A spectrometer from G 10 is used to








- Slides: 30

A spectrometer (from G 10) is used to measure the masses of small particles like atoms. It has been established that the mass of an atom is = 1, 66 x 10 -27 kg. i. e. H 0, 0000000000000166 kg This really a very small number! Spectrometer 1

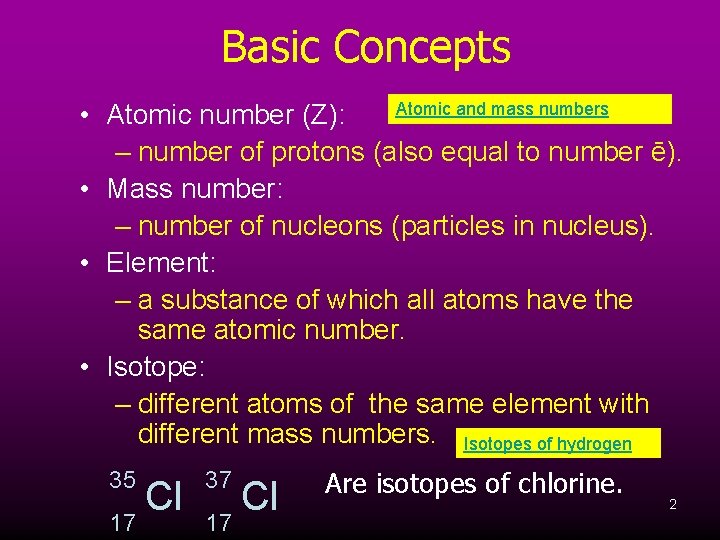
Basic Concepts Atomic and mass numbers • Atomic number (Z): – number of protons (also equal to number ē). • Mass number: – number of nucleons (particles in nucleus). • Element: – a substance of which all atoms have the same atomic number. • Isotope: – different atoms of the same element with different mass numbers. Isotopes of hydrogen 35 17 Cl 37 17 Cl Are isotopes of chlorine. 2

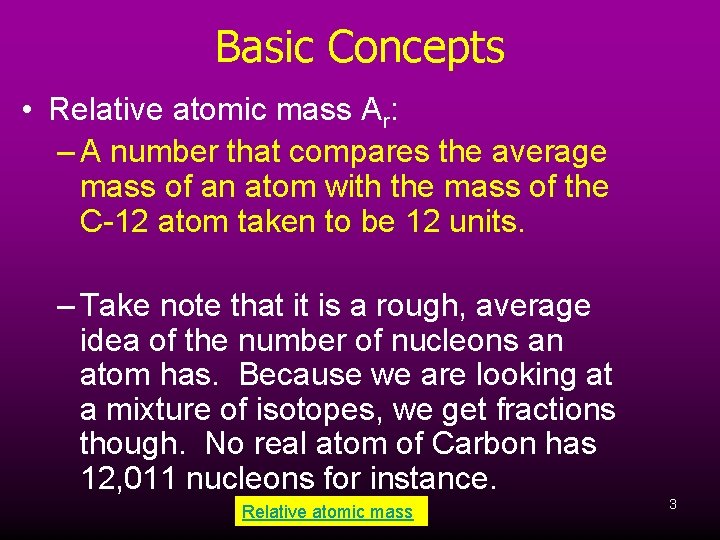
Basic Concepts • Relative atomic mass Ar: – A number that compares the average mass of an atom with the mass of the C-12 atom taken to be 12 units. – Take note that it is a rough, average idea of the number of nucleons an atom has. Because we are looking at a mixture of isotopes, we get fractions though. No real atom of Carbon has 12, 011 nucleons for instance. Relative atomic mass 3

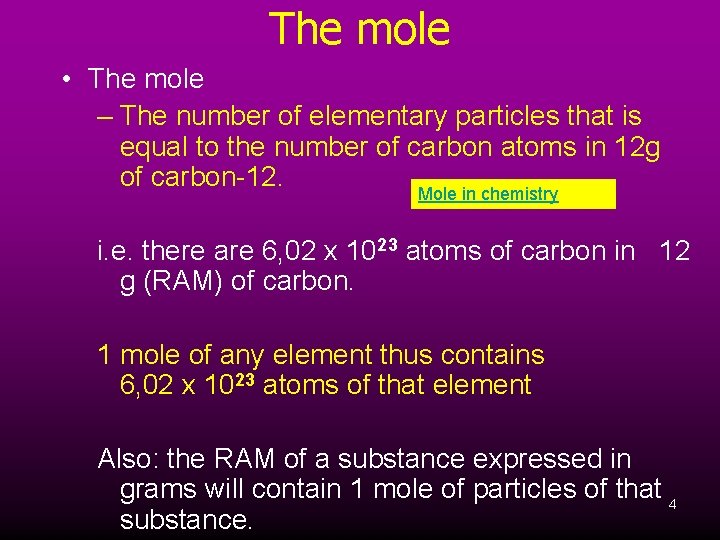
The mole • The mole – The number of elementary particles that is equal to the number of carbon atoms in 12 g of carbon-12. Mole in chemistry i. e. there are 6, 02 x 1023 atoms of carbon in 12 g (RAM) of carbon. 1 mole of any element thus contains 6, 02 x 1023 atoms of that element Also: the RAM of a substance expressed in grams will contain 1 mole of particles of that 4 substance.

