Unit 1 Matter Measurement Properties of Matter What






- Slides: 13

Unit 1: Matter & Measurement Properties of Matter

What is matter? • Anything that has mass and volume and is made of atoms. Examples: air, clouds, smoke, ice, liquid water, water vapor, DNA, proteins, etc. • Energy is NOT matter. Examples of energy: light, heat, X-rays, UV radiation, etc.

Kinetic Theory of Matter is made up of particles which are in continual random motion.

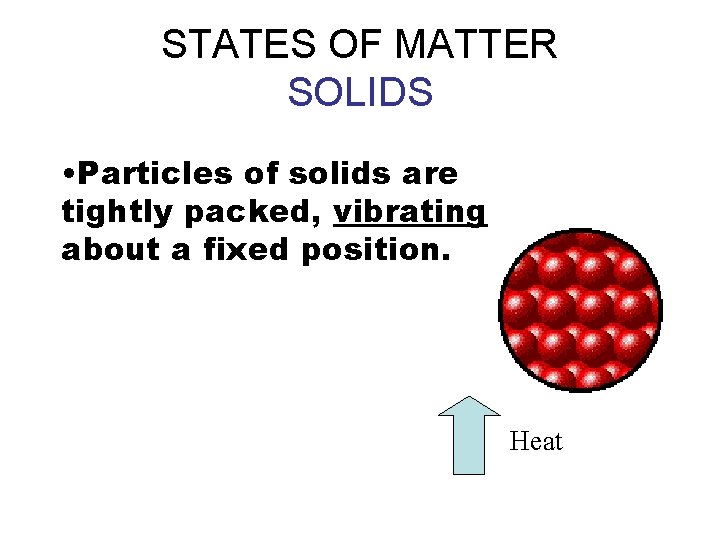
STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. Heat

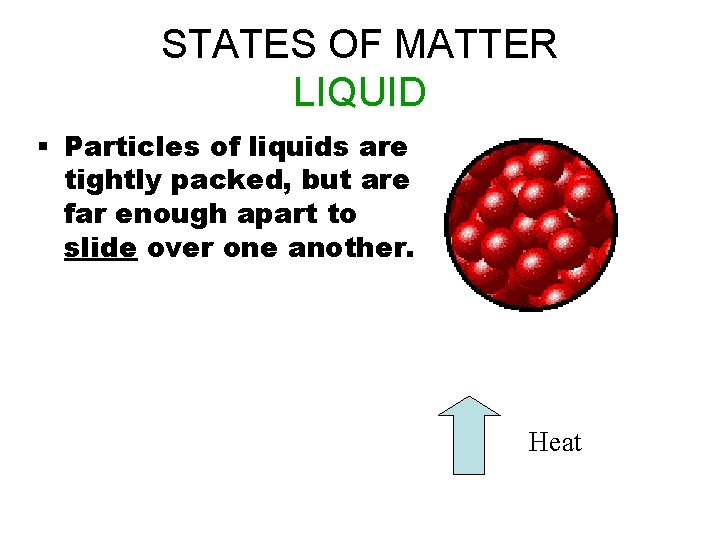
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. Heat

STATES OF MATTER GAS § Particles of gases are very far apart and move freely.

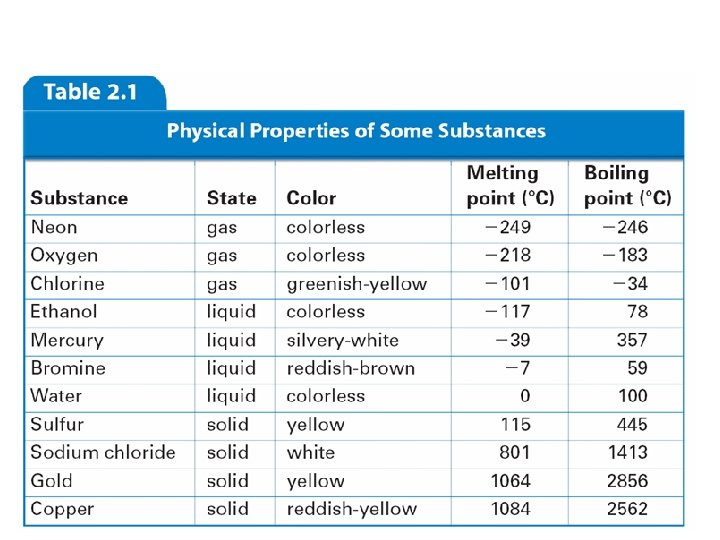
Physical properties of Matter A physical property is a quality or condition of a substance that can be observed or measured without changing the substance’s composition. Examples: - Texture - Color - State (solid, liquid, gas) - Conductivity - Ductility (ability to be stretched into a wire) - Malleability (ability to be bent into shapes) - Melting Point - Boiling Point - Density


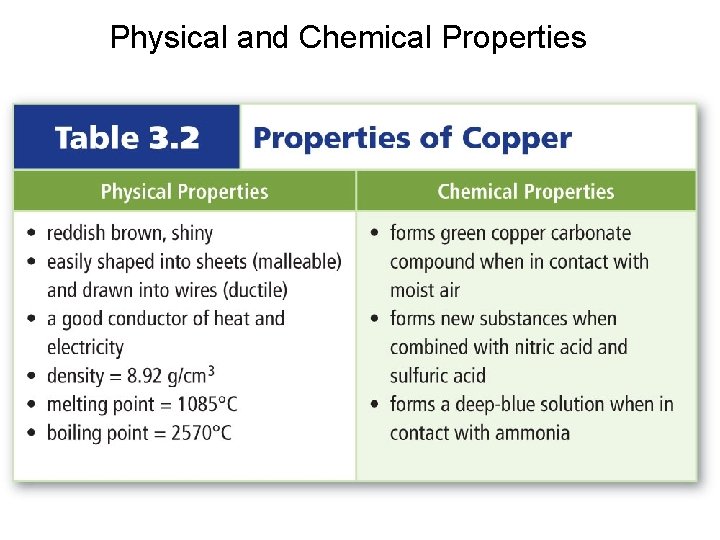
Chemical Properties of Matter The ability of a substance to combine with or change into one or more other substances is called a chemical property. – Iron forming rust – Copper turning green in the air

Physical and Chemical Properties

Quick Quiz 1. Match the states of matter with the following descriptions: (1) takes the volume and shape of its container (2) has a definite shape and volume (3) has a definite volume but an indefinite shape A. (1) liquid, (2) solid and (3) gas B. (1) gas, (2) solid, and (3) liquid C. (1) gas, (2) liquid, and (3) solid

Quick Quiz. 2. Compared to liquids and solids, gases are easily compressed because the particles in a gas… A) B) C) D) attract each other significantly are spaced relatively far apart are extremely small move in constant, random motion

Quick Quiz. 3. Which properties can be observed without changing the composition of a substance? A. all properties of a substance B. intensive properties C. chemical properties D. physical properties
 2018 geometry bootcamp answers
2018 geometry bootcamp answers The mole a measurement of matter answer key
The mole a measurement of matter answer key Matter energy and measurement
Matter energy and measurement 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Density is a qualitative measurement of matter.
Density is a qualitative measurement of matter. The mole a measurement of matter
The mole a measurement of matter Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Extensive examples
Extensive examples Chemical property of matter
Chemical property of matter Units of measurement for momentum
Units of measurement for momentum Unit of moles
Unit of moles Mole unit of measurement
Mole unit of measurement Salinity unit of measurement
Salinity unit of measurement Characteristics of pure water
Characteristics of pure water