Chemical Calculations Relative Atomic Mass The relative atomic





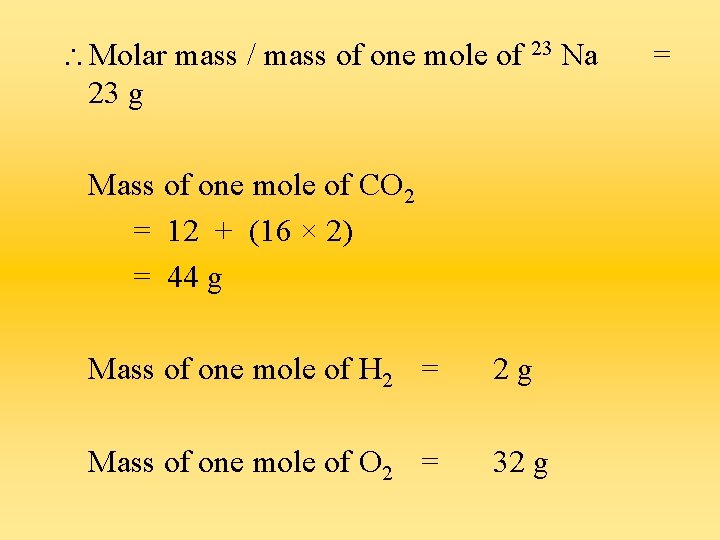

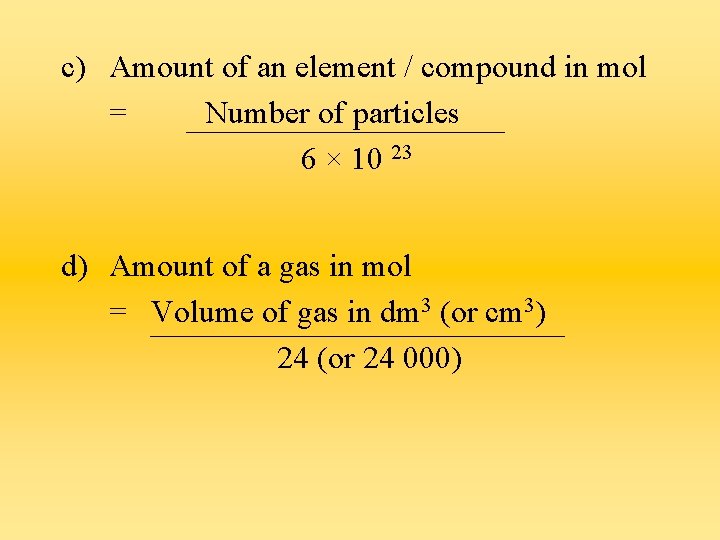
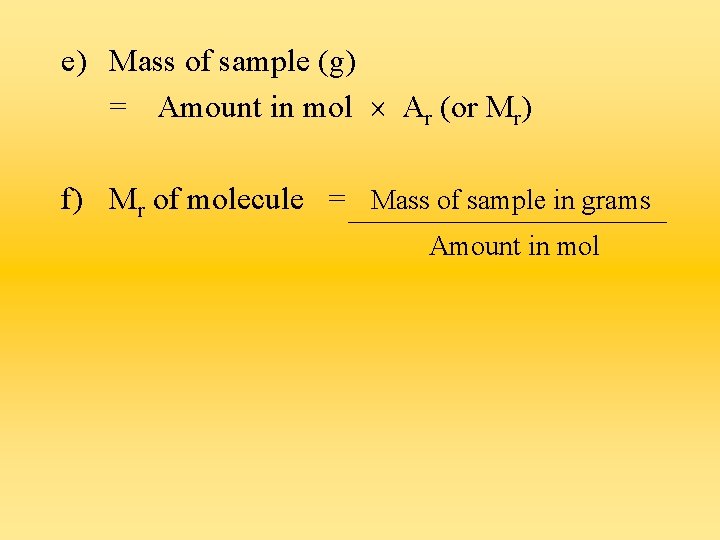


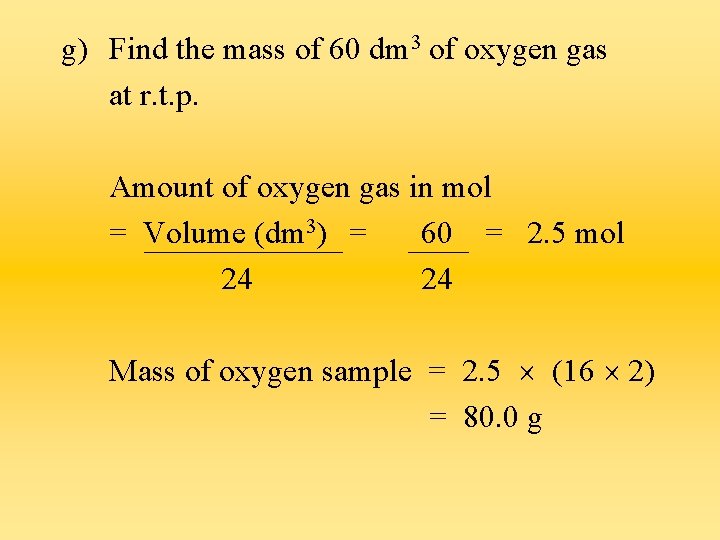
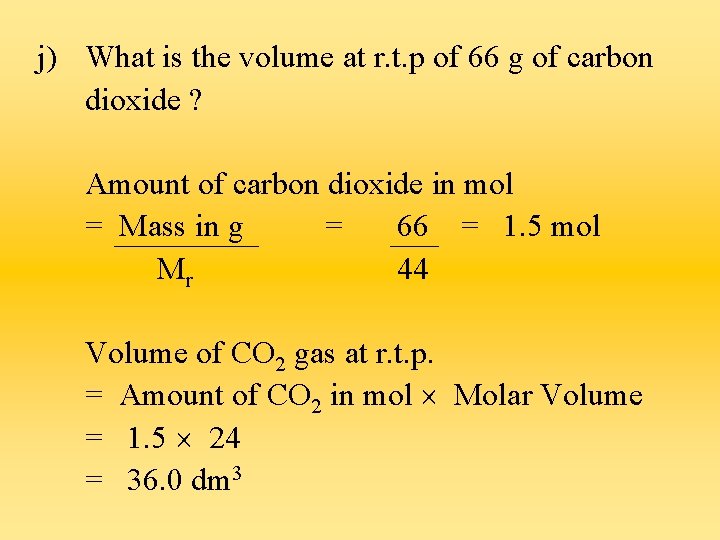














- Slides: 75

Chemical Calculations

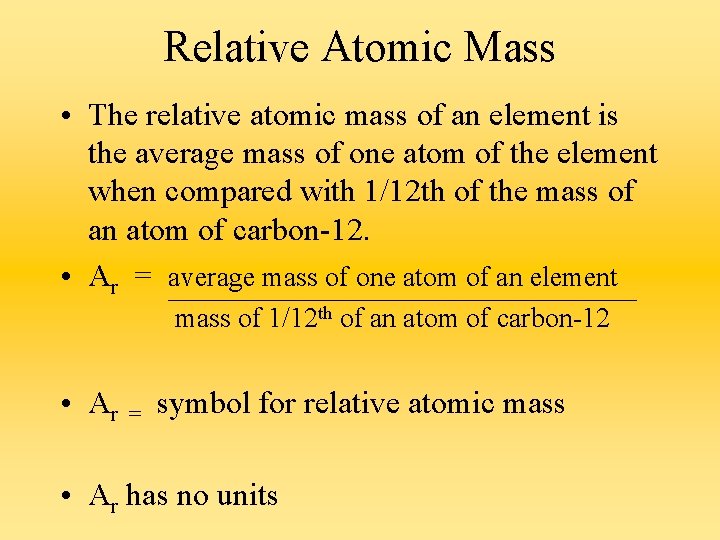
Relative Atomic Mass • The relative atomic mass of an element is the average mass of one atom of the element when compared with 1/12 th of the mass of an atom of carbon-12. • Ar = average mass of one atom of an element mass of 1/12 th of an atom of carbon-12 • Ar = symbol for relative atomic mass • Ar has no units

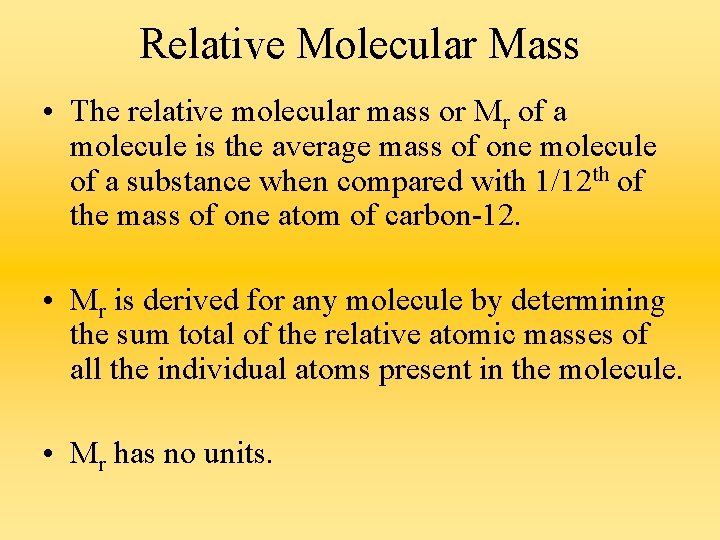
Relative Molecular Mass • The relative molecular mass or Mr of a molecule is the average mass of one molecule of a substance when compared with 1/12 th of the mass of one atom of carbon-12. • Mr is derived for any molecule by determining the sum total of the relative atomic masses of all the individual atoms present in the molecule. • Mr has no units.

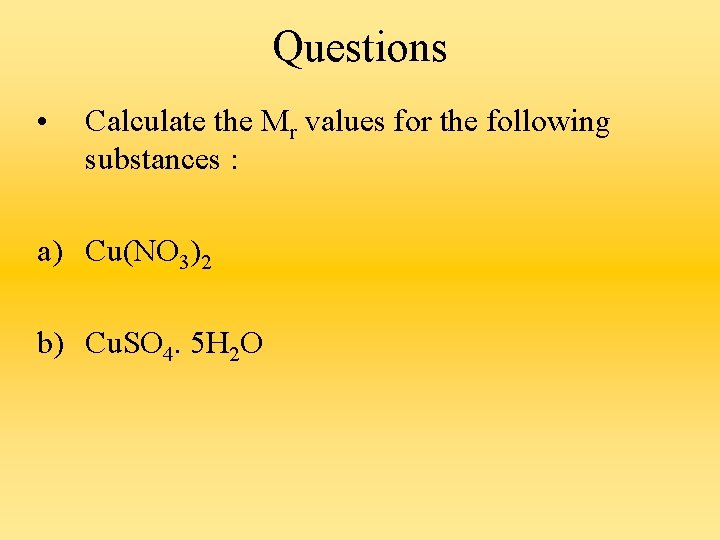
Questions • Calculate the Mr values for the following substances : a) Cu(NO 3)2 b) Cu. SO 4. 5 H 2 O

a) Mr of Cu(NO 3)2 = 64 + 2(14 + (16 × 3) ) = 188 b) Mr of Cu. SO 4. 5 H 2 O = 64 + 32 + (16 × 4) + 5((2 × 1) + 16) = 250

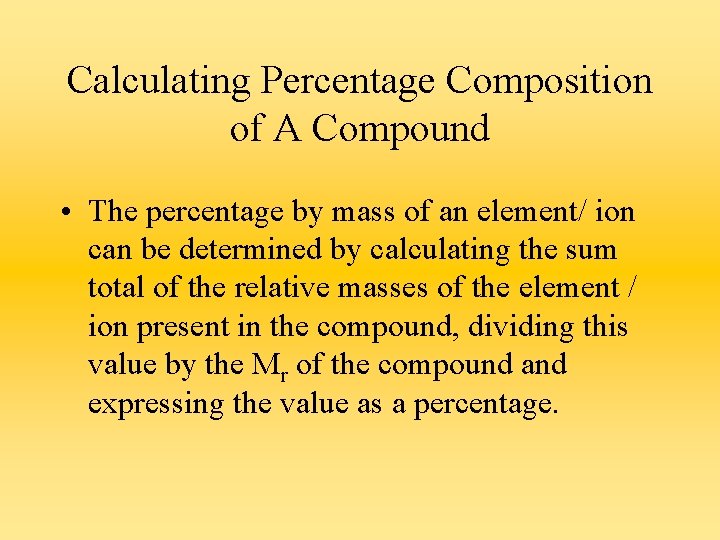
Calculating Percentage Composition of A Compound • The percentage by mass of an element/ ion can be determined by calculating the sum total of the relative masses of the element / ion present in the compound, dividing this value by the Mr of the compound and expressing the value as a percentage.

