Atomic mass 17092021 RELATIVE ATOMIC MASS Ar Mass





- Slides: 15

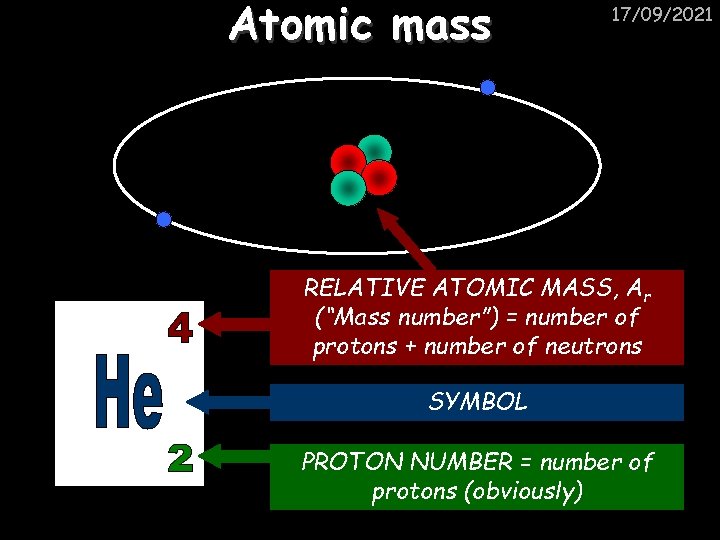
Atomic mass 17/09/2021 RELATIVE ATOMIC MASS, Ar (“Mass number”) = number of protons + number of neutrons SYMBOL PROTON NUMBER = number of protons (obviously)

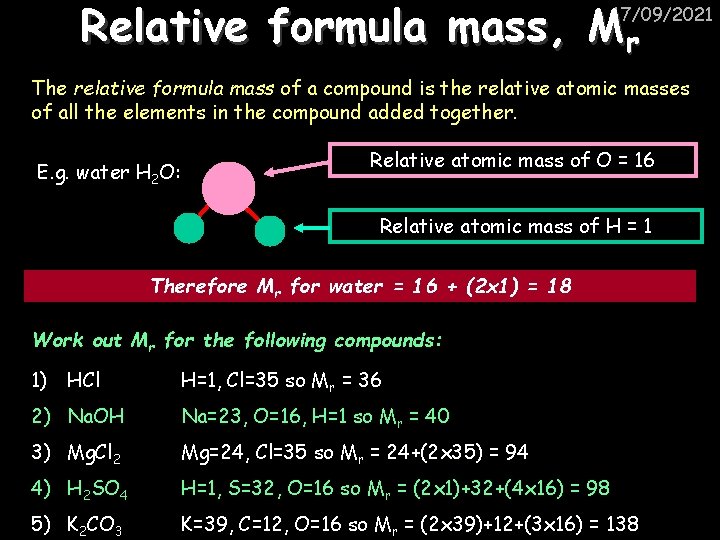
Relative formula mass, Mr 17/09/2021 The relative formula mass of a compound is the relative atomic masses of all the elements in the compound added together. E. g. water H 2 O: Relative atomic mass of O = 16 Relative atomic mass of H = 1 Therefore Mr for water = 16 + (2 x 1) = 18 Work out Mr for the following compounds: 1) HCl H=1, Cl=35 so Mr = 36 2) Na. OH Na=23, O=16, H=1 so Mr = 40 3) Mg. Cl 2 Mg=24, Cl=35 so Mr = 24+(2 x 35) = 94 4) H 2 SO 4 H=1, S=32, O=16 so Mr = (2 x 1)+32+(4 x 16) = 98 5) K 2 CO 3 K=39, C=12, O=16 so Mr = (2 x 39)+12+(3 x 16) = 138

Ca. CO 3 More examples 40 + 12 + 3 x 16 HNO 3 1 + 14 + 3 x 16 2 Mg. O 2 x (24 + 16) 3 H 2 O 3 x ((2 x 1) + 16) 17/09/2021 100 80 4 NH 3 2 KMn. O 4 3 C 2 H 5 OH 4 Ca(OH)2 Moles – The relative formula mass of a substance, in grams, is known as 1 mole of that substance. E. g. 18 g of H 2 O = 1 mole of H 2 O

