Unit 1 Matter and Measure Measure Scientific Measurement






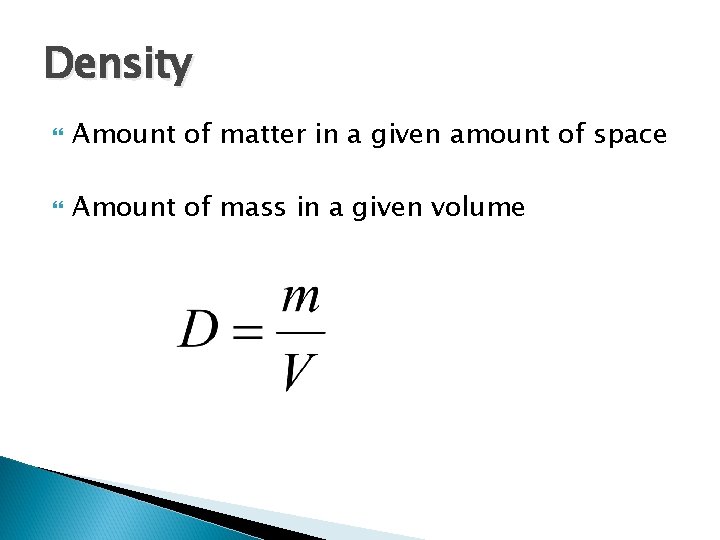
















- Slides: 45

Unit 1 Matter and Measure

Measure

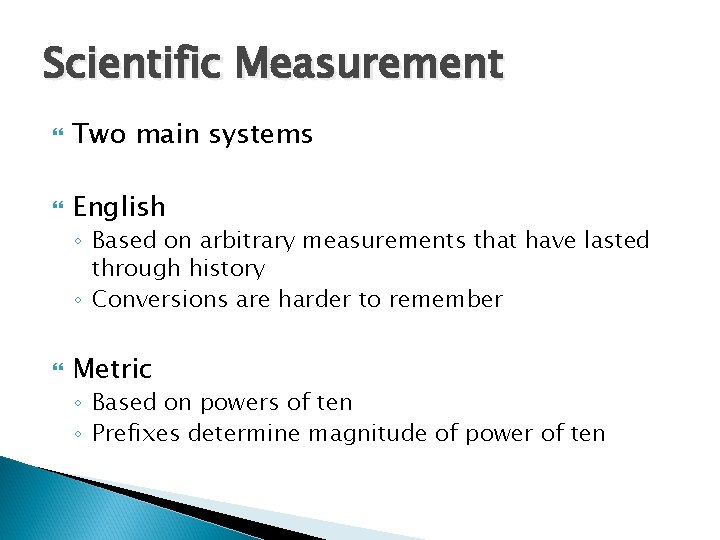
Scientific Measurement Two main systems English ◦ Based on arbitrary measurements that have lasted through history ◦ Conversions are harder to remember Metric ◦ Based on powers of ten ◦ Prefixes determine magnitude of power of ten

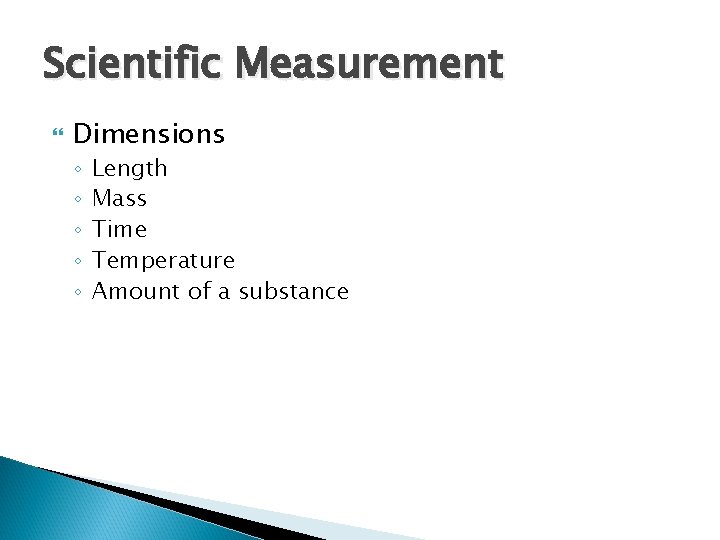
Scientific Measurement Dimensions ◦ ◦ ◦ Length Mass Time Temperature Amount of a substance

