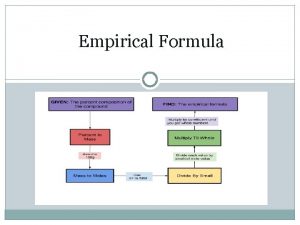
162022 Relative mass formula atomic mass and empirical







- Slides: 13

1/6/2022 Relative mass formula, atomic mass, and empirical formula www. assignmentpoint. com

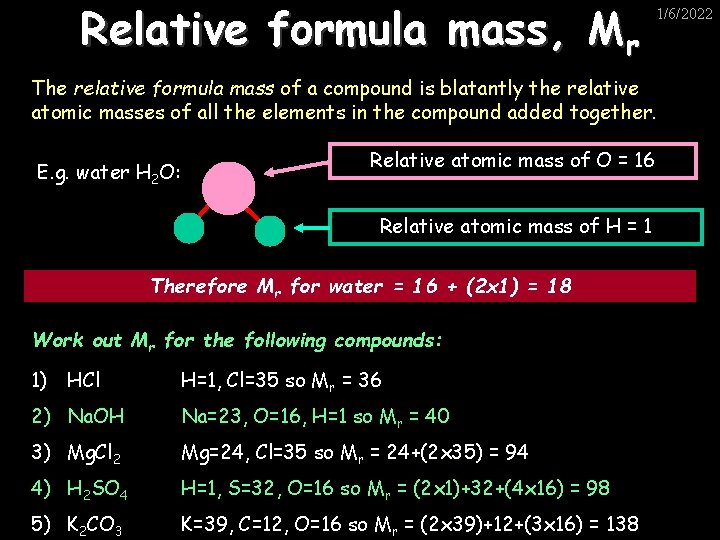
Relative formula mass, Mr 1/6/2022 The relative formula mass of a compound is blatantly the relative atomic masses of all the elements in the compound added together. E. g. water H 2 O: Relative atomic mass of O = 16 Relative atomic mass of H = 1 Therefore Mr for water = 16 + (2 x 1) = 18 Work out Mr for the following compounds: 1) HCl H=1, Cl=35 so Mr = 36 2) Na. OH Na=23, O=16, H=1 so Mr = 40 3) Mg. Cl 2 Mg=24, Cl=35 so Mr = 24+(2 x 35) = 94 4) H 2 SO 4 H=1, S=32, O=16 so Mr = (2 x 1)+32+(4 x 16) = 98 5) K 2 CO 3 K=39, C=12, O=16 so Mr = (2 x 39)+12+(3 x 16) = 138 www. assignmentpoint. com

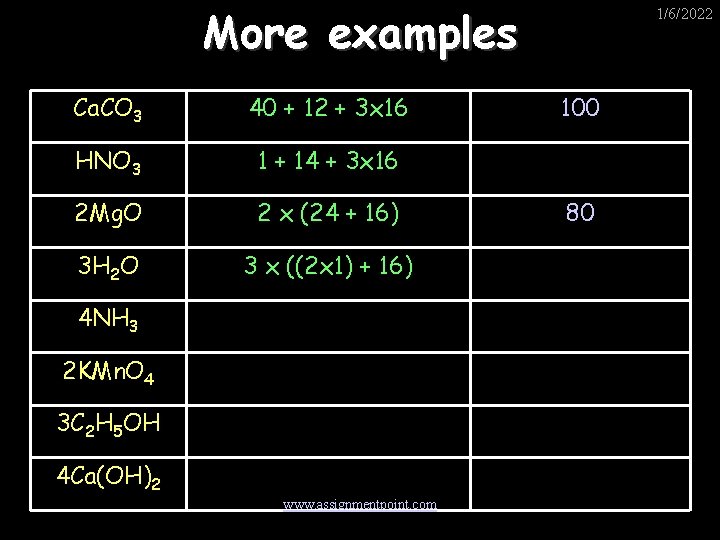
More examples Ca. CO 3 40 + 12 + 3 x 16 HNO 3 1 + 14 + 3 x 16 2 Mg. O 2 x (24 + 16) 3 H 2 O 3 x ((2 x 1) + 16) 4 NH 3 2 KMn. O 4 3 C 2 H 5 OH 4 Ca(OH)2 www. assignmentpoint. com 1/6/2022 100 80

