Relative atomic mass and relative atomic formula Watch









- Slides: 15

Relative atomic mass and relative atomic formula

Watch the video and answer the questions 1. Where will you find the relative atomic mass for an element? 2. How do you work out the relative formula mass? 3. Why is the relative formula mass for Water (H 2 O) 18 and not 17? https: //www. youtube. com/watch? v=it_f. MQu 5 ivg

Relative Atomic Mass Ar Scientists discuss the mass if an atoms relative to the mass of a Carbon atom. The mass of a carbon atom is given as 12 and the mass of other atoms are then calculated from this. This is called the relative atomic mass (Ar) Why doesn't it have any units?

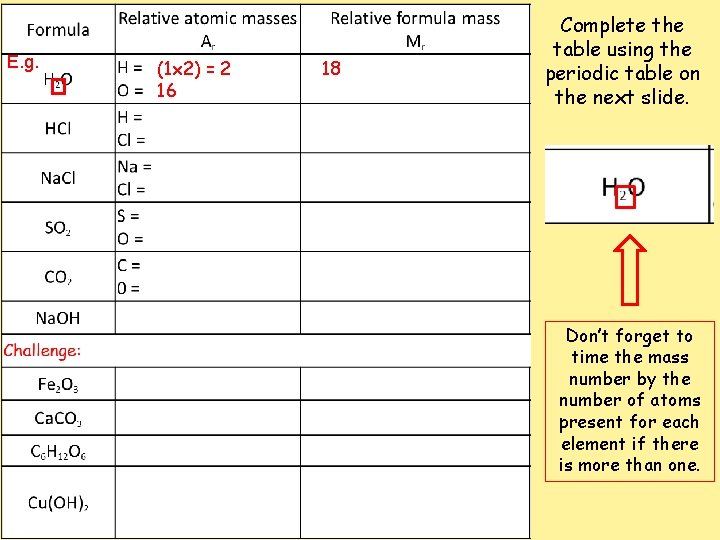
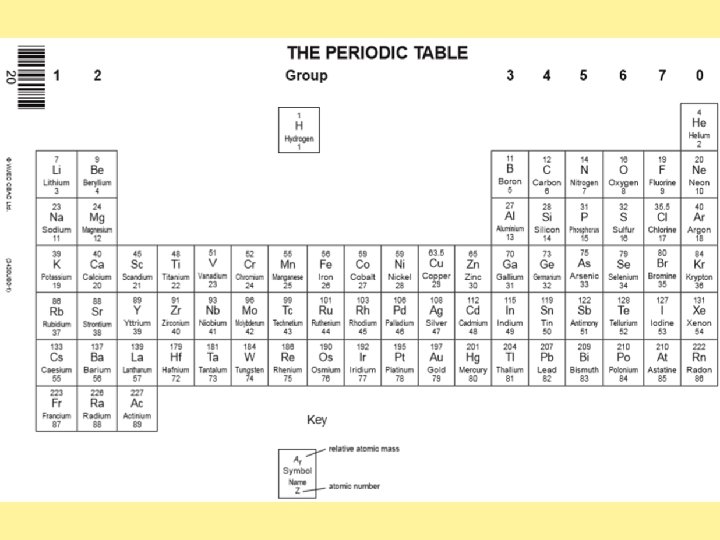
E. g. (1 x 2) = 2 16 18 Complete the table using the periodic table on the next slide. Don’t forget to time the mass number by the number of atoms present for each element if there is more than one.


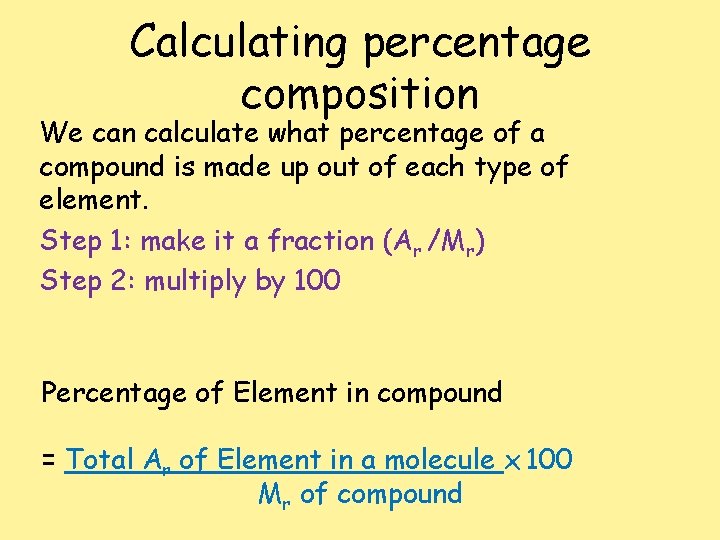
Calculating percentage composition We can calculate what percentage of a compound is made up out of each type of element. Step 1: make it a fraction (Ar /Mr) Step 2: multiply by 100 Percentage of Element in compound = Total Ar of Element in a molecule x 100 Mr of compound