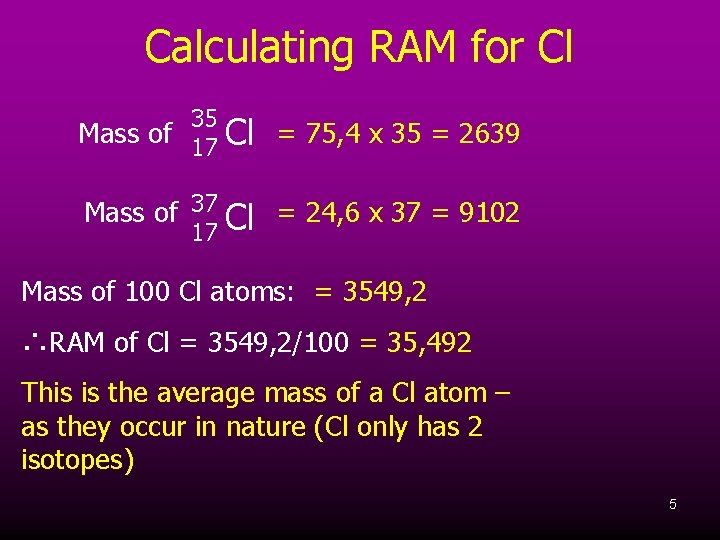
Calculating RAM for Cl 35 Mass of 17 Cl = 75, 4 x 35 = 2639 Mass of 37 Cl = 24, 6 x 37 = 9102 17 Mass of 100 Cl atoms: = 3549, 2 ∴RAM of Cl = 3549, 2/100 = 35, 492 This is the average mass of a Cl atom – as they occur in nature (Cl only has 2 isotopes) 5

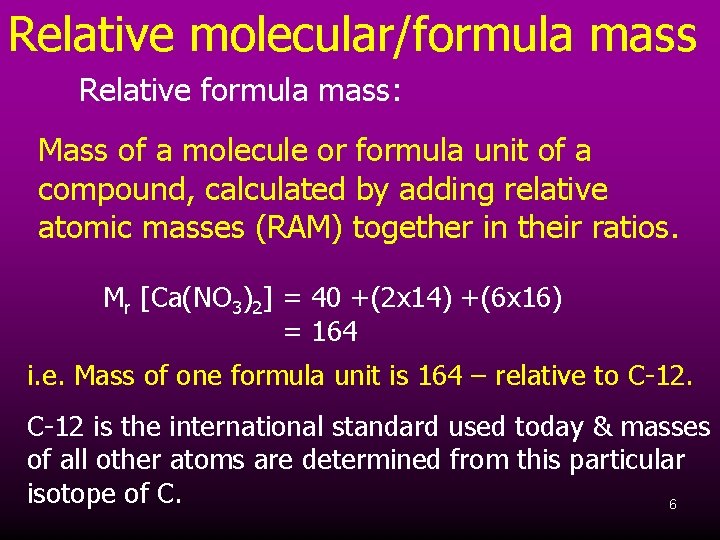
Relative molecular/formula mass Relative formula mass: Mass of a molecule or formula unit of a compound, calculated by adding relative atomic masses (RAM) together in their ratios. Mr [Ca(NO 3)2] = 40 +(2 x 14) +(6 x 16) = 164 i. e. Mass of one formula unit is 164 – relative to C-12 is the international standard used today & masses of all other atoms are determined from this particular isotope of C. 6

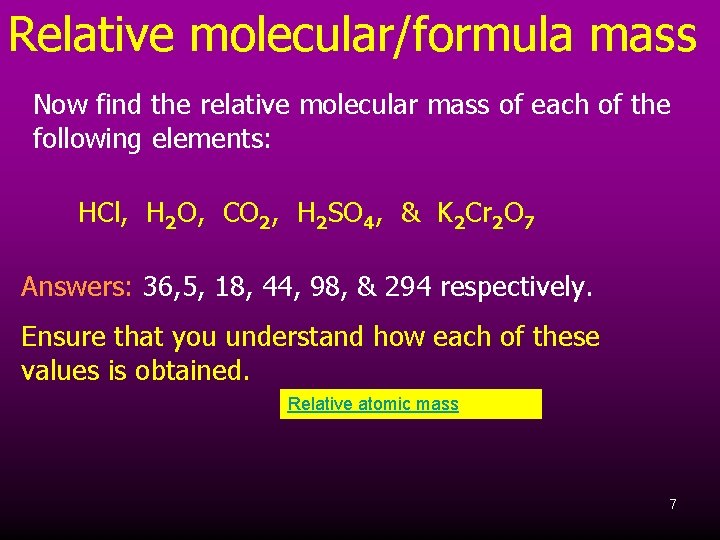
Relative molecular/formula mass Now find the relative molecular mass of each of the following elements: HCl, H 2 O, CO 2, H 2 SO 4, & K 2 Cr 2 O 7 Answers: 36, 5, 18, 44, 98, & 294 respectively. Ensure that you understand how each of these values is obtained. Relative atomic mass 7