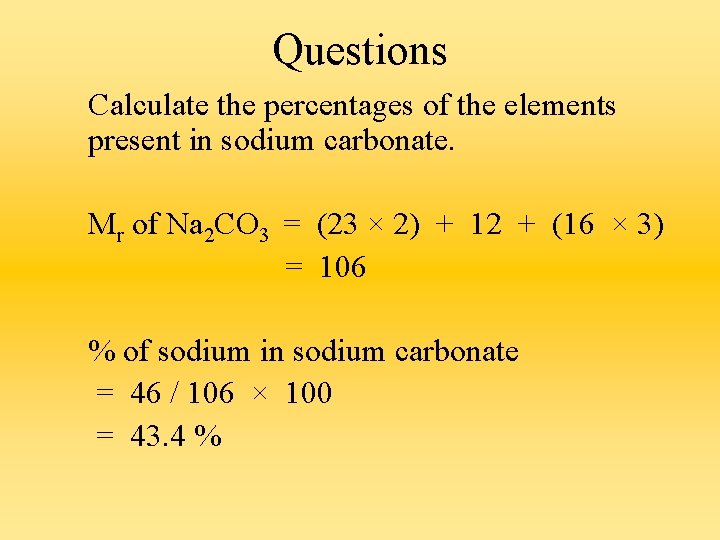
Questions Calculate the percentages of the elements present in sodium carbonate. Mr of Na 2 CO 3 = (23 × 2) + 12 + (16 × 3) = 106 % of sodium in sodium carbonate = 46 / 106 × 100 = 43. 4 %

% of carbon in sodium carbonate = 12 / 106 × 100 = 11. 3 % % of oxygen in sodium carbonate = 48 / 106 × 100 = 45. 3 % OR % of oxygen in sodium carbonate = 100 – 43. 4 – 11. 3 = 45. 3 %

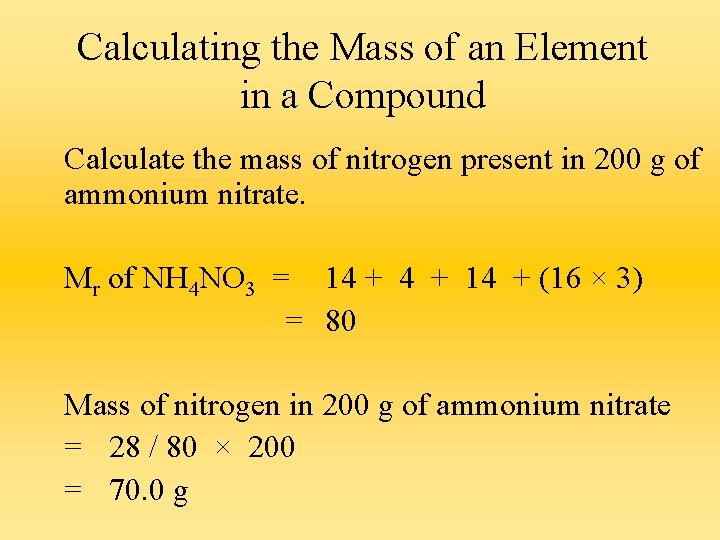
Calculating the Mass of an Element in a Compound Calculate the mass of nitrogen present in 200 g of ammonium nitrate. Mr of NH 4 NO 3 = 14 + (16 × 3) = 80 Mass of nitrogen in 200 g of ammonium nitrate = 28 / 80 × 200 = 70. 0 g

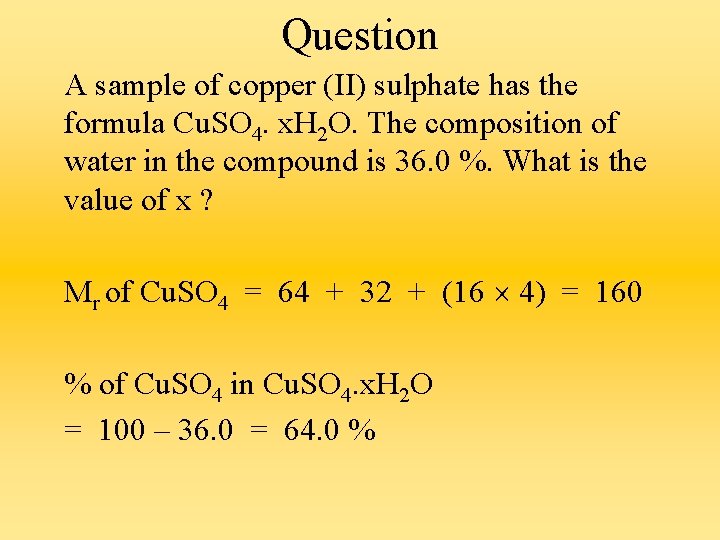
Question A sample of copper (II) sulphate has the formula Cu. SO 4. x. H 2 O. The composition of water in the compound is 36. 0 %. What is the value of x ? Mr of Cu. SO 4 = 64 + 32 + (16 4) = 160 % of Cu. SO 4 in Cu. SO 4. x. H 2 O = 100 – 36. 0 = 64. 0 %

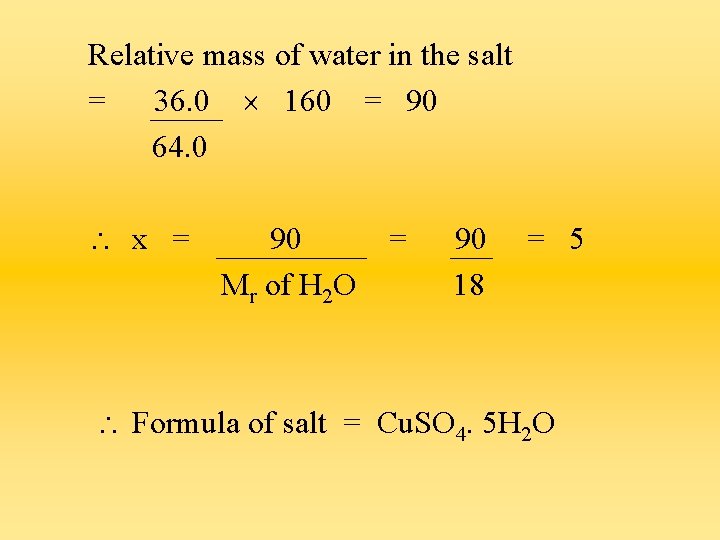
Relative mass of water in the salt = 36. 0 160 = 90 64. 0 x = 90 Mr of H 2 O = 90 18 = 5 Formula of salt = Cu. SO 4. 5 H 2 O

The Mole a) One mole of any matter (atoms, ions or molecules) contains 6 × 10 23 particles. There are no units for the mole. Symbol = mol b) The mass of one mole of any substance is called the molar mass. The value of molar mass is equal to the Ar or Mr of the substance (either element or compound) in grams.

c) The volume of one mole of any gas at room temperature and pressure is 24 dm 3 or 24 000 cm 3. This is called the molar volume at r. t. p. d) Avogadro’s Law states that equal volumes of gases contain the same number of molecules under the same conditions of temperature and pressure. e) The volume of a gas is directly proportional to the number of moles of the gas.
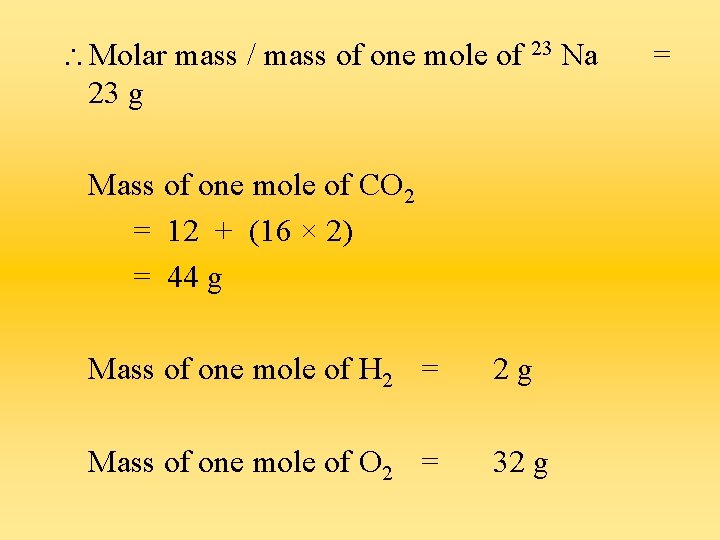
Molar mass / mass of one mole of 23 Na 23 g Mass of one mole of CO 2 = 12 + (16 × 2) = 44 g Mass of one mole of H 2 = 2 g Mass of one mole of O 2 = 32 g =

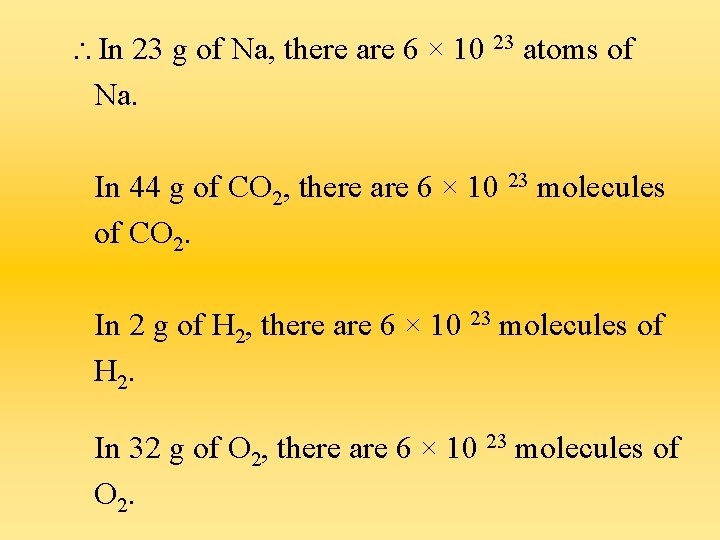
In 23 g of Na, there are 6 × 10 23 atoms of Na. In 44 g of CO 2, there are 6 × 10 23 molecules of CO 2. In 2 g of H 2, there are 6 × 10 23 molecules of H 2. In 32 g of O 2, there are 6 × 10 23 molecules of O 2.