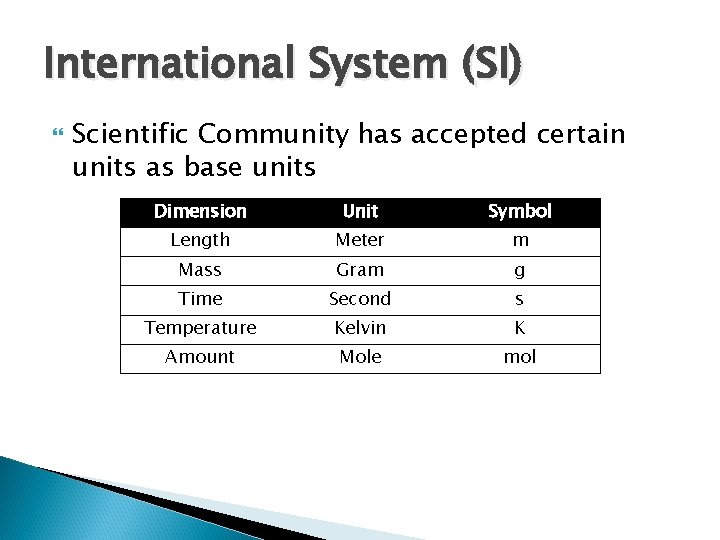
International System (SI) Scientific Community has accepted certain units as base units Dimension Unit Symbol Length Meter m Mass Gram g Time Second s Temperature Kelvin K Amount Mole mol

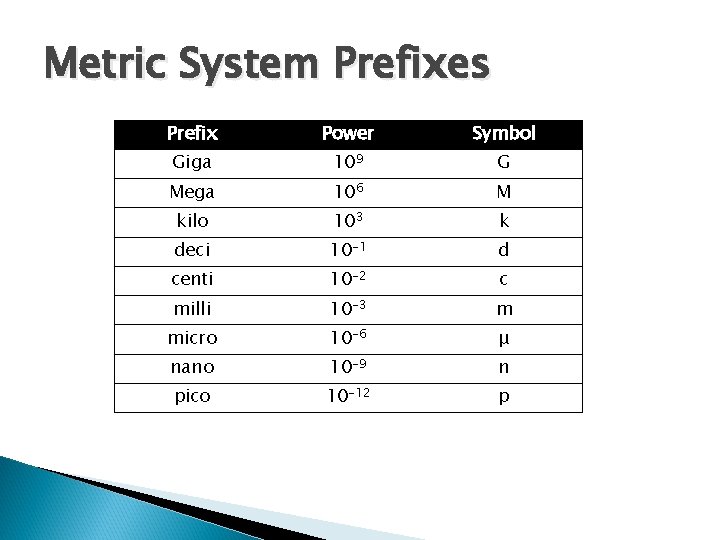
Metric System Prefixes Prefix Power Symbol Giga 109 G Mega 106 M kilo 103 k deci 10 -1 d centi 10 -2 c milli 10 -3 m micro 10 -6 μ nano 10 -9 n pico 10 -12 p

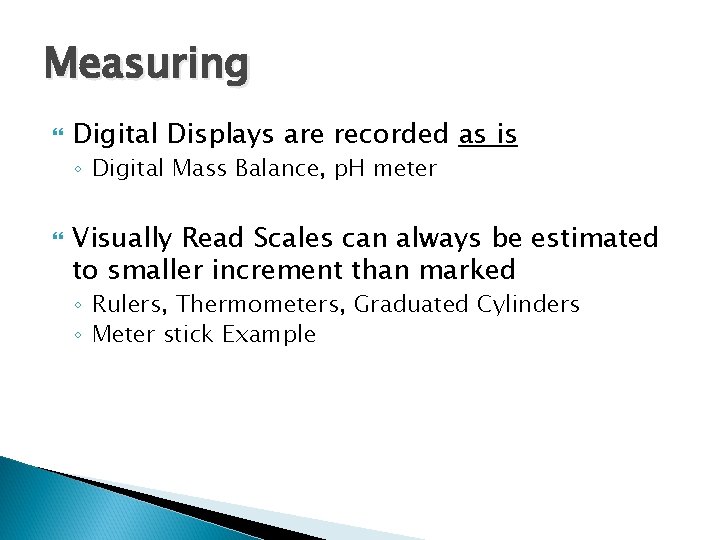
Measuring Digital Displays are recorded as is ◦ Digital Mass Balance, p. H meter Visually Read Scales can always be estimated to smaller increment than marked ◦ Rulers, Thermometers, Graduated Cylinders ◦ Meter stick Example