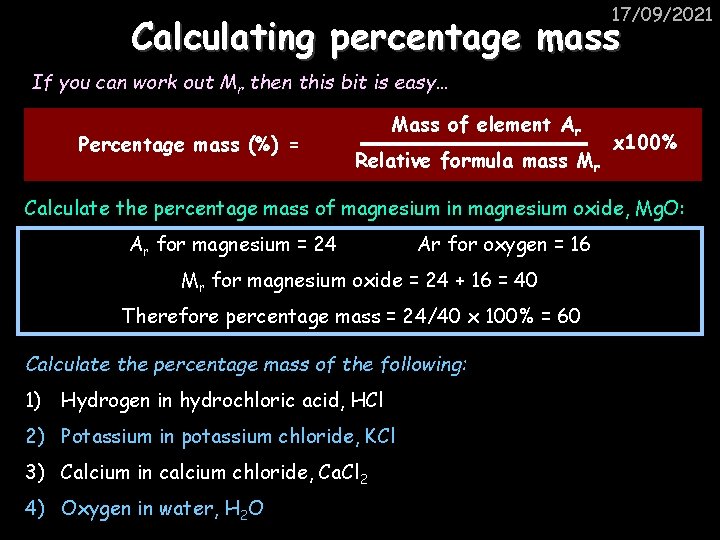
17/09/2021 Calculating percentage mass If you can work out Mr then this bit is easy… Percentage mass (%) = Mass of element Ar Relative formula mass Mr x 100% Calculate the percentage mass of magnesium in magnesium oxide, Mg. O: Ar for magnesium = 24 Ar for oxygen = 16 Mr for magnesium oxide = 24 + 16 = 40 Therefore percentage mass = 24/40 x 100% = 60% Calculate the percentage mass of the following: 1) Hydrogen in hydrochloric acid, HCl 2) Potassium in potassium chloride, KCl 3) Calcium in calcium chloride, Ca. Cl 2 4) Oxygen in water, H 2 O

Empirical formulae 17/09/2021 Empirical formulae is simply a way of showing how many atoms are in a molecule (like a chemical formula). For example, Ca. O, Ca. CO 3, H 20 and KMn. O 4 are all empirical formulae. Here’s how to work them out: A classic exam question: Find the simplest formula of 2. 24 g of iron reacting with 0. 96 g of oxygen. Step 1: Divide both masses by the relative atomic mass: For iron 2. 24/56 = 0. 04 For oxygen 0. 96/16 = 0. 06 Step 2: Write this as a ratio and simplify: 0. 04: 0. 06 is equivalent to 2: 3 Step 3: Write the formula: 2 iron atoms for 3 oxygen atoms means the formula is Fe 2 O 3

Example questions 17/09/2021 1) Find the empirical formula of magnesium oxide which contains 48 g of magnesium and 32 g of oxygen. 2) Find the empirical formula of a compound that contains 42 g of nitrogen and 9 g of hydrogen. 3) Find the empirical formula of a compound containing 20 g of calcium, 6 g of carbon and 24 g of oxygen.