44 g of CO 2 occupy 24 dm 3 at r. t. p. 2 g of H 2 occupy 24 dm 3 at r. t. p. 32 g of O 2 occupy 24 dm 3 at r. t. p. 10 cm 3 of O 2 have the same no. of molecules as 10 cm 3 of CO 2. 10 cm 3 of O 2 and 10 cm 3 of CO 2 consist of the same number of moles.

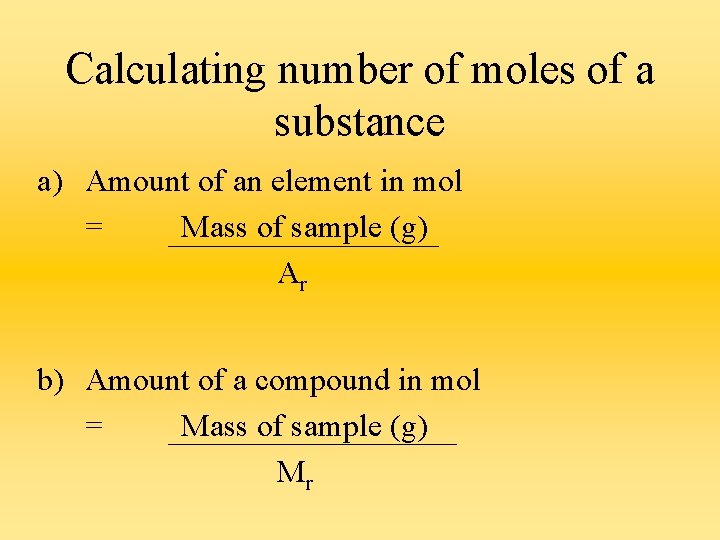
Calculating number of moles of a substance a) Amount of an element in mol = Mass of sample (g) Ar b) Amount of a compound in mol = Mass of sample (g) Mr
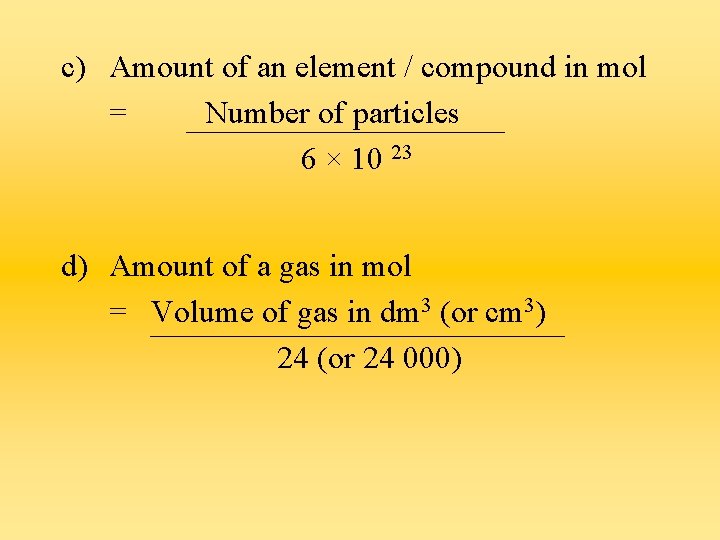
c) Amount of an element / compound in mol = Number of particles 6 × 10 23 d) Amount of a gas in mol = Volume of gas in dm 3 (or cm 3) 24 (or 24 000)
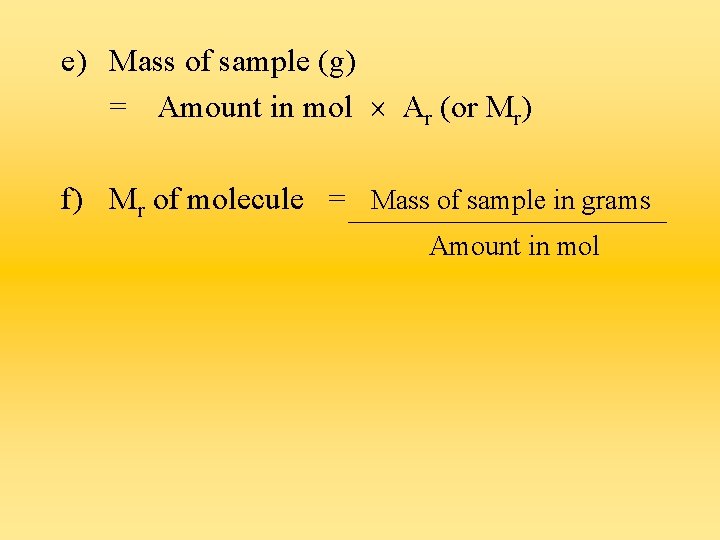
e) Mass of sample (g) = Amount in mol Ar (or Mr) f) Mr of molecule = Mass of sample in grams Amount in mol

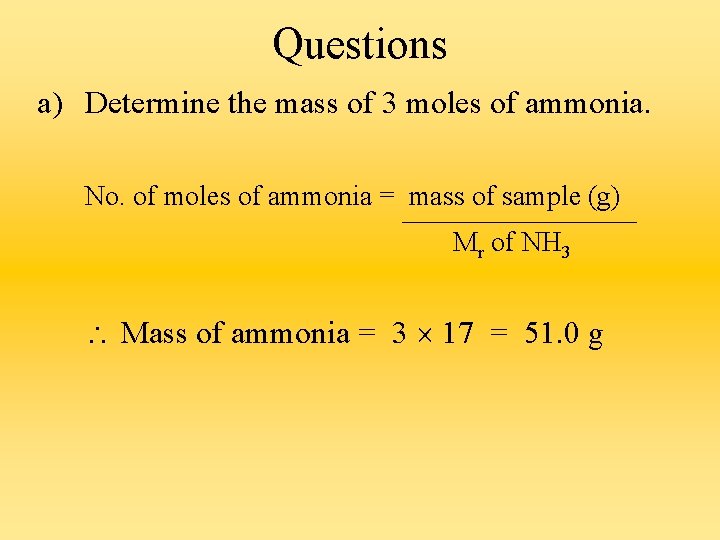
Questions a) Determine the mass of 3 moles of ammonia. No. of moles of ammonia = mass of sample (g) Mr of NH 3 Mass of ammonia = 3 17 = 51. 0 g

b) 0. 25 moles of ethene has a mass of 7 g. What is the relative molecular mass of ethene ? Mr of ethene = Mass of sample No. of moles = 7 / 0. 25 = 28

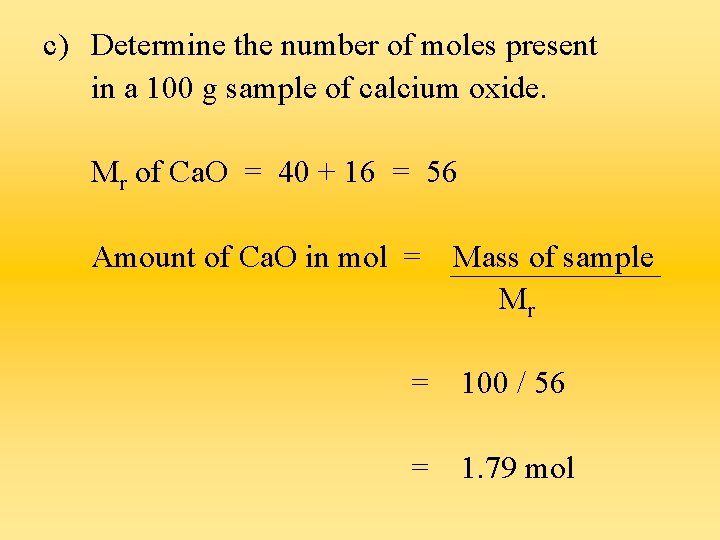
c) Determine the number of moles present in a 100 g sample of calcium oxide. Mr of Ca. O = 40 + 16 = 56 Amount of Ca. O in mol = Mass of sample Mr = 100 / 56 = 1. 79 mol

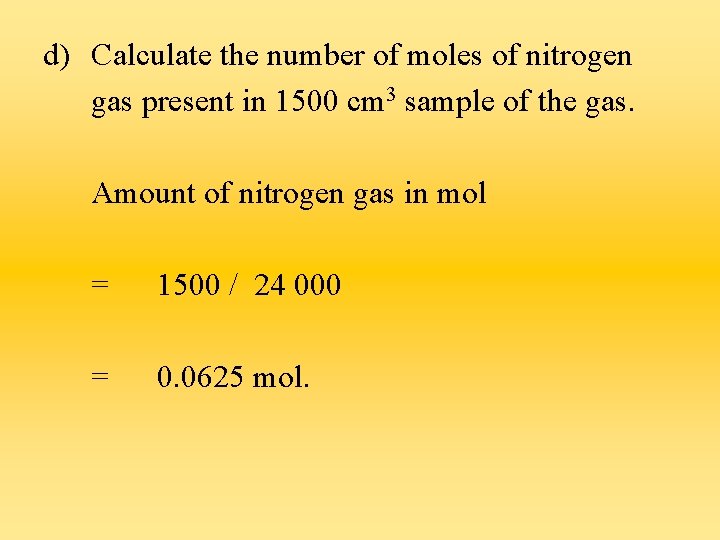
d) Calculate the number of moles of nitrogen gas present in 1500 cm 3 sample of the gas. Amount of nitrogen gas in mol = 1500 / 24 000 = 0. 0625 mol.

e) Determine the volume occupied by 0. 2 moles of sulphur dioxide gas at r. t. p. Volume of SO 2 gas at r. t. p. = Amount of SO 2 in mol Molar Volume = 0. 2 24 = 4. 80 dm 3