Relative atomic mass 1/6/2022 • The mass of an isotopic element relative to Carbon 12. • Example: chlorine occurs in isotope forms Cl-35 (75. 5%) and Cl-37 (24. 5%) • Relative atomic mass = • ((75. 5 x 35)+(24. 5 x 37))/(75. 5+24. 5)=35. 5 • Try this: neon-20 (90. 9%), neon-21 (0. 3%), and neon-22 (8. 8%) www. assignmentpoint. com

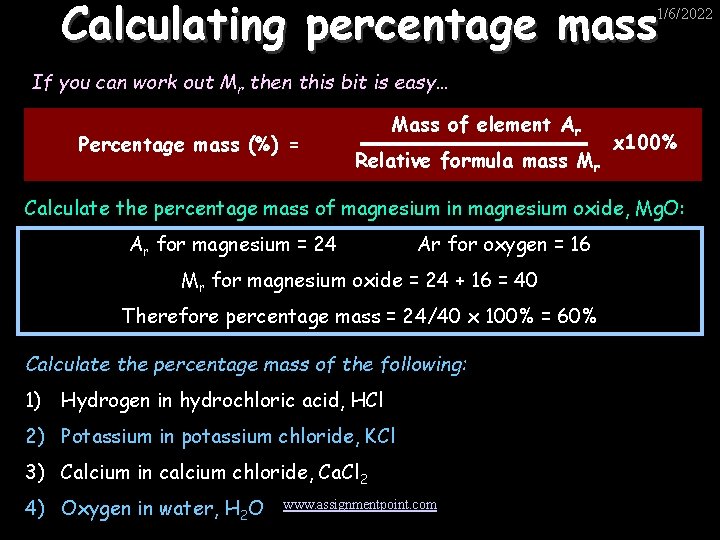
Calculating percentage mass 1/6/2022 If you can work out Mr then this bit is easy… Percentage mass (%) = Mass of element Ar Relative formula mass Mr x 100% Calculate the percentage mass of magnesium in magnesium oxide, Mg. O: Ar for magnesium = 24 Ar for oxygen = 16 Mr for magnesium oxide = 24 + 16 = 40 Therefore percentage mass = 24/40 x 100% = 60% Calculate the percentage mass of the following: 1) Hydrogen in hydrochloric acid, HCl 2) Potassium in potassium chloride, KCl 3) Calcium in calcium chloride, Ca. Cl 2 4) Oxygen in water, H 2 O www. assignmentpoint. com

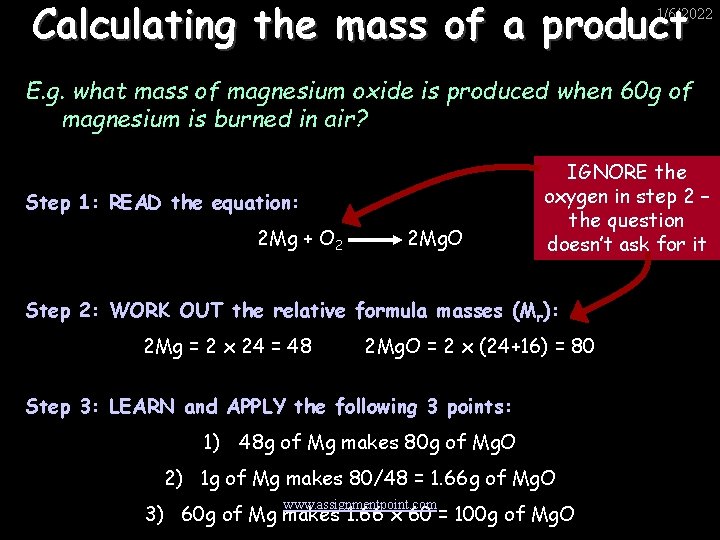
Calculating the mass of a product 1/6/2022 E. g. what mass of magnesium oxide is produced when 60 g of magnesium is burned in air? Step 1: READ the equation: 2 Mg + O 2 2 Mg. O IGNORE the oxygen in step 2 – the question doesn’t ask for it Step 2: WORK OUT the relative formula masses (Mr): 2 Mg = 2 x 24 = 48 2 Mg. O = 2 x (24+16) = 80 Step 3: LEARN and APPLY the following 3 points: 1) 48 g of Mg makes 80 g of Mg. O 2) 1 g of Mg makes 80/48 = 1. 66 g of Mg. O www. assignmentpoint. com 3) 60 g of Mg makes 1. 66 x 60 = 100 g of Mg. O

