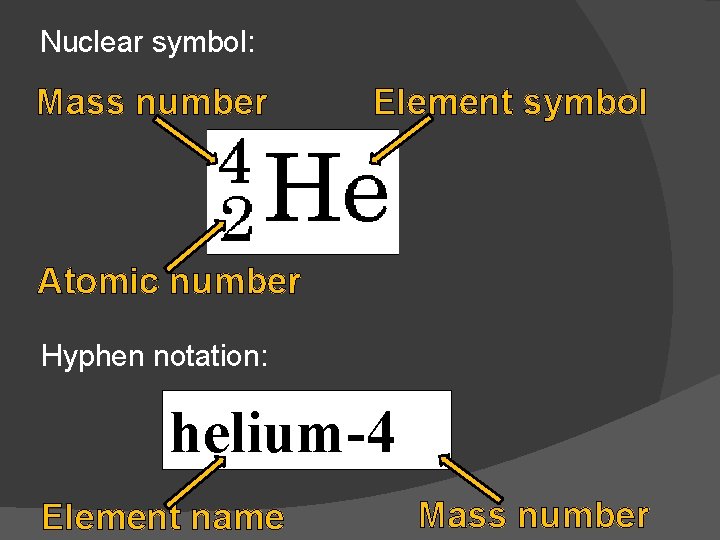
Nuclear symbol: Mass number Element symbol Atomic number Hyphen notation: helium-4 Element name Mass number

Quiz 3 A What is the atomic mass of fluorine (F)? 2. What is the molar mass of Tin (Sn)? 3. What is the molar mass of magnesium chloride (Mg. Cl 2)? 4. What is the molecular mass (mass of one molecule) of propane (C 3 H 8)? 1. Show all work for all calculations!

Quiz 3 B

Quiz 3 C
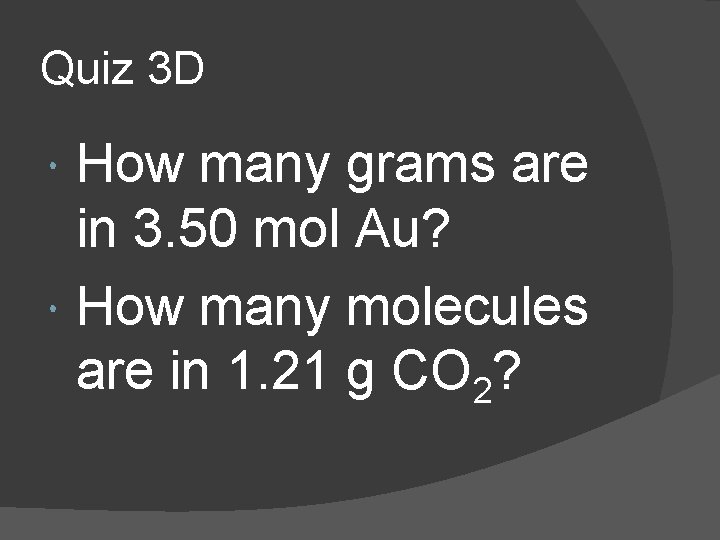
Quiz 3 D How many grams are in 3. 50 mol Au? How many molecules are in 1. 21 g CO 2?