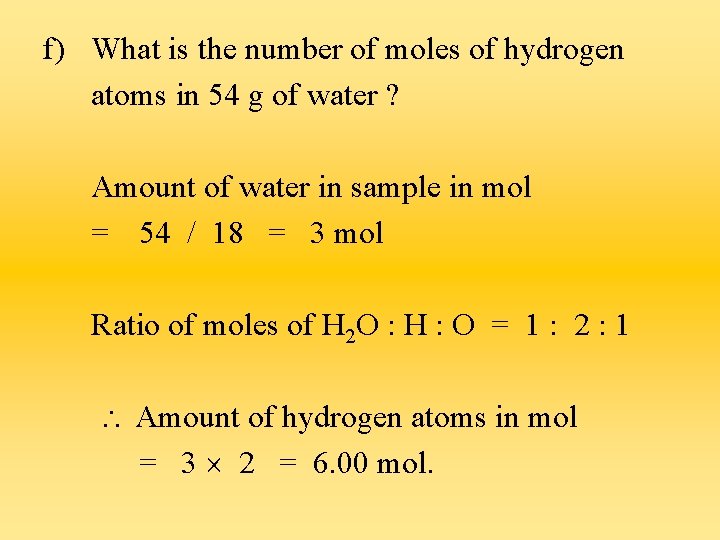
f) What is the number of moles of hydrogen atoms in 54 g of water ? Amount of water in sample in mol = 54 / 18 = 3 mol Ratio of moles of H 2 O : H : O = 1 : 2 : 1 Amount of hydrogen atoms in mol = 3 2 = 6. 00 mol.
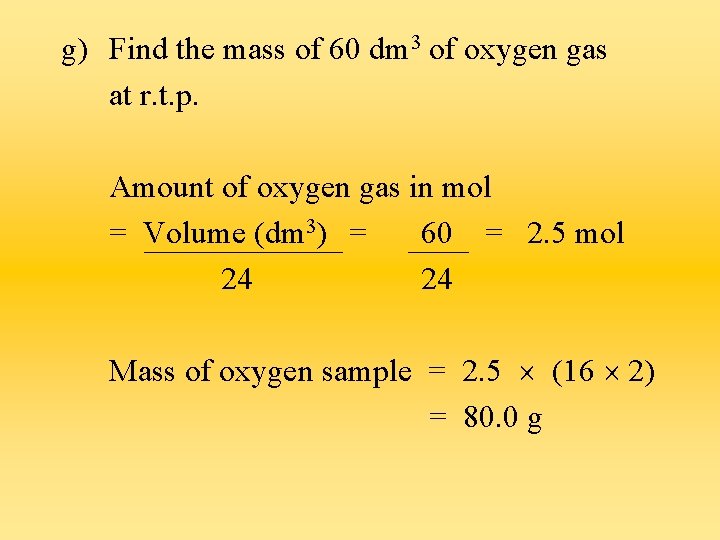
g) Find the mass of 60 dm 3 of oxygen gas at r. t. p. Amount of oxygen gas in mol = Volume (dm 3) = 60 = 2. 5 mol 24 24 Mass of oxygen sample = 2. 5 (16 2) = 80. 0 g

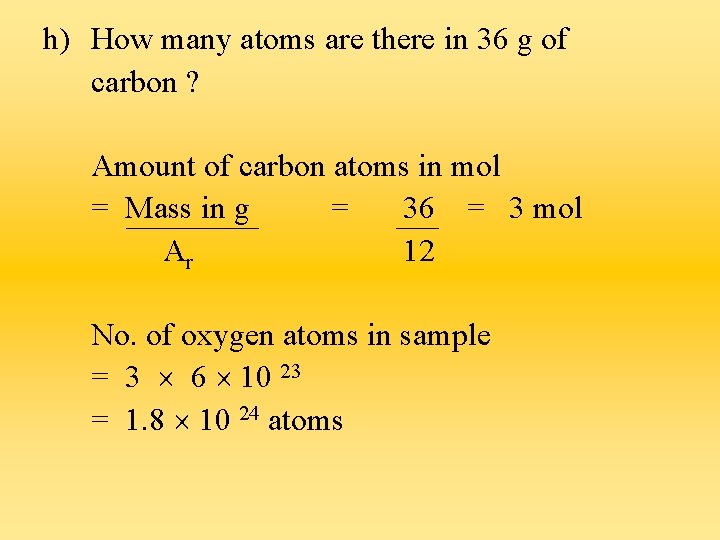
h) How many atoms are there in 36 g of carbon ? Amount of carbon atoms in mol = Mass in g = 36 = 3 mol Ar 12 No. of oxygen atoms in sample = 3 6 10 23 = 1. 8 10 24 atoms

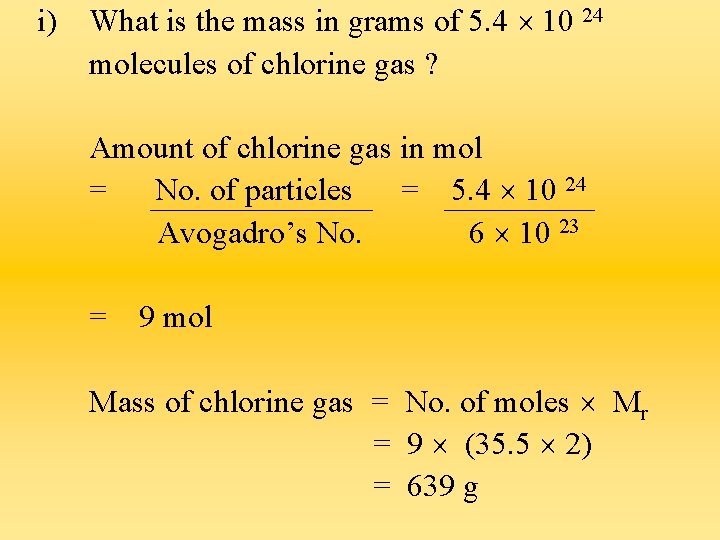
i) What is the mass in grams of 5. 4 10 24 molecules of chlorine gas ? Amount of chlorine gas in mol = No. of particles = 5. 4 10 24 Avogadro’s No. 6 10 23 = 9 mol Mass of chlorine gas = No. of moles Mr = 9 (35. 5 2) = 639 g
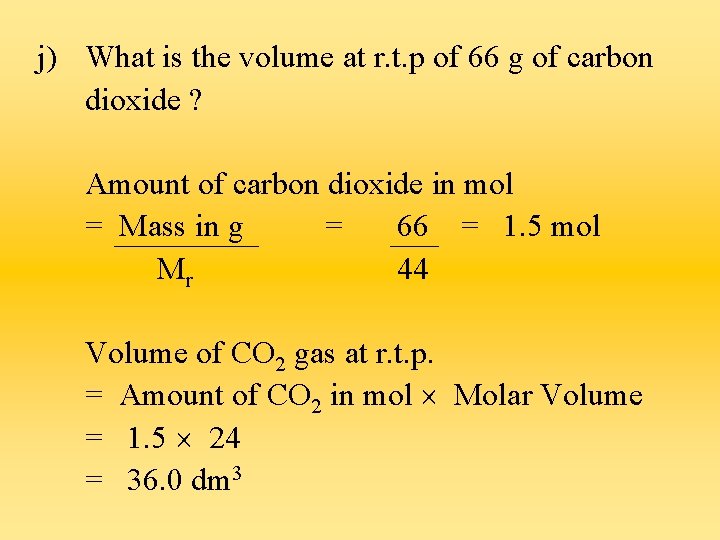
j) What is the volume at r. t. p of 66 g of carbon dioxide ? Amount of carbon dioxide in mol = Mass in g = 66 = 1. 5 mol Mr 44 Volume of CO 2 gas at r. t. p. = Amount of CO 2 in mol Molar Volume = 1. 5 24 = 36. 0 dm 3

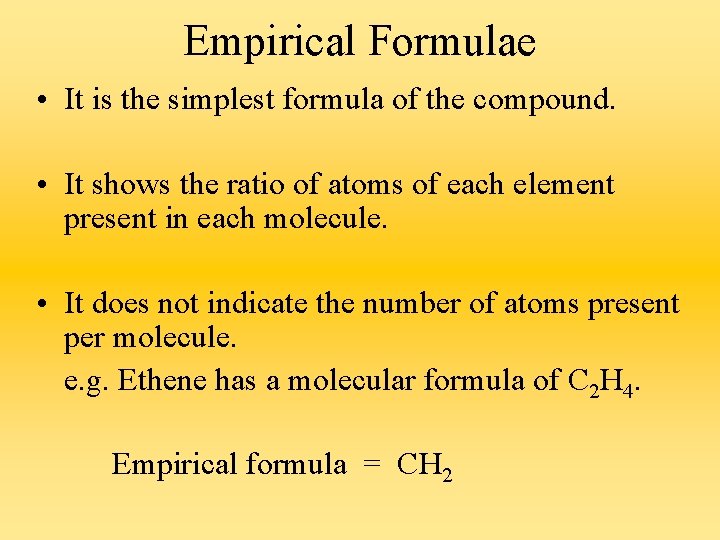
Empirical Formulae • It is the simplest formula of the compound. • It shows the ratio of atoms of each element present in each molecule. • It does not indicate the number of atoms present per molecule. e. g. Ethene has a molecular formula of C 2 H 4. Empirical formula = CH 2

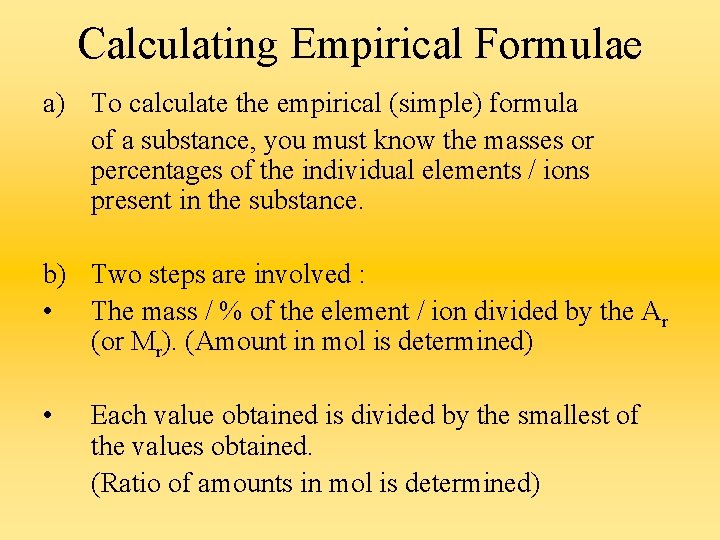
Calculating Empirical Formulae a) To calculate the empirical (simple) formula of a substance, you must know the masses or percentages of the individual elements / ions present in the substance. b) Two steps are involved : • The mass / % of the element / ion divided by the Ar (or Mr). (Amount in mol is determined) • Each value obtained is divided by the smallest of the values obtained. (Ratio of amounts in mol is determined)

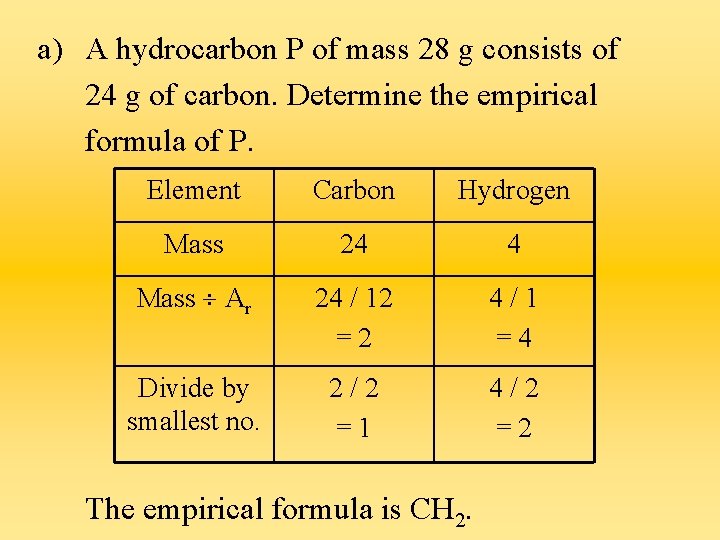
a) A hydrocarbon P of mass 28 g consists of 24 g of carbon. Determine the empirical formula of P. Element Carbon Hydrogen Mass 24 4 Mass Ar 24 / 12 =2 4/1 =4 Divide by smallest no. 2/2 =1 4/2 =2 The empirical formula is CH 2.