Counting in Chemistry You have come across the words: pairs, dozens & century. In chemistry we use huge numbers and we count numbers of atoms, molecules, ions, protons & electrons etc in moles. 1 mole = 6, 02 x 1023 particles. i. e. 602 000 000. 0 particles This really huge! The mole is also called the Avogadro number or Avogadro constant. There are only about 1 x 1014 people on earth! 8

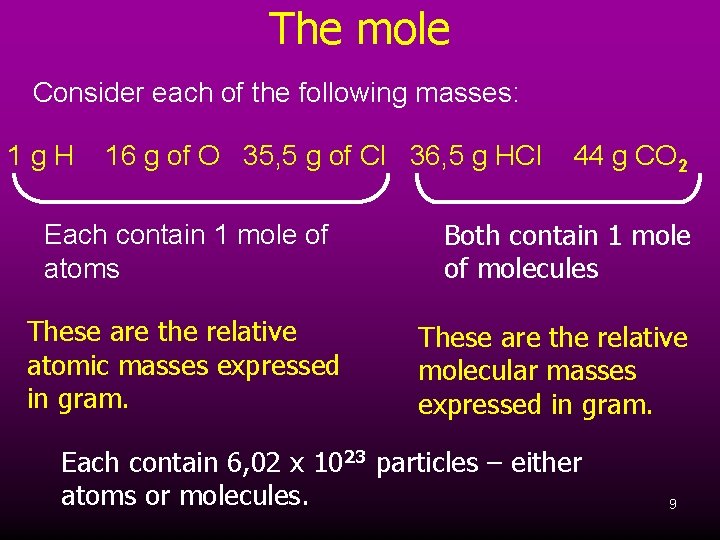
The mole Consider each of the following masses: 1 g. H 16 g of O 35, 5 g of Cl 36, 5 g HCl Each contain 1 mole of atoms These are the relative atomic masses expressed in gram. 44 g CO 2 Both contain 1 mole of molecules These are the relative molecular masses expressed in gram. Each contain 6, 02 x 1023 particles – either atoms or molecules. 9

The mole 1 g of H contains 6, 02 x 1023 atoms or 1 g of H contains ½ (6, 02 x 1023 ) molecules (Since H is diatomic: H 2) 2 g of H contains 6, 02 x 1023 molecules or 2 g of H contains 2(6, 02 x 1023) atoms Find the following: The number of molecules in 16 g of oxygen. The number of molecules in 142 g of chlorine. The number of atoms in 64 g of oxygen 10

Molar mass Mr Whereas the mass of 1 molecule is called the relative molecular mass and the mass of 1 formula unit is called the relative formula mass, the mass of 1 mole of molecules is called the molar mass and is expressed in g∙mol-1. Consider CO 2. 12 + 2(16) = 44 = mass of 1 molecule of CO 2. But 44 g∙mol-1 is the molar mass (Mr) = mass of 1 mole of CO 2 molecules i. e. 6, 02 x 1023 molecules. Molar mass 11

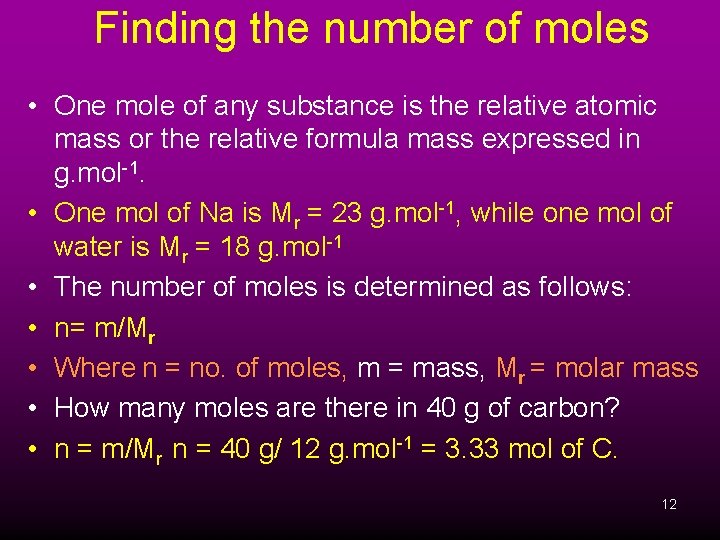
Finding the number of moles • One mole of any substance is the relative atomic mass or the relative formula mass expressed in g. mol-1. • One mol of Na is Mr = 23 g. mol-1, while one mol of water is Mr = 18 g. mol-1 • The number of moles is determined as follows: • n= m/Mr • Where n = no. of moles, m = mass, Mr = molar mass • How many moles are there in 40 g of carbon? • n = m/Mr n = 40 g/ 12 g. mol-1 = 3. 33 mol of C. 12