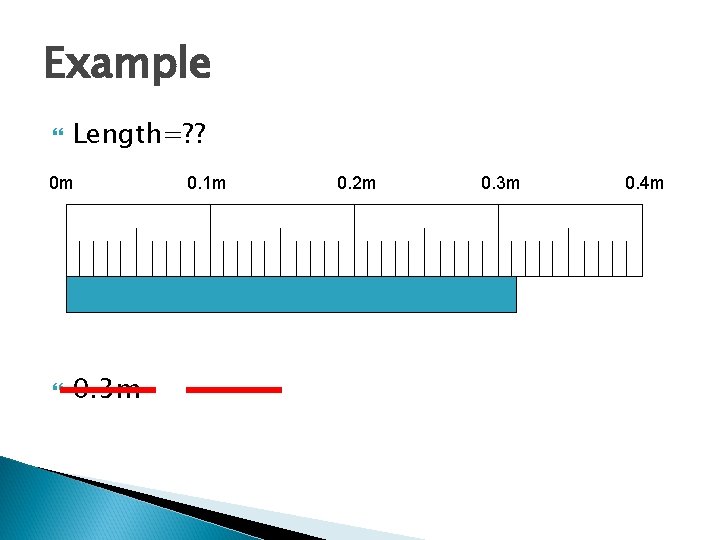
Example Length=? ? 0 m 0. 3 m 0. 1 m 0. 31 m 0. 2 m 0. 314 m 0. 3 m 0. 4 m

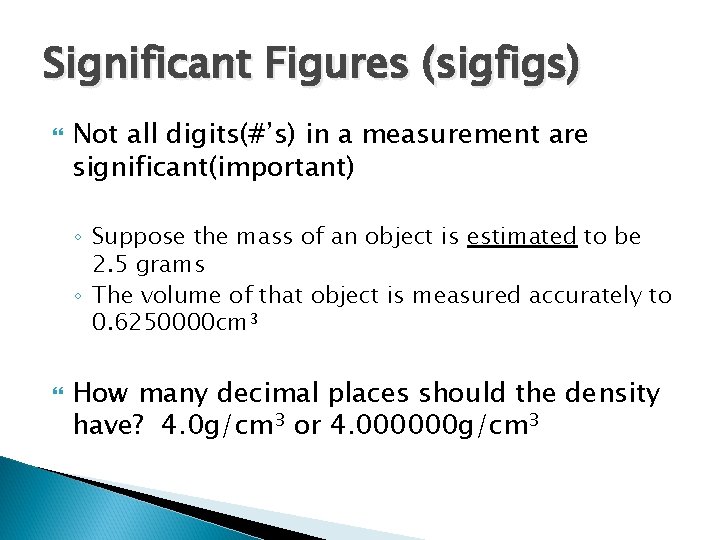
Significant Figures (sigfigs) Not all digits(#’s) in a measurement are significant(important) ◦ Suppose the mass of an object is estimated to be 2. 5 grams ◦ The volume of that object is measured accurately to 0. 6250000 cm 3 How many decimal places should the density have? 4. 0 g/cm 3 or 4. 000000 g/cm 3

Significant Figures (cont) Rules on pages 66 -71 of textbook Shorthand ◦ If the decimal point is present, start counting digits from the Pacific (left) side, starting with the first non-zero digit. 123 0. 00310 (3 sig. figs. )

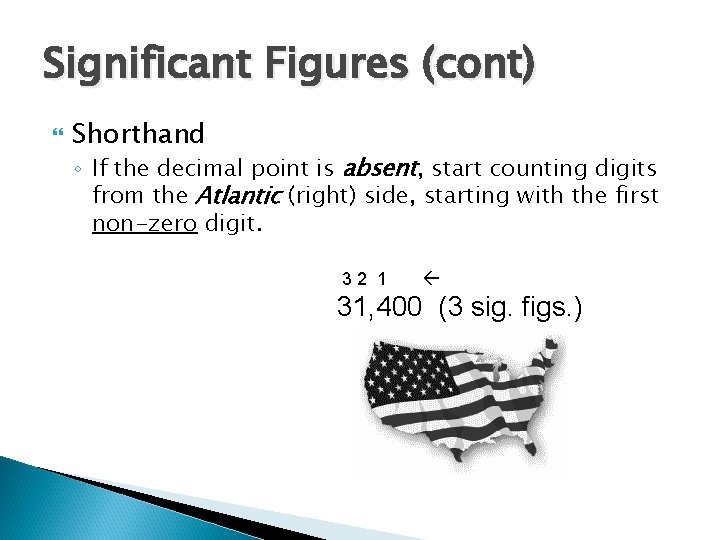
Significant Figures (cont) Shorthand ◦ If the decimal point is absent, start counting digits from the Atlantic (right) side, starting with the first non-zero digit. 32 1 31, 400 (3 sig. figs. )