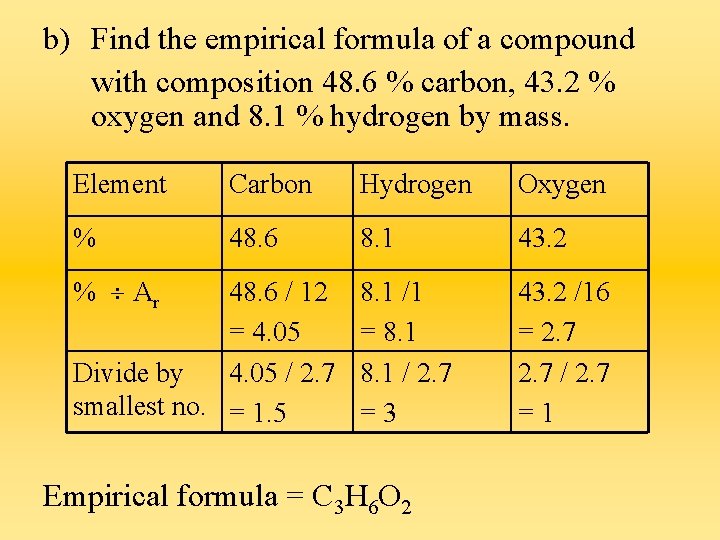
b) Find the empirical formula of a compound with composition 48. 6 % carbon, 43. 2 % oxygen and 8. 1 % hydrogen by mass. Element Carbon Hydrogen Oxygen % 48. 6 8. 1 43. 2 8. 1 /1 = 8. 1 / 2. 7 =3 43. 2 /16 = 2. 7 / 2. 7 =1 % Ar 48. 6 / 12 = 4. 05 Divide by 4. 05 / 2. 7 smallest no. = 1. 5 Empirical formula = C 3 H 6 O 2

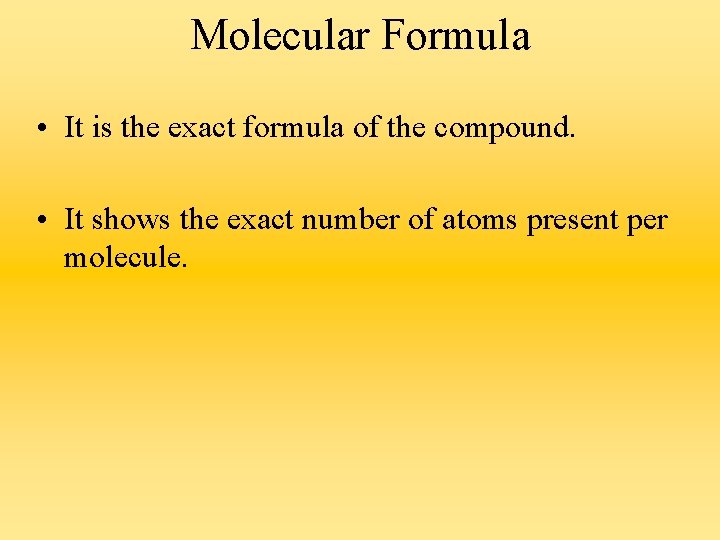
Molecular Formula • It is the exact formula of the compound. • It shows the exact number of atoms present per molecule.

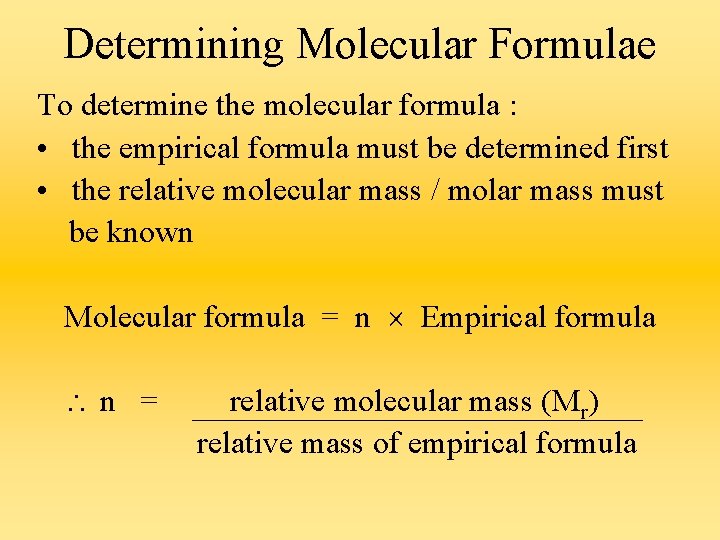
Determining Molecular Formulae To determine the molecular formula : • the empirical formula must be determined first • the relative molecular mass / molar mass must be known Molecular formula = n Empirical formula n = relative molecular mass (Mr) relative mass of empirical formula

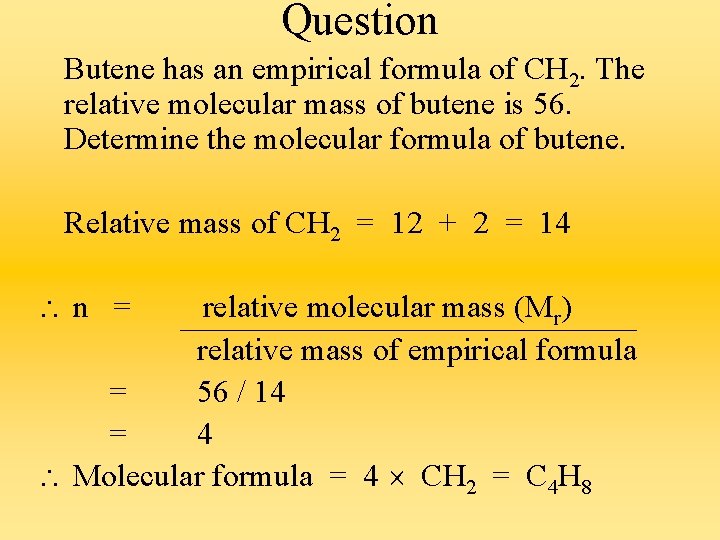
Question Butene has an empirical formula of CH 2. The relative molecular mass of butene is 56. Determine the molecular formula of butene. Relative mass of CH 2 = 12 + 2 = 14 n = relative molecular mass (Mr) relative mass of empirical formula = 56 / 14 = 4 Molecular formula = 4 CH 2 = C 4 H 8

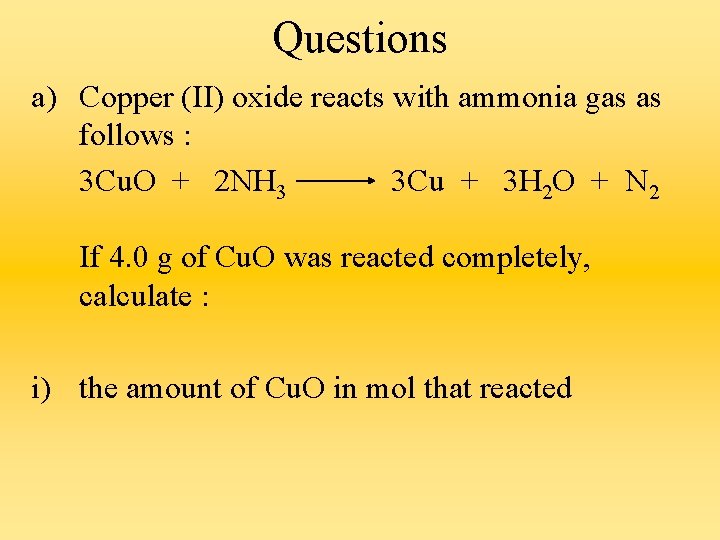
Calculations from Equations tell us : a) what the reactants and products of a reaction are. b) the ratio of amount in mol of the reactants to the products. c) the ratio of volumes of reactants to products in reactions involving gases.

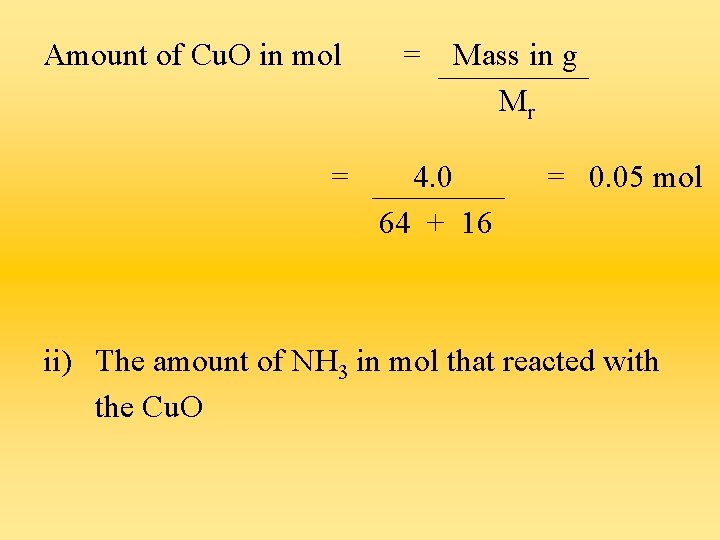
Questions a) Copper (II) oxide reacts with ammonia gas as follows : 3 Cu. O + 2 NH 3 3 Cu + 3 H 2 O + N 2 If 4. 0 g of Cu. O was reacted completely, calculate : i) the amount of Cu. O in mol that reacted

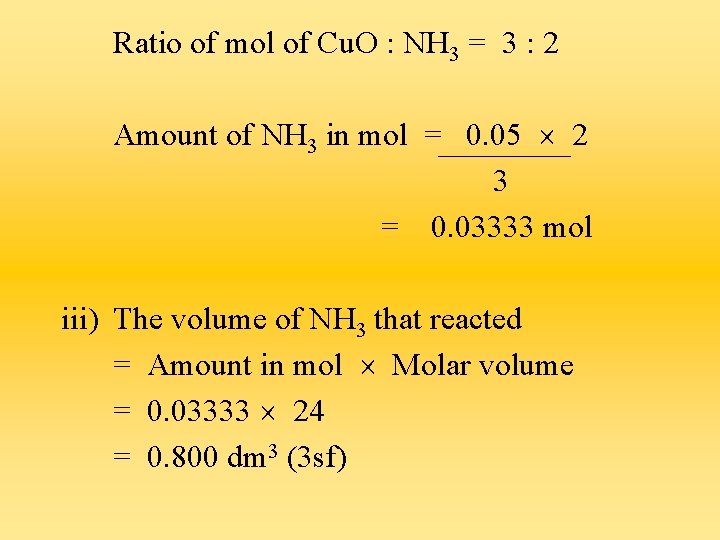
Amount of Cu. O in mol = = Mass in g Mr 4. 0 64 + 16 = 0. 05 mol ii) The amount of NH 3 in mol that reacted with the Cu. O

Ratio of mol of Cu. O : NH 3 = 3 : 2 Amount of NH 3 in mol = 0. 05 2 3 = 0. 03333 mol iii) The volume of NH 3 that reacted = Amount in mol Molar volume = 0. 03333 24 = 0. 800 dm 3 (3 sf)