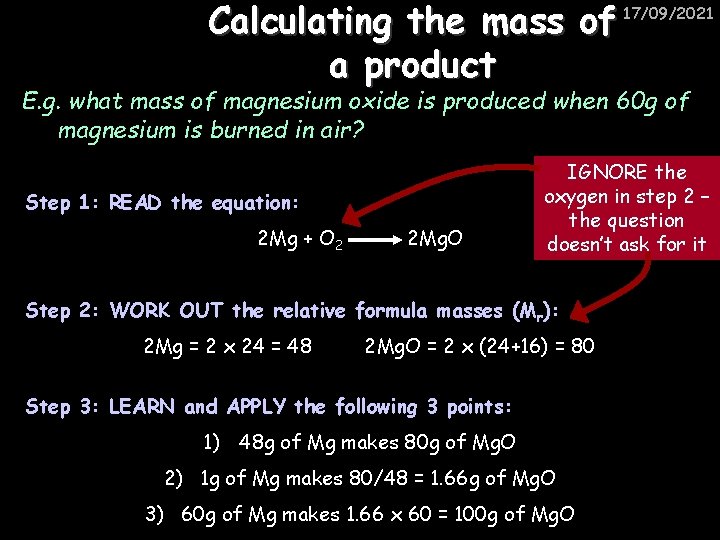
Calculating the mass of a product 17/09/2021 E. g. what mass of magnesium oxide is produced when 60 g of magnesium is burned in air? Step 1: READ the equation: 2 Mg + O 2 2 Mg. O IGNORE the oxygen in step 2 – the question doesn’t ask for it Step 2: WORK OUT the relative formula masses (Mr): 2 Mg = 2 x 24 = 48 2 Mg. O = 2 x (24+16) = 80 Step 3: LEARN and APPLY the following 3 points: 1) 48 g of Mg makes 80 g of Mg. O 2) 1 g of Mg makes 80/48 = 1. 66 g of Mg. O 3) 60 g of Mg makes 1. 66 x 60 = 100 g of Mg. O

17/09/2021 1) When water is electrolysed it breaks down into hydrogen and oxygen: 2 H 2 O 2 H 2 + O 2 What mass of hydrogen is produced by the electrolysis of 6 g of water? Work out Mr: 2 H 2 O = 2 x ((2 x 1)+16) = 36 2 H 2 = 2 x 2 = 4 1. 36 g of water produces 4 g of hydrogen 2. So 1 g of water produces 4/36 = 0. 11 g of hydrogen 3. 6 g of water will produce (4/36) x 6 = 0. 66 g of hydrogen 2) What mass of calcium oxide is produced when 10 g of calcium burns? 2 Ca + O 2 Mr: 2 Ca = 2 x 40 = 80 2 Ca. O = 2 x (40+16) = 112 80 g produces 112 g so 10 g produces (112/80) x 10 = 14 g of Ca. O 3) What mass of aluminium is produced from 100 g of aluminium oxide? 2 Al 2 O 3 4 Al + 3 O 2 Mr: 2 Al 2 O 3 = 2 x((2 x 27)+(3 x 16)) = 204 4 Al = 4 x 27 = 108 204 g produces 108 g so 100 g produces (108/204) x 100 = 52. 9 g of Al 2 O 3

Actual Yield 17/09/2021 Even though no atoms are ever gained or lost in a chemical reaction, it is not always possible to obtain the calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction

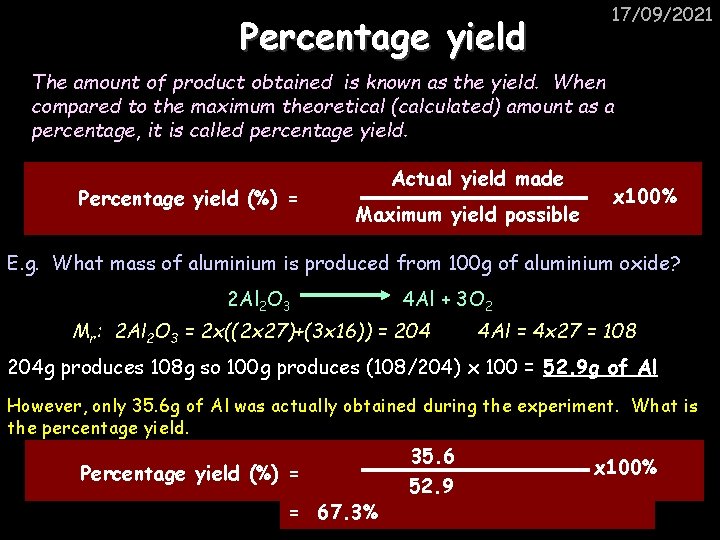
Percentage yield 17/09/2021 The amount of product obtained is known as the yield. When compared to the maximum theoretical (calculated) amount as a percentage, it is called percentage yield. Percentage yield (%) = Actual yield made Maximum yield possible x 100% E. g. What mass of aluminium is produced from 100 g of aluminium oxide? 2 Al 2 O 3 4 Al + 3 O 2 Mr: 2 Al 2 O 3 = 2 x((2 x 27)+(3 x 16)) = 204 4 Al = 4 x 27 = 108 204 g produces 108 g so 100 g produces (108/204) x 100 = 52. 9 g of Al However, only 35. 6 g of Al was actually obtained during the experiment. What is the percentage yield. Percentage yield (%) = = 67. 3% 35. 6 52. 9 x 100%