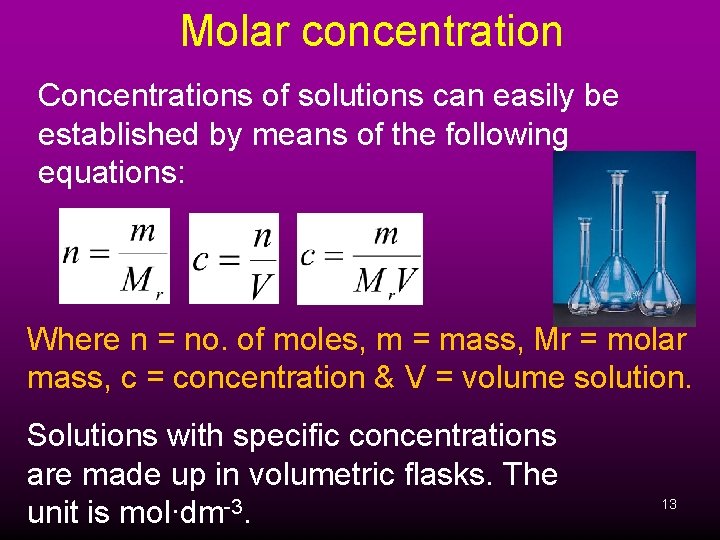
Molar concentration Concentrations of solutions can easily be established by means of the following equations: Where n = no. of moles, m = mass, Mr = molar mass, c = concentration & V = volume solution. Solutions with specific concentrations are made up in volumetric flasks. The unit is mol∙dm-3. 13

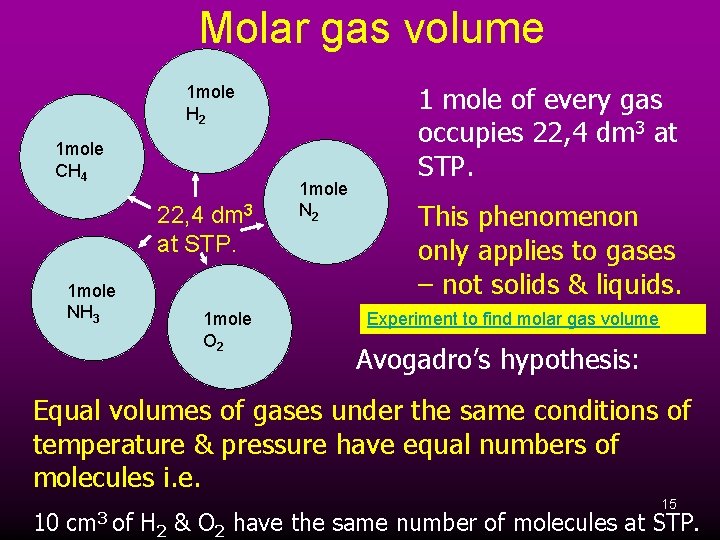
Molar gas volume This is the volume of 1 mole of any gas at STP. Using densities, we can find the volume of any gas at STP. 32 g 3 m = 22, 4 dm V = D = 1, 43 g. dm-3 For oxygen: Using the masses and densities of other gases, we find that 1 mole of any/every gas occupies 22, 4 dm 3 at STP. This value is known as molar gas volume – the volume of 1 mole of every gas at STP. Molar volumes 14

Molar gas volume 1 mole H 2 1 mole CH 4 22, 4 dm 3 at STP. 1 mole NH 3 1 mole O 2 1 mole N 2 1 mole of every gas occupies 22, 4 dm 3 at STP. This phenomenon only applies to gases – not solids & liquids. Experiment to find molar gas volume Avogadro’s hypothesis: Equal volumes of gases under the same conditions of temperature & pressure have equal numbers of molecules i. e. 15 10 cm 3 of H 2 & O 2 have the same number of molecules at STP.