1/6/2022 1) When water is electrolysed it breaks down into hydrogen and oxygen: 2 H 2 O 2 H 2 + O 2 What mass of hydrogen is produced by the electrolysis of 6 g of water? Work out Mr: 2 H 2 O = 2 x ((2 x 1)+16) = 36 2 H 2 = 2 x 2 = 4 1. 36 g of water produces 4 g of hydrogen 2. So 1 g of water produces 4/36 = 0. 11 g of hydrogen 3. 6 g of water will produce (4/36) x 6 = 0. 66 g of hydrogen 2) What mass of calcium oxide is produced when 10 g of calcium burns? 2 Ca + O 2 Mr: 2 Ca = 2 x 40 = 80 2 Ca. O = 2 x (40+16) = 112 80 g produces 112 g so 10 g produces (112/80) x 10 = 14 g of Ca. O 3) What mass of aluminium is produced from 100 g of aluminium oxide? 2 Al 2 O 3 4 Al + 3 O 2 Mr: 2 Al 2 O 3 = 2 x((2 x 27)+(3 x 16)) = 204 www. assignmentpoint. com 4 Al = 4 x 27 = 108 204 g produces 108 g so 100 g produces (108/204) x 100 = 52. 9 g of Al 2 O 3

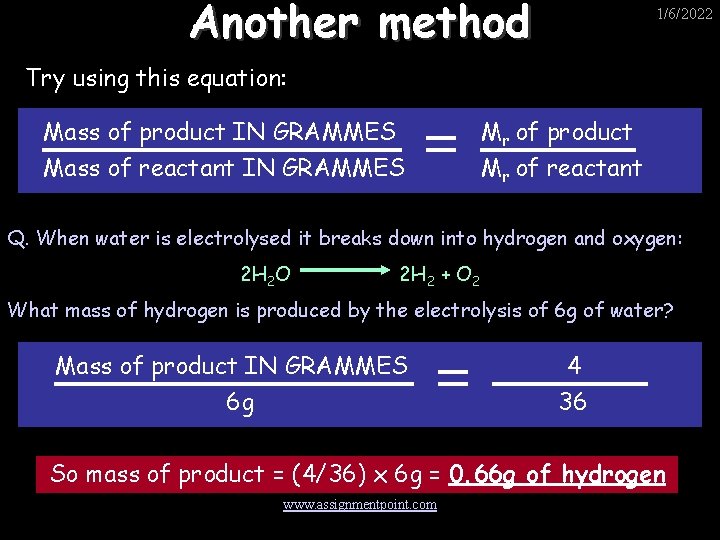
Another method 1/6/2022 Try using this equation: Mass of product IN GRAMMES Mass of reactant IN GRAMMES Mr of product Mr of reactant Q. When water is electrolysed it breaks down into hydrogen and oxygen: 2 H 2 O 2 H 2 + O 2 What mass of hydrogen is produced by the electrolysis of 6 g of water? Mass of product IN GRAMMES 6 g 4 36 So mass of product = (4/36) x 6 g = 0. 66 g of hydrogen www. assignmentpoint. com

Calculating the volume of a product 1/6/2022 At normal temperature and pressure the Relative Formula Mass (M r) of a gas will occupy a volume of 24 litres e. g. 2 g of H 2 has a volume of 24 litres 32 g of O 2 has a volume of 24 litres 44 g of CO 2 has a volume of 24 litres etc Q. When water is electrolysed it breaks down into hydrogen and oxygen: 2 H 2 O 2 H 2 + O 2 What VOLUME of hydrogen is produced by the electrolysis of 6 g of water? • On the previous page we said that the MASS of hydrogen produced was 0. 66 g • 2 g of hydrogen (H 2) will occupy 24 litres (from the red box above), • So 0. 66 g will occupy 0. 66/2 x 24 = 8 litres www. assignmentpoint. com