Atom economy 17/09/2021 This is simply a measure of the amount of starting materials that end up as useful products. It is important for sustainable development and economical reasons that industrial reactions have High Atom Economy (%) = Total Mr of useful products Total Mr of reactants x 100%

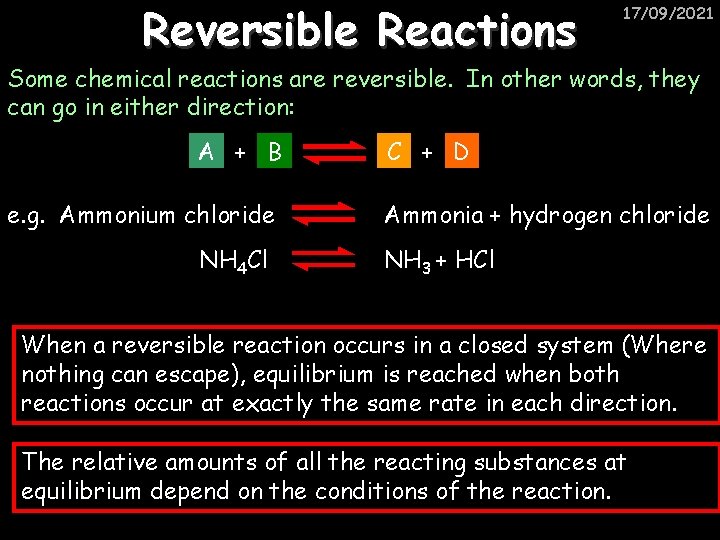
Reversible Reactions 17/09/2021 Some chemical reactions are reversible. In other words, they can go in either direction: A + B e. g. Ammonium chloride NH 4 Cl C + D Ammonia + hydrogen chloride NH 3 + HCl When a reversible reaction occurs in a closed system (Where nothing can escape), equilibrium is reached when both reactions occur at exactly the same rate in each direction. The relative amounts of all the reacting substances at equilibrium depend on the conditions of the reaction.

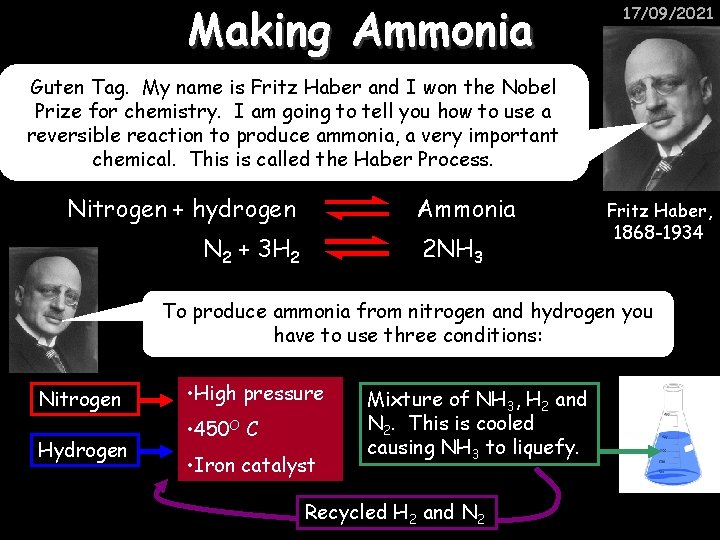
Making Ammonia 17/09/2021 Guten Tag. My name is Fritz Haber and I won the Nobel Prize for chemistry. I am going to tell you how to use a reversible reaction to produce ammonia, a very important chemical. This is called the Haber Process. Nitrogen + hydrogen Ammonia N 2 + 3 H 2 2 NH 3 Fritz Haber, 1868 -1934 To produce ammonia from nitrogen and hydrogen you have to use three conditions: Nitrogen Hydrogen • High pressure • 450 O C • Iron catalyst Mixture of NH 3, H 2 and N 2. This is cooled causing NH 3 to liquefy. Recycled H 2 and N 2