Avogadro’s hypothesis He said: equal volumes of different gases (at the same temp & pressure) contain the same number of molecules. The ratio of masses of equal volumes of gases must be the same ratio as their relative molecular masses. You can thus also determine the relative molecular mass of a gas by using volumes & the known relative molecular mass of another gas. Avogadro's law 16

Calculation Fertilizers need to be applied to lawns & ground where crops are to be grown. Most fertilizers have a proportion of some of nitrogen, phosphorus, potassium, calcium, magnesium, hydrogen, chlorine & oxygen. Common fertilizers are: NH 4 NO 3, Ca(H 2 PO 4)2 & KCl We need to be able to correctly calculate the fertilizer required for certain crops in certain soils lacking particular elements. 17

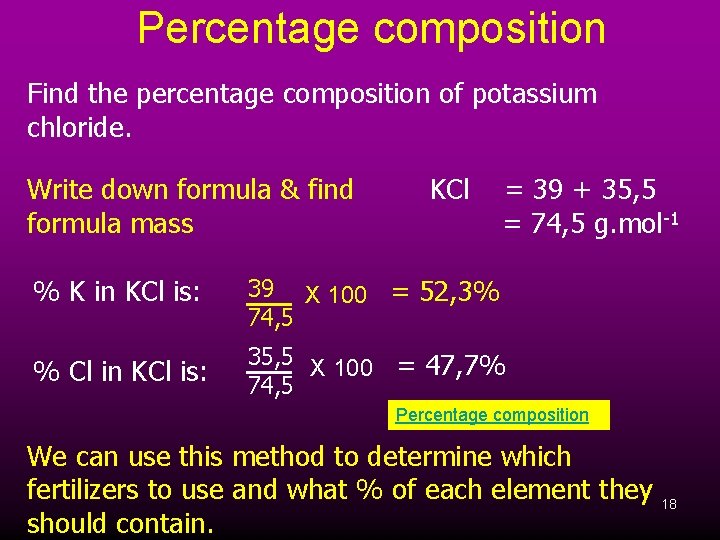
Percentage composition Find the percentage composition of potassium chloride. Write down formula & find formula mass KCl = 39 + 35, 5 = 74, 5 g. mol-1 % K in KCl is: 39 X 100 = 52, 3% 74, 5 % Cl in KCl is: 35, 5 X 100 = 47, 7% 74, 5 Percentage composition We can use this method to determine which fertilizers to use and what % of each element they 18 should contain.

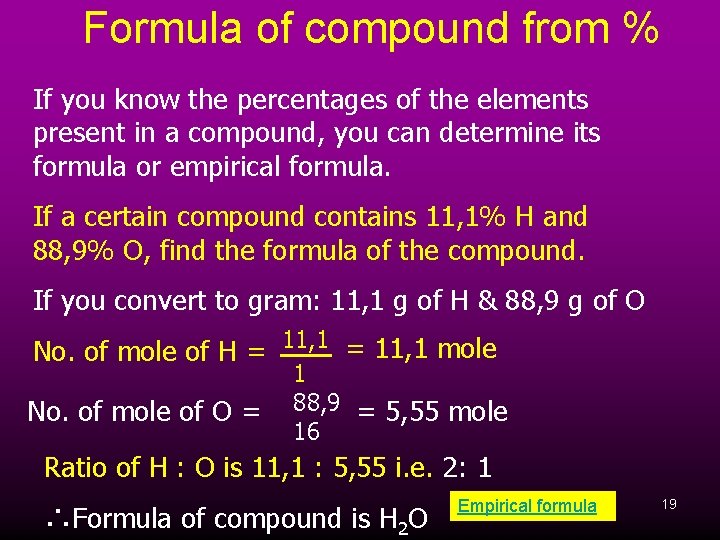
Formula of compound from % If you know the percentages of the elements present in a compound, you can determine its formula or empirical formula. If a certain compound contains 11, 1% H and 88, 9% O, find the formula of the compound. If you convert to gram: 11, 1 g of H & 88, 9 g of O No. of mole of H = 11, 1 mole No. of mole of O = 1 88, 9 = 5, 55 mole 16 Ratio of H : O is 11, 1 : 5, 55 i. e. 2: 1 ∴Formula of compound is H 2 O Empirical formula 19

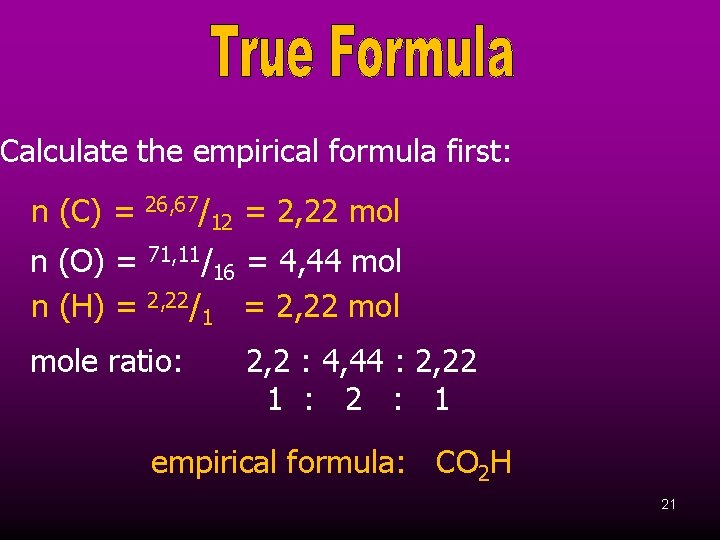
A compound consists of 26, 67 % carbon, 71, 11 % oxygen and 2, 22 % hydrogen by mass. If the relative formula mass of the compound is 90, calculate its true formula. 20