Sig. Figs for Math Addition and Subtraction ◦ Answer has to have the same number of decimal places as least decimal places in what you are adding or subtracting Example ◦ 15. 62 -7. 248 = ? ? ? Calculator 8. 372 Science 8. 37

Sig. Figs for Math Multiplication and Division ◦ Answer has to have same number of Sigfigs as least number of Sigfigs in what you are multiplying or dividing Example ◦ 7. 55*0. 34 = ? ? ? Calculator 2. 567 Science 2. 6

Scientific Notation Short hand way of writing very large and very small numbers ◦ Uses only sigfigs Examples: ◦ 602, 000, 000, 000 ◦ 6. 02 x 1023 ◦ 0. 00000567 ◦ 5. 67 x 10 -10

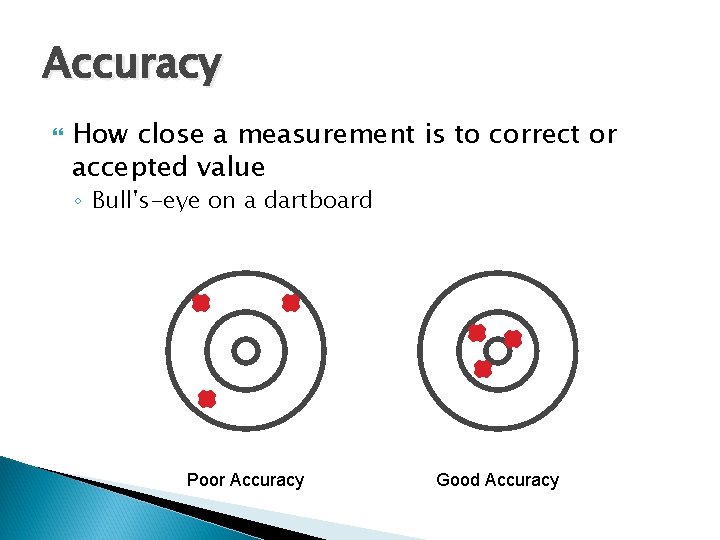
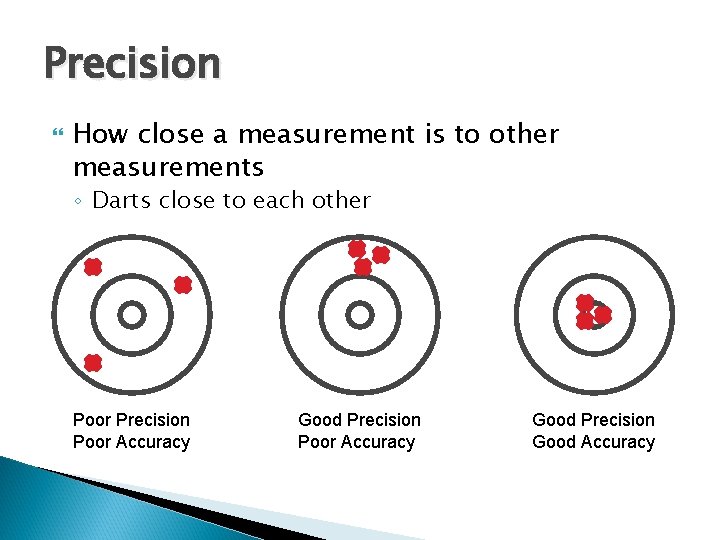
Accuracy How close a measurement is to correct or accepted value ◦ Bull's-eye on a dartboard Poor Accuracy Good Accuracy

Precision How close a measurement is to other measurements ◦ Darts close to each other Poor Precision Poor Accuracy Good Precision Good Accuracy