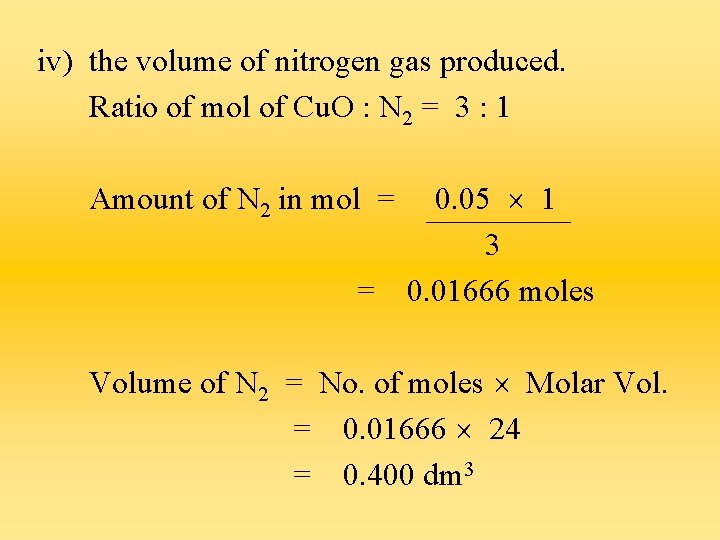
iv) the volume of nitrogen gas produced. Ratio of mol of Cu. O : N 2 = 3 : 1 Amount of N 2 in mol = = 0. 05 1 3 0. 01666 moles Volume of N 2 = No. of moles Molar Vol. = 0. 01666 24 = 0. 400 dm 3

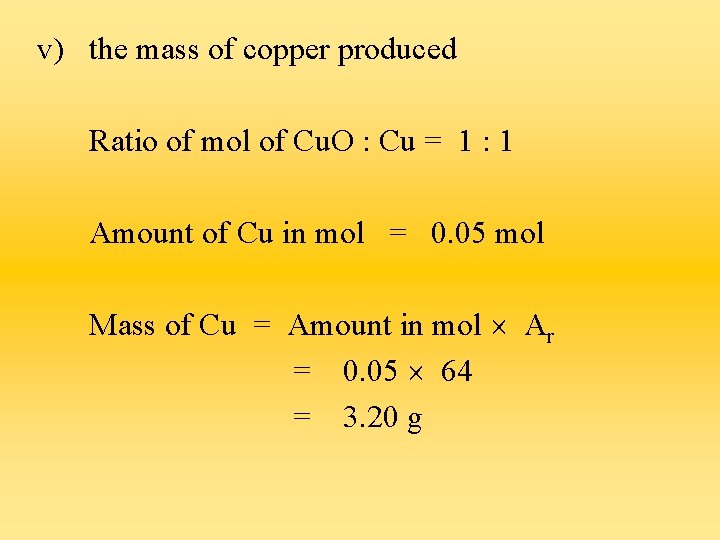
v) the mass of copper produced Ratio of mol of Cu. O : Cu = 1 : 1 Amount of Cu in mol = 0. 05 mol Mass of Cu = Amount in mol Ar = 0. 05 64 = 3. 20 g

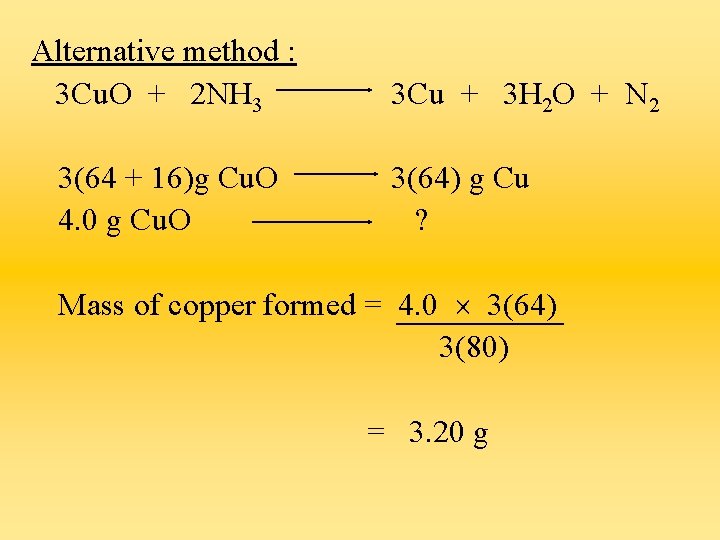
Alternative method : 3 Cu. O + 2 NH 3 3(64 + 16)g Cu. O 4. 0 g Cu. O 3 Cu + 3 H 2 O + N 2 3(64) g Cu ? Mass of copper formed = 4. 0 3(64) 3(80) = 3. 20 g

b) Nitrogen combines with hydrogen to form ammonia as shown below : N 2 + 3 H 2 2 NH 3 30 cm 3 of nitrogen gas is reacted with 60 cm 3 of hydrogen gas at r. t. p. i) What is the volume of ammonia gas formed ? ii) What is the total volume of gaseous products formed ?

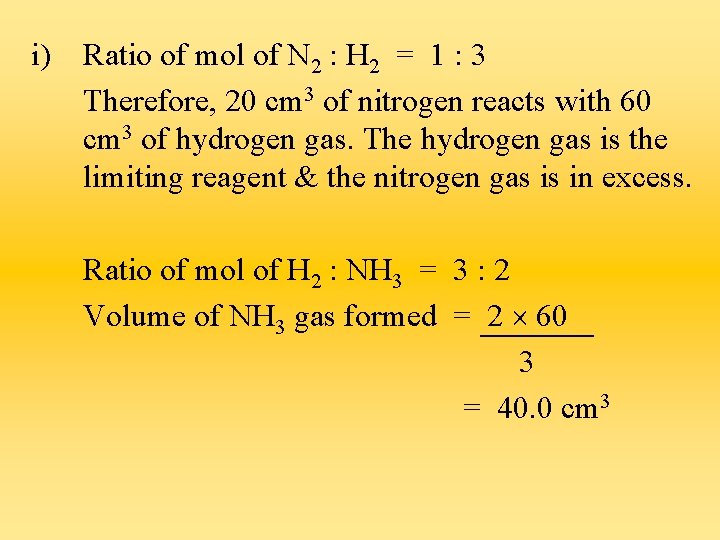
i) Ratio of mol of N 2 : H 2 = 1 : 3 Therefore, 20 cm 3 of nitrogen reacts with 60 cm 3 of hydrogen gas. The hydrogen gas is the limiting reagent & the nitrogen gas is in excess. Ratio of mol of H 2 : NH 3 = 3 : 2 Volume of NH 3 gas formed = 2 60 3 = 40. 0 cm 3

ii) Volume of excess nitrogen = 30 – 20 = 10. 0 cm 3 Total volume of gaseous products = 10 + 40 = 50. 0 cm 3

Calculating Percentage Yield in a Reaction 1) During experiments, the amount of chemicals produced is always less than the maximum amount you would expect. This is expressed as the percentage yield. 2) Percentage yield of substance = Experimental mass of substance 100 % Maximum possible mass of product

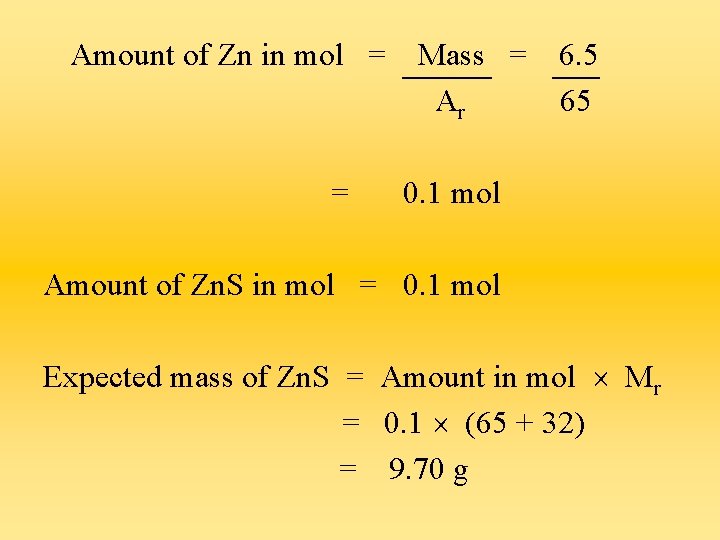
Questions a) Zinc reacts with sulphur according to the equation : Zn + S Zn. S 6. 5 g of zinc was reacted with sulphur to make zinc sulphide. 9. 0 g of zinc sulphide was obtained. Calculate the % yield.

Amount of Zn in mol = = Mass = Ar 6. 5 65 0. 1 mol Amount of Zn. S in mol = 0. 1 mol Expected mass of Zn. S = Amount in mol Mr = 0. 1 (65 + 32) = 9. 70 g

% yield of Zn. S = 9. 0 100 % = 9. 7 92. 8 %

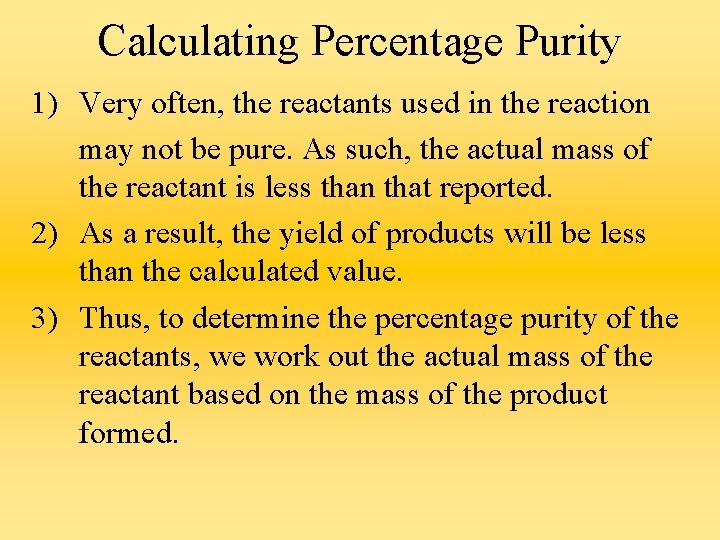
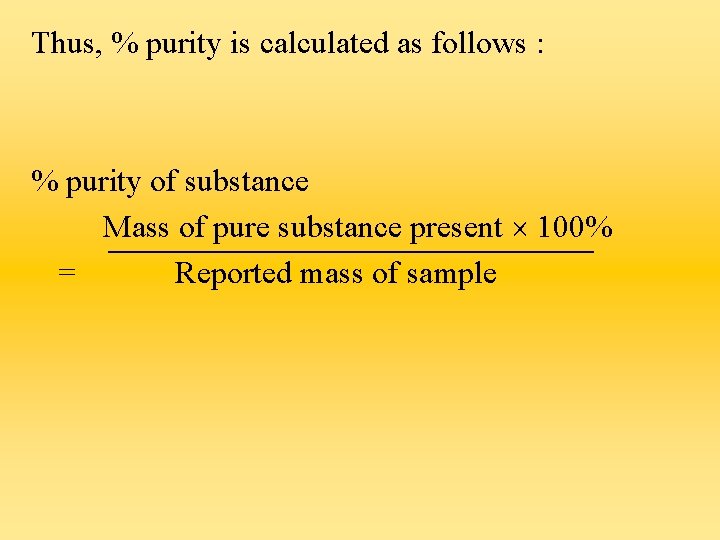
Calculating Percentage Purity 1) Very often, the reactants used in the reaction may not be pure. As such, the actual mass of the reactant is less than that reported. 2) As a result, the yield of products will be less than the calculated value. 3) Thus, to determine the percentage purity of the reactants, we work out the actual mass of the reactant based on the mass of the product formed.