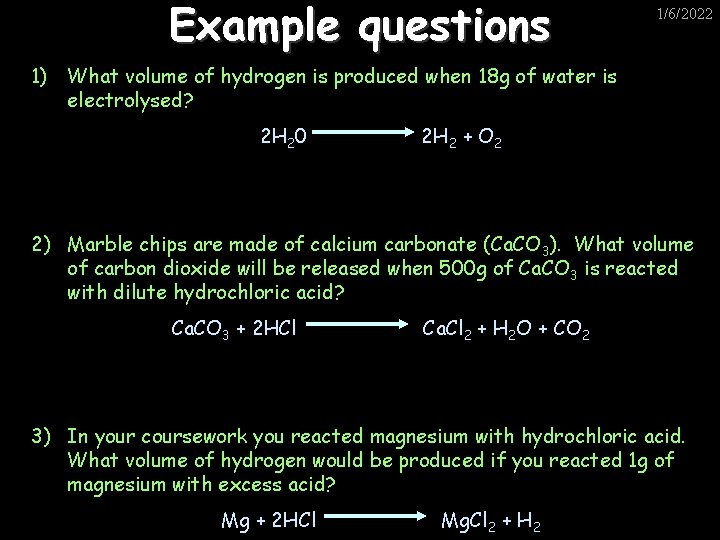
Example questions 1/6/2022 1) What volume of hydrogen is produced when 18 g of water is electrolysed? 2 H 20 2 H 2 + O 2 2) Marble chips are made of calcium carbonate (Ca. CO 3). What volume of carbon dioxide will be released when 500 g of Ca. CO 3 is reacted with dilute hydrochloric acid? Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 O + CO 2 3) In your coursework you reacted magnesium with hydrochloric acid. What volume of hydrogen would be produced if you reacted 1 g of magnesium with excess acid? www. assignmentpoint. com Mg + 2 HCl Mg. Cl 2 + H 2

Empirical formulae 1/6/2022 Empirical formulae is simply a way of showing how many atoms are in a molecule (like a chemical formula). For example, Ca. O, Ca. CO 3, H 20 and KMn. O 4 are all empirical formulae. Here’s how to work them out: A classic exam question: Find the simplest formula of 2. 24 g of iron reacting with 0. 96 g of oxygen. Step 1: Divide both masses by the relative atomic mass: For iron 2. 24/56 = 0. 04 For oxygen 0. 96/16 = 0. 06 Step 2: Write this as a ratio and simplify: 0. 04: 0. 06 is equivalent to 2: 3 Step 3: Write the formula: www. assignmentpoint. com 2 iron atoms for 3 oxygen atoms means the formula is Fe 2 O 3

Example questions 1/6/2022 1) Find the empirical formula of magnesium oxide which contains 48 g of magnesium and 32 g of oxygen. 2) Find the empirical formula of a compound that contains 42 g of nitrogen and 9 g of hydrogen. 3) Find the empirical formula of a compound containing 20 g of calcium, 6 g of carbon and 24 g of oxygen. www. assignmentpoint. com

1/6/2022 This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. www. assignmentpoint. com
 Relative atomic mass of beryllium
Relative atomic mass of beryllium Difference between atomic mass and mass number
Difference between atomic mass and mass number Atomic mass and atomic number difference
Atomic mass and atomic number difference Relative atomic mass of barium
Relative atomic mass of barium Relative atomic mass of boron
Relative atomic mass of boron Define relative atomic mass
Define relative atomic mass Relative atomic mass
Relative atomic mass Mass and atomic number
Mass and atomic number How to find empirical formula from percentages
How to find empirical formula from percentages Derive semi empirical mass formula
Derive semi empirical mass formula Semi empirical mass formula
Semi empirical mass formula Converting mass to moles
Converting mass to moles Atomic mass vs molar mass
Atomic mass vs molar mass Molecular formula and empirical formula
Molecular formula and empirical formula