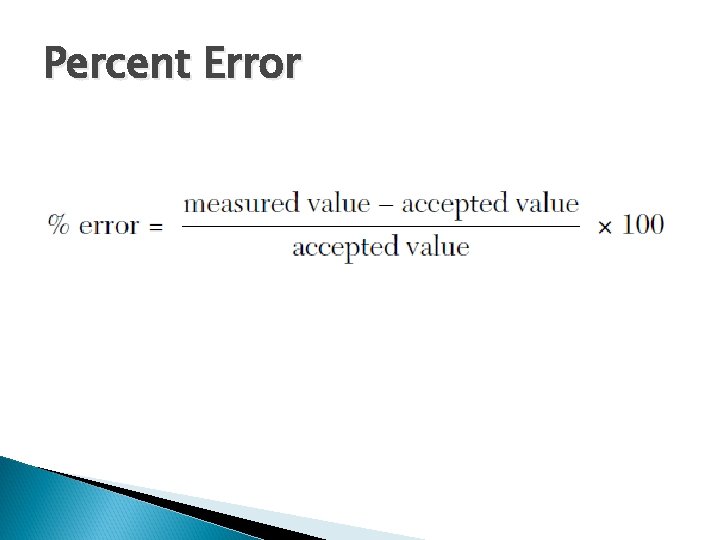
Percent Error

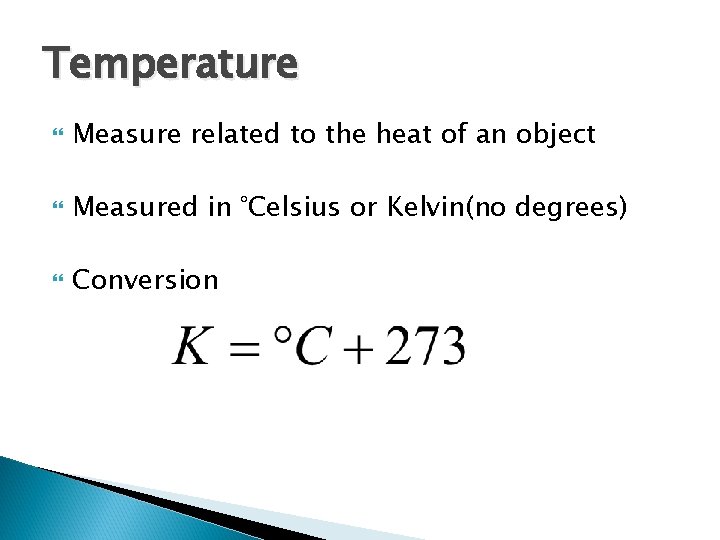
Temperature Measure related to the heat of an object Measured in °Celsius or Kelvin(no degrees) Conversion
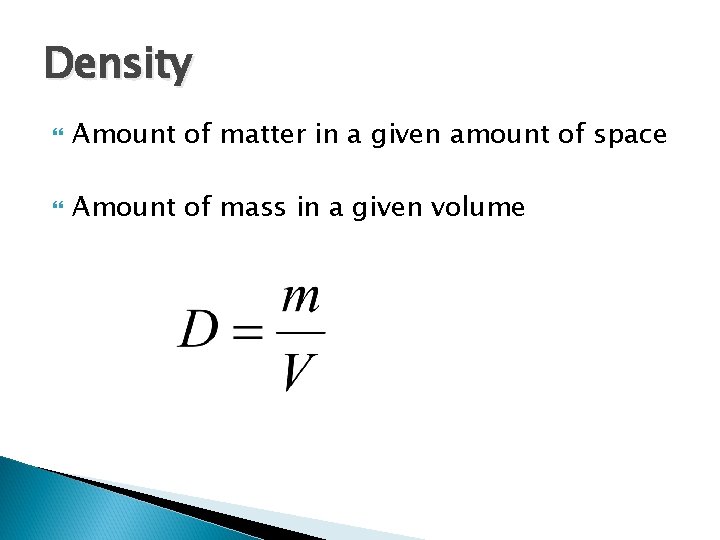
Density Amount of matter in a given amount of space Amount of mass in a given volume


Matter

Chemistry What is Chemistry? ◦ Study of matter and the changes it undergoes Branches ◦ ◦ ◦ Organic Physical Analytical Biochemical Inorganic

IUPAC International Union of Pure and Applied Chemistry Group that names elements and compounds Meets every few years