Calculate the empirical formula first: n (C) = 26, 67/ n (O) = n (H) = 71, 11/ 2, 22/ mole ratio: 12 16 1 = 2, 22 mol = 4, 44 mol = 2, 22 mol 2, 2 : 4, 44 : 2, 22 1 : 2 : 1 empirical formula: CO 2 H 21

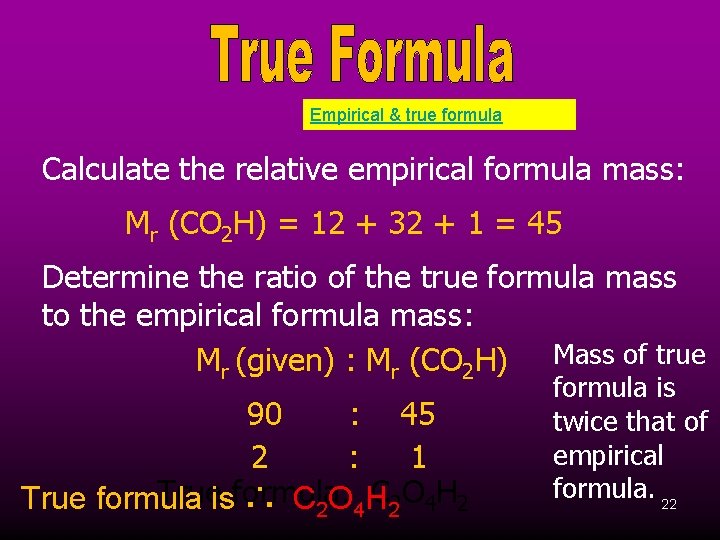
Empirical & true formula Calculate the relative empirical formula mass: Mr (CO 2 H) = 12 + 32 + 1 = 45 Determine the ratio of the true formula mass to the empirical formula mass: Mr (given) : Mr (CO 2 H) Mass of true 90 : 45 2 : 1 Trueisformula: C 22 O 4 H 2 True formula ∴ C 2 O 4 H formula is twice that of empirical formula. 22

Chemical equations are an integral part of Chemistry and give us the following information about the reacting substances: Reactants reacting Products formed Numbers of moles of reactants & products involved Proportional of masses of reactants & products Proportional volume of gases that react or are formed The physical sate (g, ℓ, aq, or s) of reactants & products 23

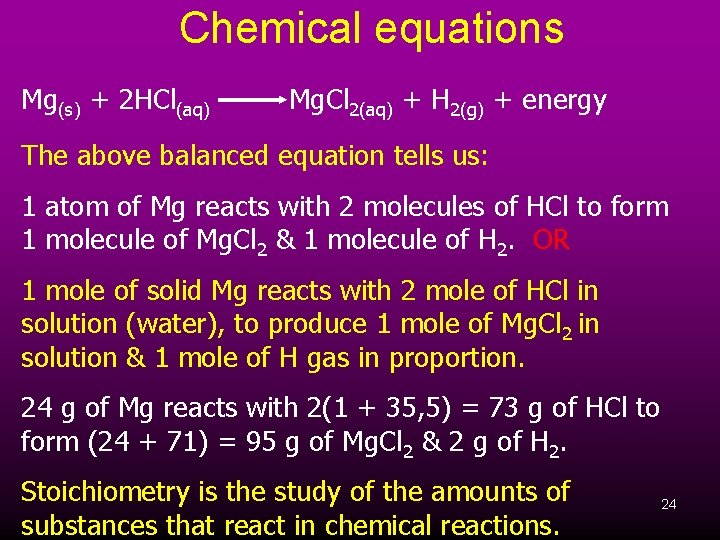
Chemical equations Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) + energy The above balanced equation tells us: 1 atom of Mg reacts with 2 molecules of HCl to form 1 molecule of Mg. Cl 2 & 1 molecule of H 2. OR 1 mole of solid Mg reacts with 2 mole of HCl in solution (water), to produce 1 mole of Mg. Cl 2 in solution & 1 mole of H gas in proportion. 24 g of Mg reacts with 2(1 + 35, 5) = 73 g of HCl to form (24 + 71) = 95 g of Mg. Cl 2 & 2 g of H 2. Stoichiometry is the study of the amounts of substances that react in chemical reactions. 24