Thus, % purity is calculated as follows : % purity of substance Mass of pure substance present 100% = Reported mass of sample

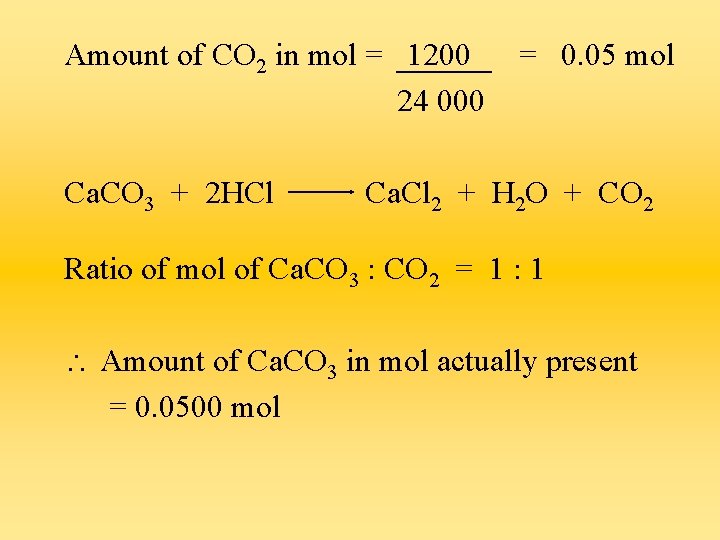
Questions a) An impure sample of calcium carbonate contains calcium sulphate as an impurity. When excess hydrochloric acid was added to 6 g of the sample, 1200 cm 3 of gas were produced at r. t. p. Calculate the % purity of the calcium carbonate sample. Mass of impure calcium carbonate = 6 g Volume of CO 2 = 1200 cm 3

Amount of CO 2 in mol = 1200 24 000 Ca. CO 3 + 2 HCl = 0. 05 mol Ca. Cl 2 + H 2 O + CO 2 Ratio of mol of Ca. CO 3 : CO 2 = 1 : 1 Amount of Ca. CO 3 in mol actually present = 0. 0500 mol

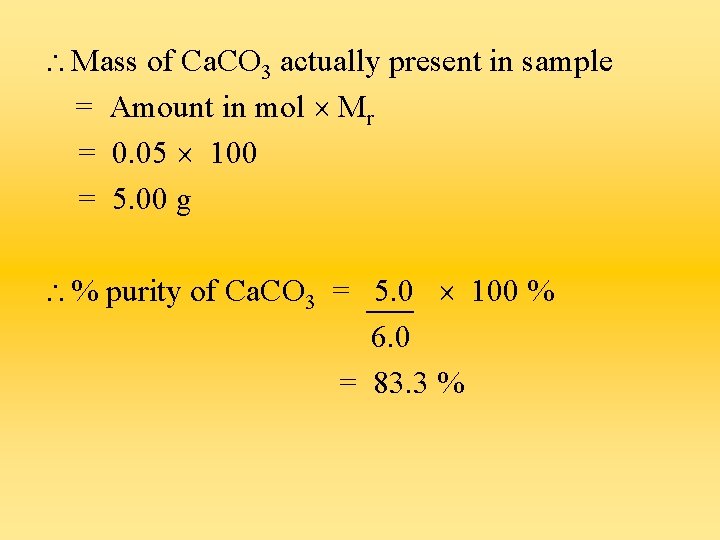
Mass of Ca. CO 3 actually present in sample = Amount in mol Mr = 0. 05 100 = 5. 00 g % purity of Ca. CO 3 = 5. 0 100 % 6. 0 = 83. 3 %

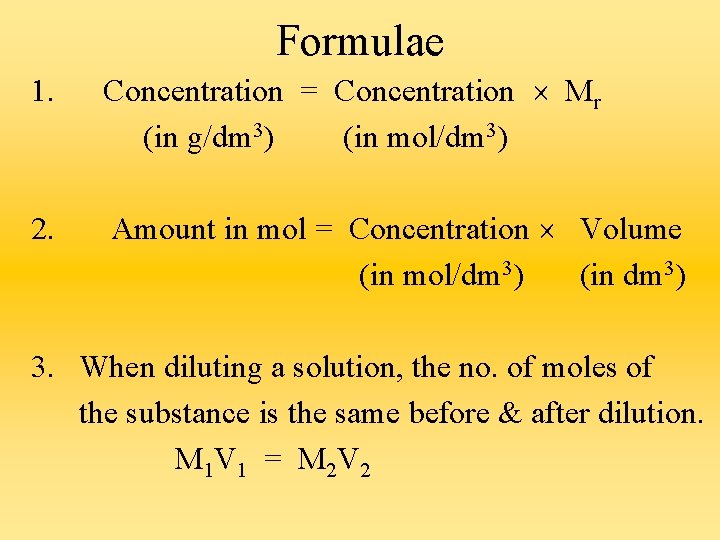
Concentration of Solutions 1) The concentration of a solution refers to the amount of solute (either in grams or in moles) dissolved in a fixed volume of the solvent. 2) Concentration can be expressed in : a) grams per cm 3 / grams per dm 3 b) moles per dm 3

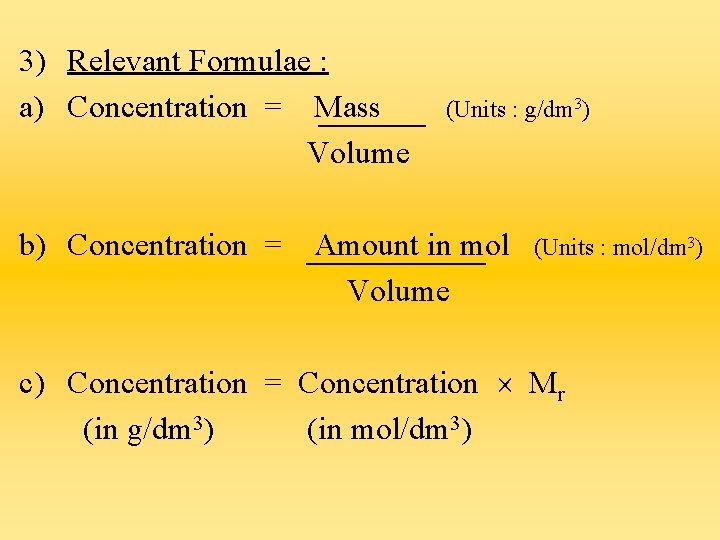
3) Relevant Formulae : a) Concentration = Mass Volume b) Concentration = (Units : g/dm 3) Amount in mol Volume (Units : mol/dm 3) c) Concentration = Concentration Mr (in g/dm 3) (in mol/dm 3)

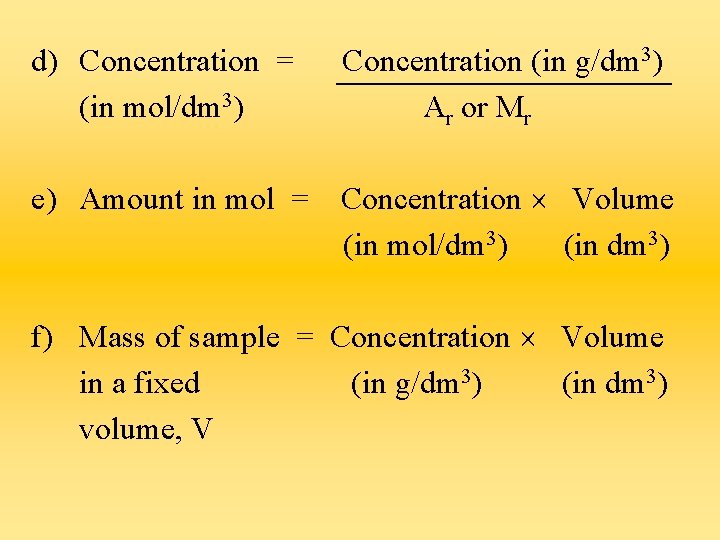
d) Concentration = (in mol/dm 3) Concentration (in g/dm 3) Ar or Mr e) Amount in mol = Concentration Volume (in mol/dm 3) (in dm 3) f) Mass of sample = Concentration Volume in a fixed (in g/dm 3) (in dm 3) volume, V

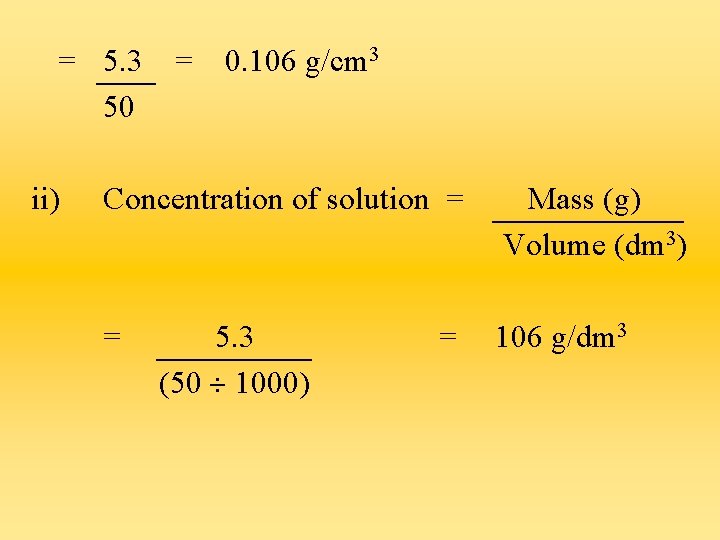
Questions a) A student dissolved 5. 3 g of sodium nitrate in 50 cm 3 of water. Determine : i) the concentration of the solution in g/cm 3 ii) the concentration of the solution in g/dm 3 iii) the concentration of the solution in mol/dm 3 i) Concentration of solution = Mass (g) Volume (cm 3)

= 5. 3 50 ii) = 0. 106 g/cm 3 Concentration of solution = Mass (g) Volume (dm 3) = 106 g/dm 3 5. 3 (50 1000) =

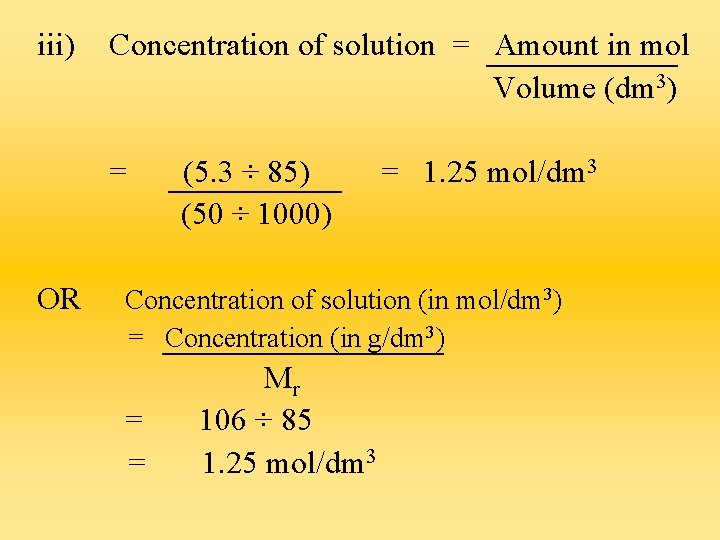
iii) Concentration of solution = Amount in mol Volume (dm 3) = OR (5. 3 ÷ 85) (50 ÷ 1000) = 1. 25 mol/dm 3 Concentration of solution (in mol/dm 3) = Concentration (in g/dm 3) = = Mr 106 ÷ 85 1. 25 mol/dm 3

b) You are provided with a solution of sodium chloride of concentration 0. 8 mol/dm 3. Calculate the concentration in g/dm 3. Concentration of solution (in g/dm 3) = Concentration (in mol/dm 3) Mr = = 0. 8 (23 + 35. 5) 46. 8 g/dm 3

3) Calculate the number of moles of calcium chloride present in 50 cm 3 of 0. 5 mol/dm 3 solution of calcium chloride. Amount of Ca. Cl 2 in mol = Conc. (mol/dm 3) Vol. (dm 3) = 0. 5 (50 ÷ 1000) = 0. 0250 moles. 1000 cm 3 50 cm 3 0. 5 mol ?