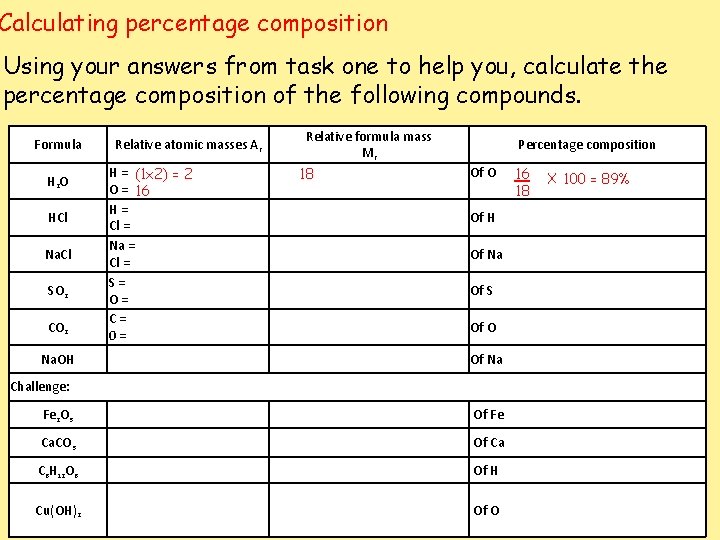
Calculating percentage composition Using your answers from task one to help you, calculate the percentage composition of the following compounds. Formula H 2 O HCl Na. Cl SO 2 CO 2 Na. OH Relative atomic masses Ar H = (1 x 2) = 2 O = 16 H= Cl = Na = Cl = S= O= C= 0= Relative formula mass Mr 18 Percentage composition Of O Of H Of Na Of S Of O Of Na Challenge: Fe 2 O 3 Of Fe Ca. CO 3 Of Ca C 6 H 12 O 6 Of H Cu(OH)2 Of O 16 18 X 100 = 89%

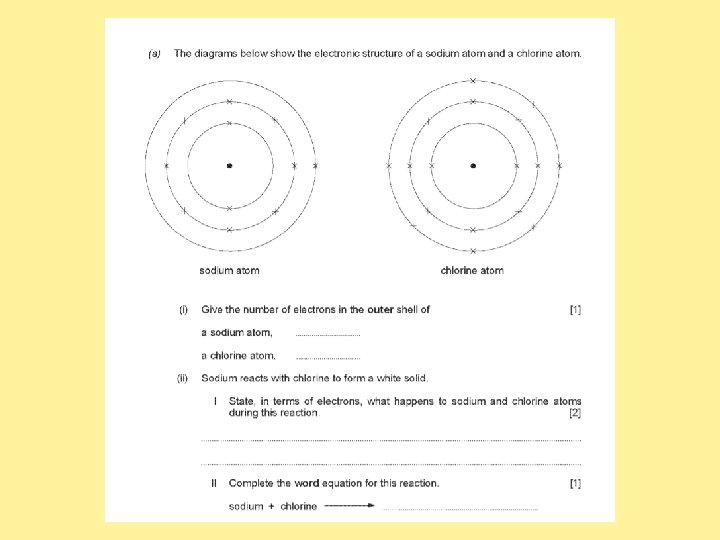
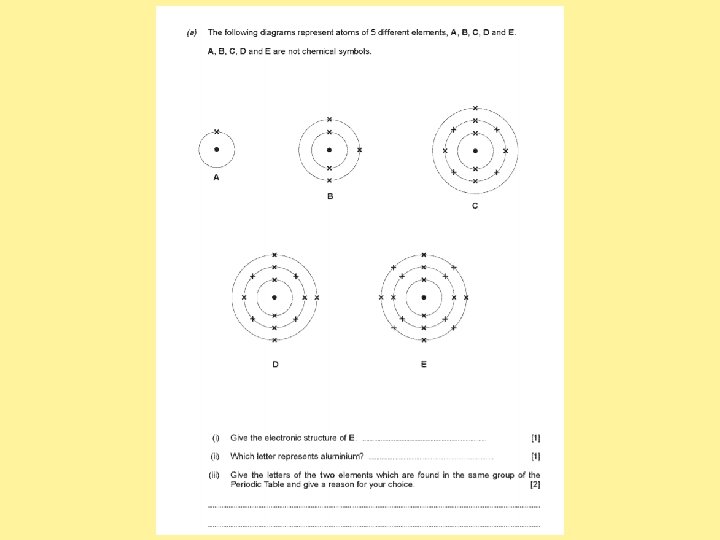
Answer the past paper questions





Word equations

A word equation is used to show what is happening in a chemical reaction. reactants products What is the word equation for hydrogen reacting with oxygen to form water?

Write a word equation for the following. 1) Methane gas burns in oxygen to produce carbon dioxide and water. 2) Lead nitrate reacts with potassium iodide. This produces a yellow solid called lead iodide and a clear liquid called potassium nitrate 3) In a car, when the engine is started enough energy is produced to combine nitrogen from the air with oxygen to form nitrogen dioxide. 4) To make sodium chloride one possible method is to react hydrochloric acid and sodium hydroxide together. This reaction also produces water 5) Often during an experiment, we find that a gas is produced. An example of this is the production of hydrogen when a metal, such as magnesium, reacts with an acid, such as hydrochloric acid. In this reaction magnesium chloride and hydrogen will be produced. 6) The colour of fireworks comes from the reactions of different metals with oxygen. The production of magnesium oxide gives a bright white colour. This comes from the reaction between magnesium and oxygen. Heat energy is needed for this to happen.