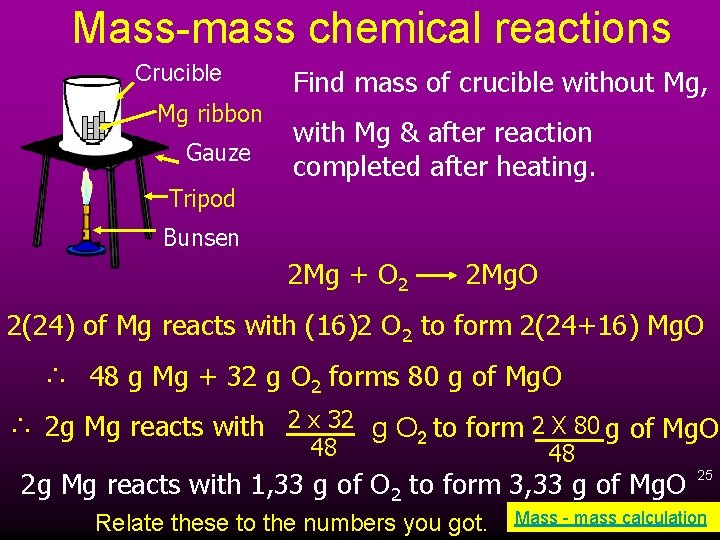
Mass-mass chemical reactions Crucible Mg ribbon Gauze Find mass of crucible without Mg, with Mg & after reaction completed after heating. Tripod Bunsen 2 Mg + O 2 2 Mg. O 2(24) of Mg reacts with (16)2 O 2 to form 2(24+16) Mg. O ∴ 48 g Mg + 32 g O 2 forms 80 g of Mg. O ∴ 2 g Mg reacts with 2 x 32 g O 2 to form 2 X 80 g of Mg. O 48 48 2 g Mg reacts with 1, 33 g of O 2 to form 3, 33 g of Mg. O Relate these to the numbers you got. 25 Mass - mass calculation

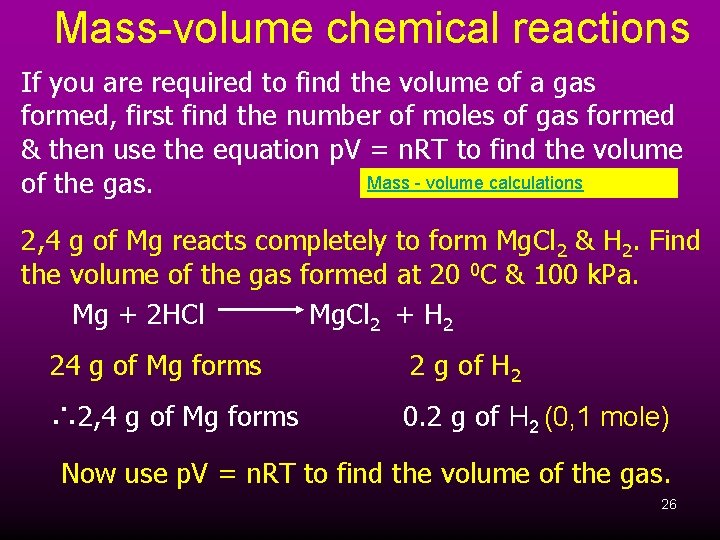
Mass-volume chemical reactions If you are required to find the volume of a gas formed, first find the number of moles of gas formed & then use the equation p. V = n. RT to find the volume Mass - volume calculations of the gas. 2, 4 g of Mg reacts completely to form Mg. Cl 2 & H 2. Find the volume of the gas formed at 20 0 C & 100 k. Pa. Mg + 2 HCl Mg. Cl 2 + H 2 24 g of Mg forms 2 g of H 2 ∴ 2, 4 g of Mg forms 0. 2 g of H 2 (0, 1 mole) Now use p. V = n. RT to find the volume of the gas. 26