Matter Anything that has mass and takes up space, volume

Atom Simplest form of matter Made up of Subatomic Particles Different atoms have different properties

Pure Substances Element ◦ simplest form of matter that has a unique set of properties. ◦ arranged into a table, called the periodic table ◦ Can’t be broken down by chemical means ◦ denoted using chemical symbols, O, Cu, Fe Symbols always have the first letter capitalized If needed, any additional letters are not capitalized

Pure Substances (Cont) Compounds ◦ substance of two or more elements chemically combined in a fixed proportion ◦ Ex. H 2 O, C 6 H 12 O 6 ◦ Can be broken down by chemical means

Mixtures Physical blend of two or more substances Two Types: ◦ Homogeneous ◦ Heterogeneous

Mixtures (cont) Homogeneous ◦ ◦ Composition is uniform throughout Examples: Air, Olive Oil, Stainless Steel Solution is a homogeneous mixture Aqueous Solution is something mixed in water Heterogeneous ◦ Composition is not uniform throughout ◦ Examples: Salad Dressing, Chicken Noodle Soup

Separating Mixtures Differences in physical properties can be used to separate mixtures ◦ Filtration – Separates solids from liquids in heterogeneous mixtures ◦ Distillation – Separates homogeneous liquid mixtures based on different boiling points

Separating Mixtures ◦ Evaporation – evaporate away liquid to leave solid ◦ Chromatography – separation of substances based on polarity

Phases(States) of Matter Solid ◦ Definite shape and volume ◦ Particles are packed tightly together in a regular geometric pattern ◦ (s) used after chemical formulas ◦ Cu(s)

Phases of Matter Liquid ◦ Definite volume, takes shape of container ◦ Particles can slide past each other ◦ (l) used after chemical formulas ◦ H 2 O(l)

Phases of Matter Gas ◦ Takes shape and volume of container ◦ Particles are spread very far apart ◦ (g) used after chemical formulas ◦ H 2 O(g)

Aqueous Solutions (aq) used after chemical symbols ◦ Na. Cl(aq)

Phase Changes Solid Liquid Solid Liquid Gas Liquid Solid Gas Solid Melting Freezing Vaporization Condensation Sublimation Deposition Temperature does NOT change during a phase change

Identifying Substances Physical Property ◦ quality or condition of a substance that can be observed or measured without changing the substance’s composition ◦ Ex: Color, shape, size, mass Physical Change ◦ some properties change, but the composition remains the same ◦ Can be reversible or irreversible ◦ Ex: melting, freezing, tearing

Identifying Substances (cont) Chemical Change ◦ change that produces matter with a different composition than the original matter ◦ Ex. burning, rusting, decomposing, exploding, corroding Chemical property ◦ property that can only be observed by changing the composition of the substance. ◦ Ex: Reactivity with acids, reactivity with oxygen

Physical or Chemical ? Boiling Point Green color Shiny Conductivity Solubility Reacts with acid Reacts with O 2 Physical Physical Chemical

Describing Matter Extensive Properties ◦ property that depends on the amount of matter in a sample. ◦ Ex: mass, weight, volume Intensive Properties ◦ property that depends on the type of matter in a sample, not the amount of matter ◦ Ex: Density, hardness, viscosity

Energy Capacity to do work Ability to do something Types: ◦ ◦ ◦ Chemical Electrical Mechanical Potential Kinetic

Energy Exchanges Exothermic ◦ Process when energy is released or given off ◦ Ex: Burning, freezing Endothermic ◦ Process when energy is absorbed or taken in ◦ Ex: Melting

Scientific Method Observation ◦ using five senses to make observations. Hypothesis ◦ proposed explanation for an observation. Experiment ◦ procedure used to test a hypothesis.