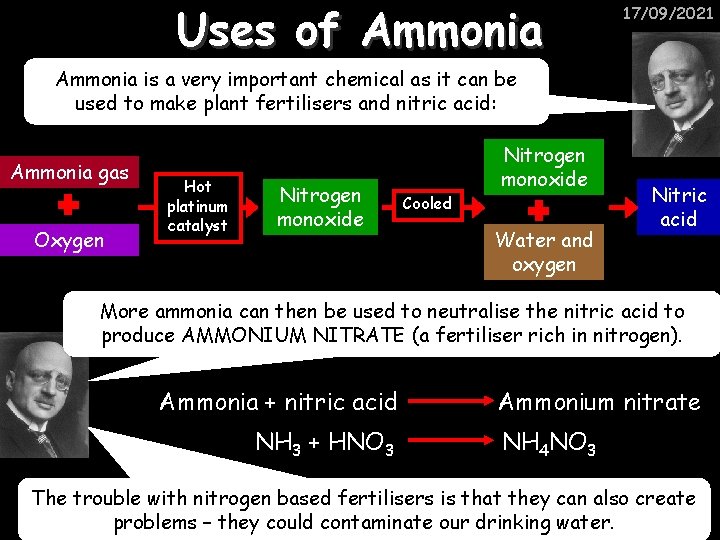
Uses of Ammonia 17/09/2021 Ammonia is a very important chemical as it can be used to make plant fertilisers and nitric acid: Ammonia gas Oxygen Hot platinum catalyst Nitrogen monoxide Cooled Nitrogen monoxide Water and oxygen Nitric acid More ammonia can then be used to neutralise the nitric acid to produce AMMONIUM NITRATE (a fertiliser rich in nitrogen). Ammonia + nitric acid NH 3 + HNO 3 Ammonium nitrate NH 4 NO 3 The trouble with nitrogen based fertilisers is that they can also create problems – they could contaminate our drinking water.

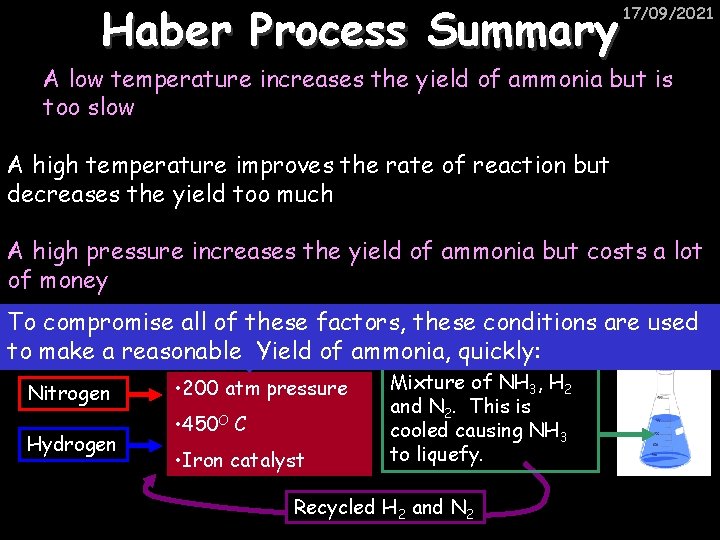
Haber Process Summary 17/09/2021 A low temperature increases the yield of ammonia but is too slow A high temperature improves the rate of reaction but decreases the yield too much A high pressure increases the yield of ammonia but costs a lot of money To compromise all of these factors, these conditions are used to make a reasonable Yield of ammonia, quickly: Nitrogen Hydrogen • 200 atm pressure • 450 O C • Iron catalyst Mixture of NH 3, H 2 and N 2. This is cooled causing NH 3 to liquefy. Recycled H 2 and N 2