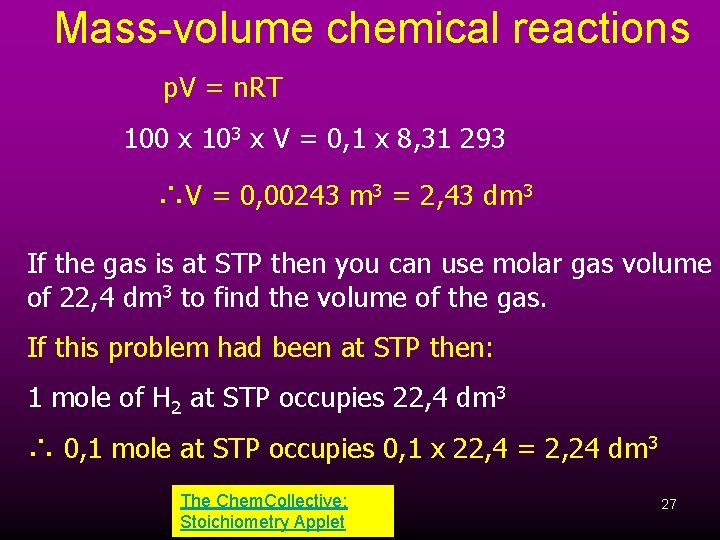
Mass-volume chemical reactions p. V = n. RT 100 x 103 x V = 0, 1 x 8, 31 293 ∴V = 0, 00243 m 3 = 2, 43 dm 3 If the gas is at STP then you can use molar gas volume of 22, 4 dm 3 to find the volume of the gas. If this problem had been at STP then: 1 mole of H 2 at STP occupies 22, 4 dm 3 ∴ 0, 1 mole at STP occupies 0, 1 x 22, 4 = 2, 24 dm 3 The Chem. Collective: Stoichiometry Applet 27

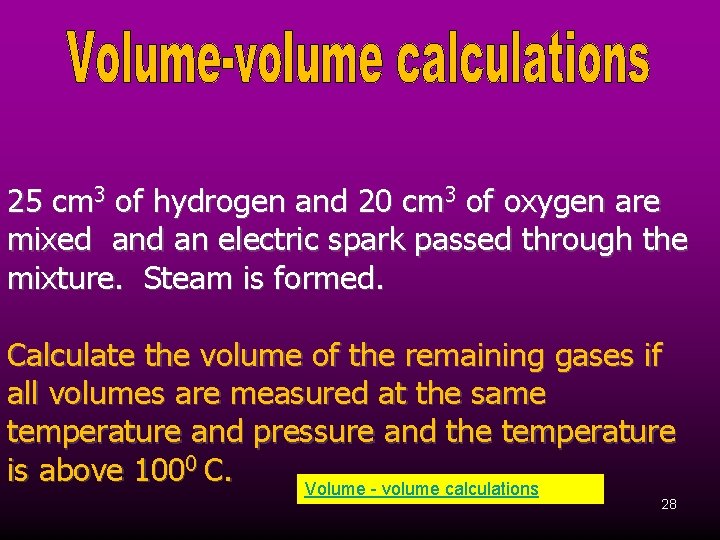
25 cm 3 of hydrogen and 20 cm 3 of oxygen are mixed an electric spark passed through the mixture. Steam is formed. Calculate the volume of the remaining gases if all volumes are measured at the same temperature and pressure and the temperature is above 1000 C. Volume - volume calculations 28

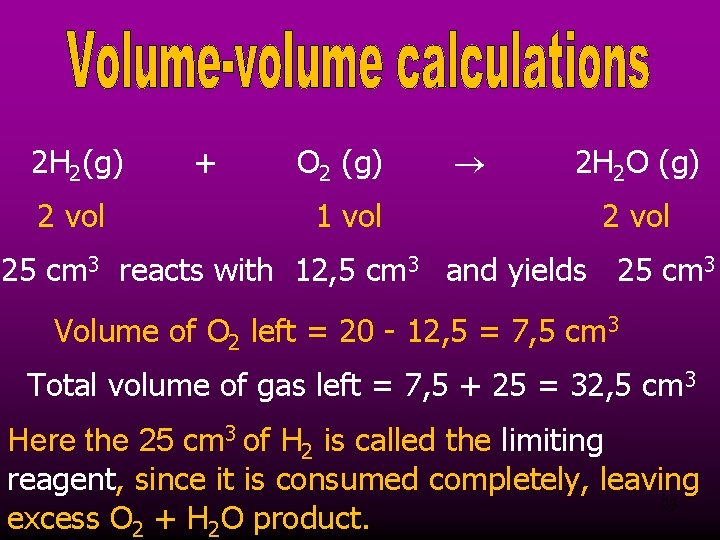
2 H 2(g) 2 vol + O 2 (g) 1 vol 2 H 2 O (g) 2 vol 25 cm 3 reacts with 12, 5 cm 3 and yields 25 cm 3 Volume of O 2 left = 20 - 12, 5 = 7, 5 cm 3 Total volume of gas left = 7, 5 + 25 = 32, 5 cm 3 Here the 25 cm 3 of H 2 is called the limiting reagent, since it is consumed completely, leaving 29 excess O 2 + H 2 O product.

Certain chemical reactions are explosive and produce a very large number of gaseous product molecules. Mining: 2 NH 4 NO 3 2 N 2(g) + 4 H 2 O(g) + O 2(g) Car engine: 2 C 8 H 18 +25 O 2 16 CO 2(g) +18 H 2 O(g) 2 STROKE PETROL ENGINE Sodium azide in car airbags: 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) Airbag operation 30