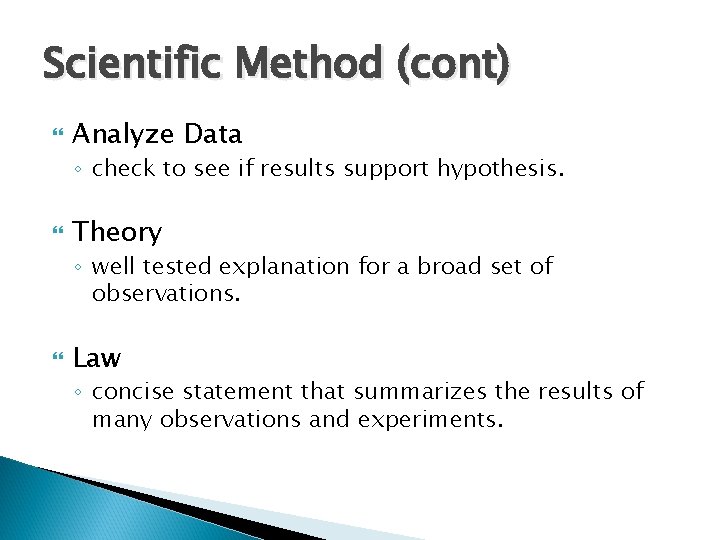
Scientific Method (cont) Analyze Data ◦ check to see if results support hypothesis. Theory ◦ well tested explanation for a broad set of observations. Law ◦ concise statement that summarizes the results of many observations and experiments.

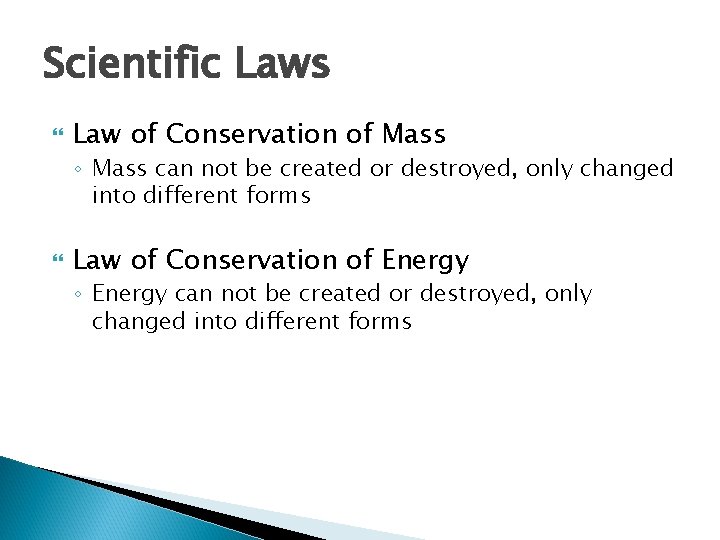
Scientific Laws Law of Conservation of Mass ◦ Mass can not be created or destroyed, only changed into different forms Law of Conservation of Energy ◦ Energy can not be created or destroyed, only changed into different forms
 Matter energy and measurement
Matter energy and measurement Measurement and scientific tools lesson 2 answer key
Measurement and scientific tools lesson 2 answer key Lesson 1 understanding science answer key
Lesson 1 understanding science answer key Measurement and scientific tools lesson 2
Measurement and scientific tools lesson 2 Grey matter reliaquest
Grey matter reliaquest Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter Ncl caudatus
Ncl caudatus The mole: a measurement of matter answer key
The mole: a measurement of matter answer key 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Density is qualitative or quantitative
Density is qualitative or quantitative The mole a measurement of matter
The mole a measurement of matter Chemistry chapter 3 scientific measurement
Chemistry chapter 3 scientific measurement Chapter 3 scientific measurement
Chapter 3 scientific measurement Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Flow of energy vs flow of matter
Flow of energy vs flow of matter What is scientific matter
What is scientific matter Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Measure vs measurement
Measure vs measurement What weather instrument measures air temperature
What weather instrument measures air temperature Brainpop measurement
Brainpop measurement How to measure matter
How to measure matter A measurement includes both a number and a unit.
A measurement includes both a number and a unit. Volume formula
Volume formula Unit 6 review questions
Unit 6 review questions Unit 2 matter and change
Unit 2 matter and change Unit 2 matter and energy
Unit 2 matter and energy Is measure for measure a comedy
Is measure for measure a comedy What are the units for momentum? *
What are the units for momentum? * Mole unit of measurement
Mole unit of measurement Mole unit of measurement
Mole unit of measurement Cohesion bond
Cohesion bond Characteristics of pure water
Characteristics of pure water Unit of measurement for power
Unit of measurement for power Derived quantity
Derived quantity Ostwald viscometer diagram
Ostwald viscometer diagram Miniature inertial measurement unit
Miniature inertial measurement unit Gtt unit of measurement
Gtt unit of measurement Climatell
Climatell Metric measurement chart
Metric measurement chart Unit of measurement
Unit of measurement Limitation of accuracy
Limitation of accuracy