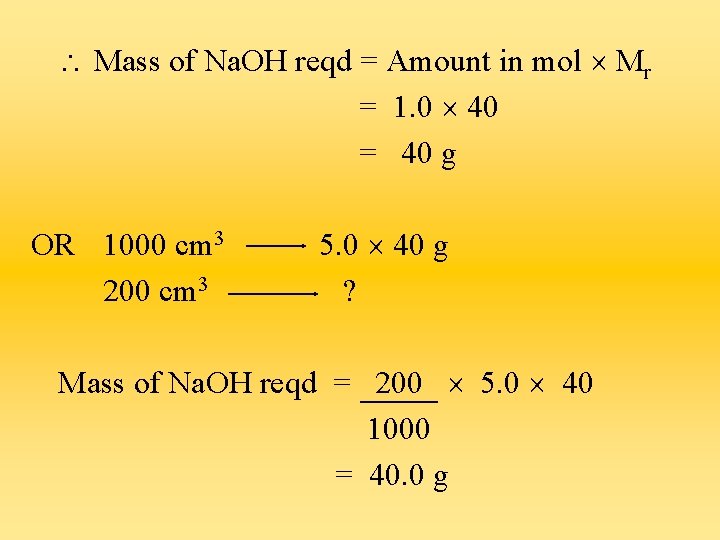
4) What mass of sodium hydroxide must be dissolved in 200 cm 3 of water to prepare a 5. 0 mol/dm 3 solution? Mr of Na. OH = 23 + 16 + 1 = 40 Amount of Na. OH in mol that is reqd = Conc. (mol/dm 3) Vol. (dm 3) = 5. 0 (200 ÷ 1000) = 1. 0 mol

Mass of Na. OH reqd = Amount in mol Mr = 1. 0 40 = 40 g OR 1000 cm 3 200 cm 3 5. 0 40 g ? Mass of Na. OH reqd = 200 5. 0 40 1000 = 40. 0 g

5) Express the concentration of a 0. 064 mol/dm 3 solution of sodium hydroxide in g/dm 3. Mr of Na. OH = 23 + 16 + 1 = 40 Concentration of Na. OH in g/dm 3 = Conc. (mol/dm 3) × Mr = 0. 064 × 40 = 2. 56 g/dm 3.

6) Express the concentration of a 20 g/dm 3 solution of sodium hydroxide in mol/dm 3. Concentration of Na. OH in mol/dm 3 = Conc. (g/dm 3) ÷ Mr = 20 ÷ 40 = 0. 500 mol/dm 3

Volumetric Analysis A method of determining the quantity of a substance present in a solid or in a solution using a technique called titration.

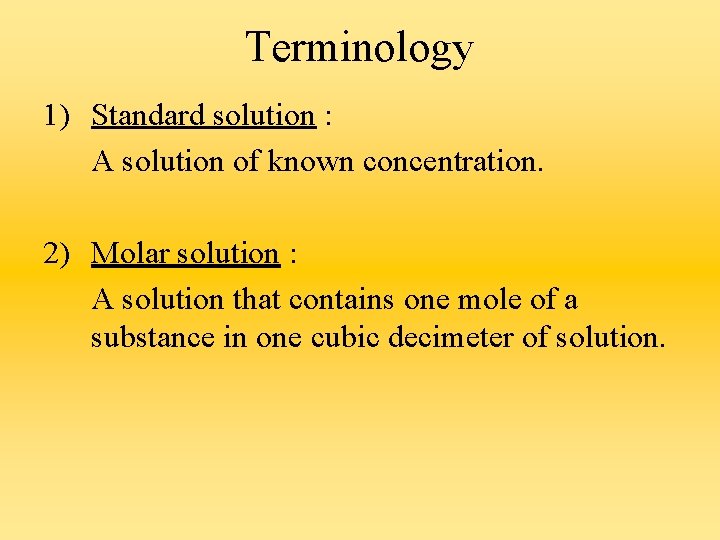
Terminology 1) Standard solution : A solution of known concentration. 2) Molar solution : A solution that contains one mole of a substance in one cubic decimeter of solution.

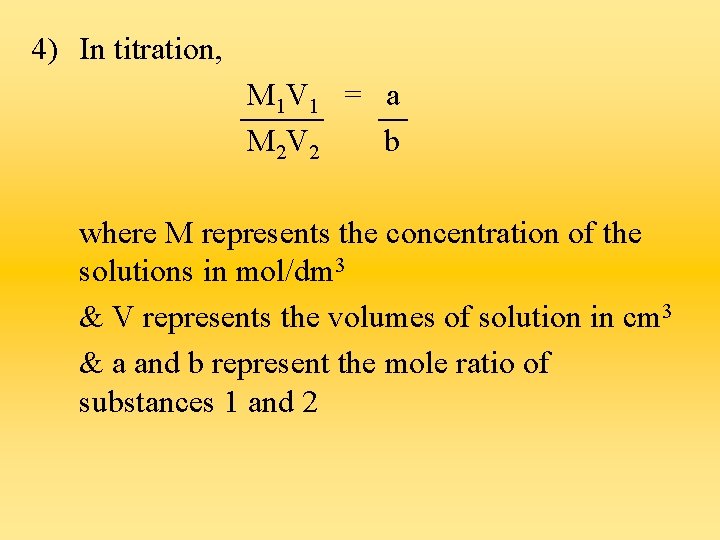
Formulae 1. Concentration = Concentration Mr (in g/dm 3) (in mol/dm 3) 2. Amount in mol = Concentration Volume (in mol/dm 3) (in dm 3) 3. When diluting a solution, the no. of moles of the substance is the same before & after dilution. M 1 V 1 = M 2 V 2

4) In titration, M 1 V 1 = a M 2 V 2 b where M represents the concentration of the solutions in mol/dm 3 & V represents the volumes of solution in cm 3 & a and b represent the mole ratio of substances 1 and 2

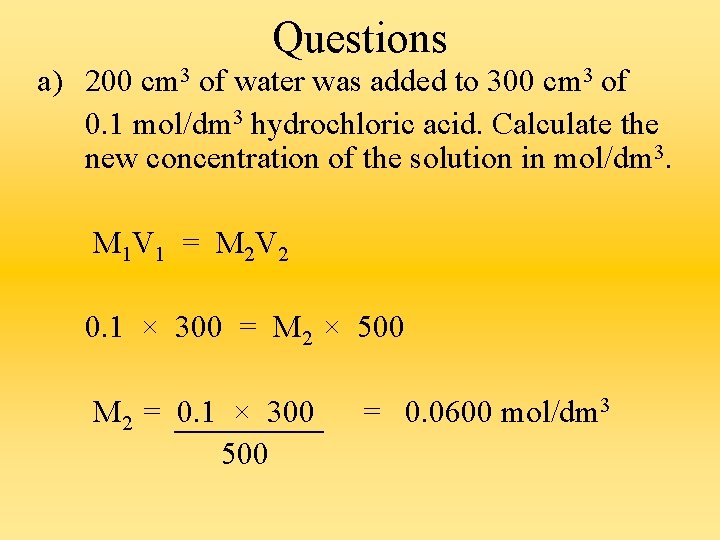
Questions a) 200 cm 3 of water was added to 300 cm 3 of 0. 1 mol/dm 3 hydrochloric acid. Calculate the new concentration of the solution in mol/dm 3. M 1 V 1 = M 2 V 2 0. 1 × 300 = M 2 × 500 M 2 = 0. 1 × 300 500 = 0. 0600 mol/dm 3

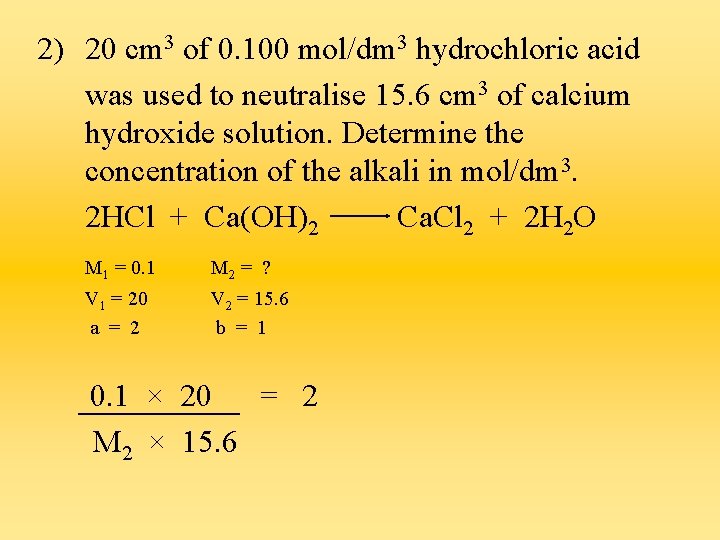
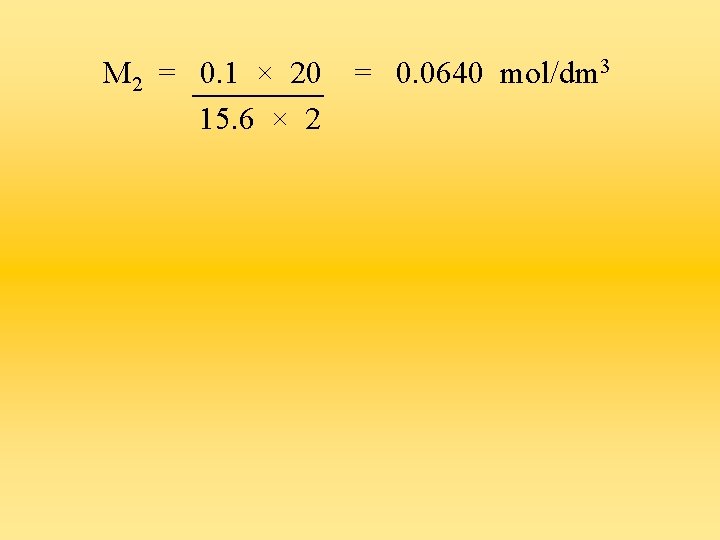
2) 20 cm 3 of 0. 100 mol/dm 3 hydrochloric acid was used to neutralise 15. 6 cm 3 of calcium hydroxide solution. Determine the concentration of the alkali in mol/dm 3. 2 HCl + Ca(OH)2 Ca. Cl 2 + 2 H 2 O M 1 = 0. 1 M 2 = ? V 1 = 20 a = 2 V 2 = 15. 6 b = 1 0. 1 × 20 = 2 M 2 × 15. 6

M 2 = 0. 1 × 20 15. 6 × 2 = 0. 0640 mol/dm 3

Choice of indicators for VA Reaction 1. Strong acid – strong alkali Indicator Litmus Methyl orange Phenolphthalein 2. Weak acid – strong alkali Phenolphthalein Litmus 3. Strong acid – weak alkali Methyl orange Litmus 4. Strong acid – soluble carbonate Methyl orange