Density Qualitative and Quantitative Density is a ratio







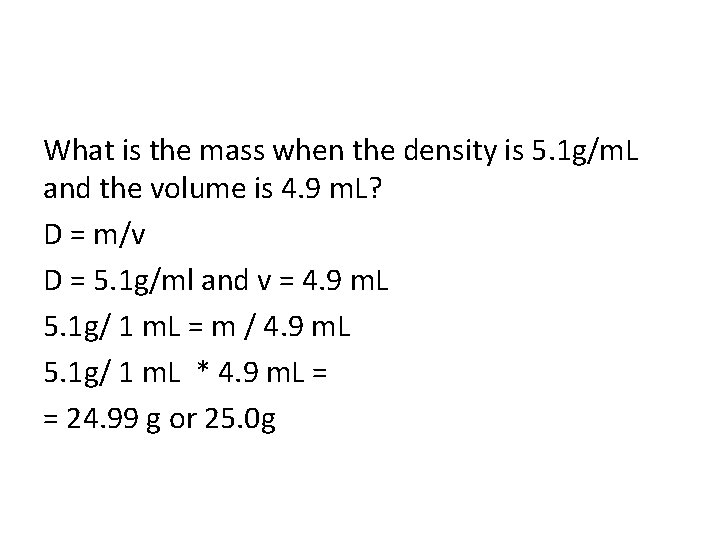
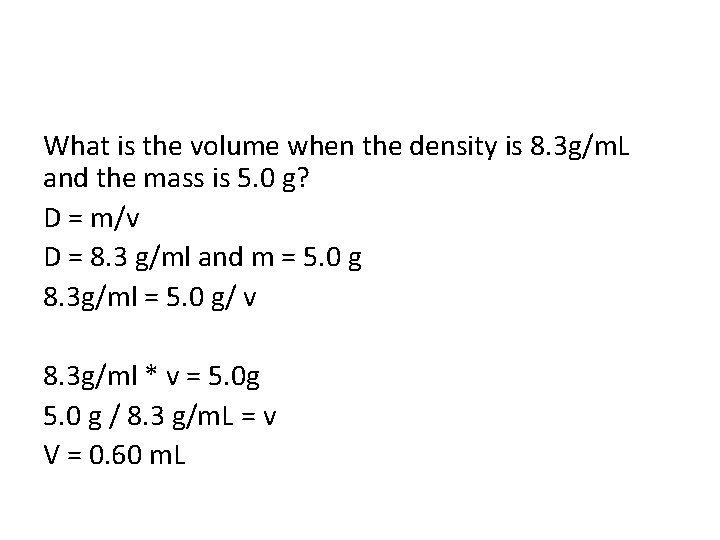
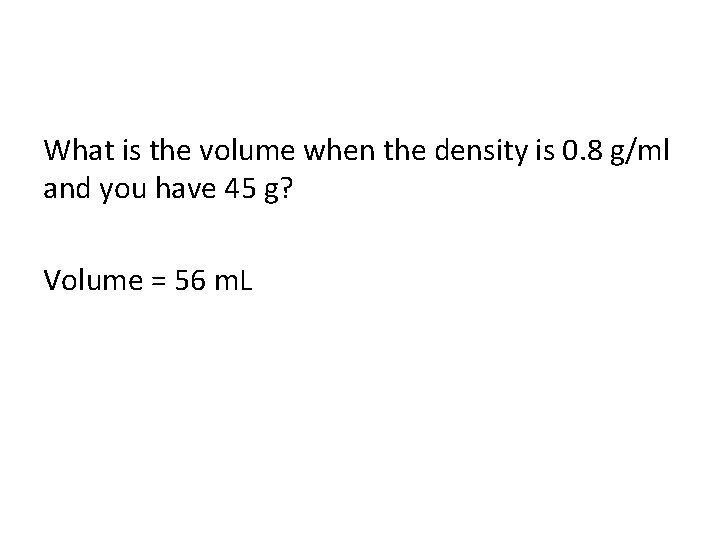
- Slides: 15

Density Qualitative and Quantitative

Density is a ratio between the amount of space occupied by matter (measured by mass) and the volume this matter has to spread out. Density is a property which can be used as one piece of data to identify an unknown. It is a general trend found on the periodic table.

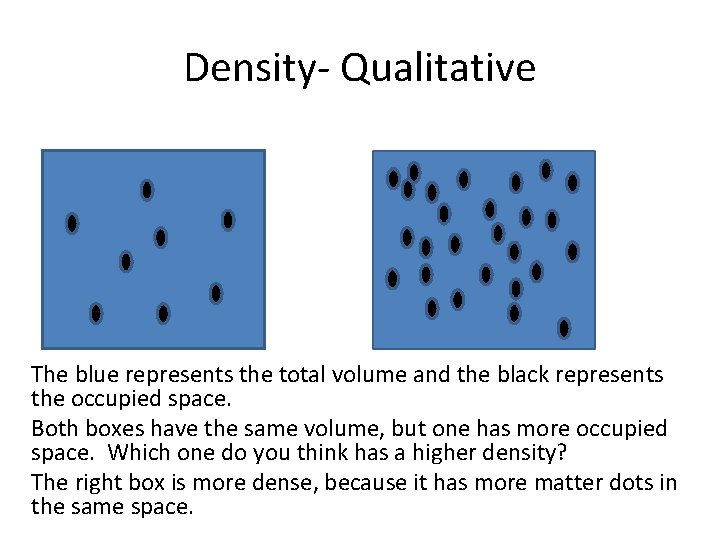
Density- Qualitative The blue represents the total volume and the black represents the occupied space. Both boxes have the same volume, but one has more occupied space. Which one do you think has a higher density? The right box is more dense, because it has more matter dots in the same space.

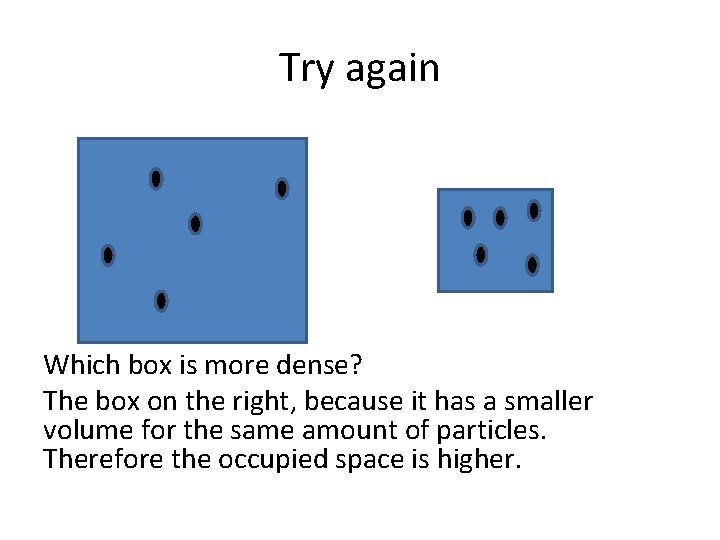
Try again Which box is more dense? The box on the right, because it has a smaller volume for the same amount of particles. Therefore the occupied space is higher.

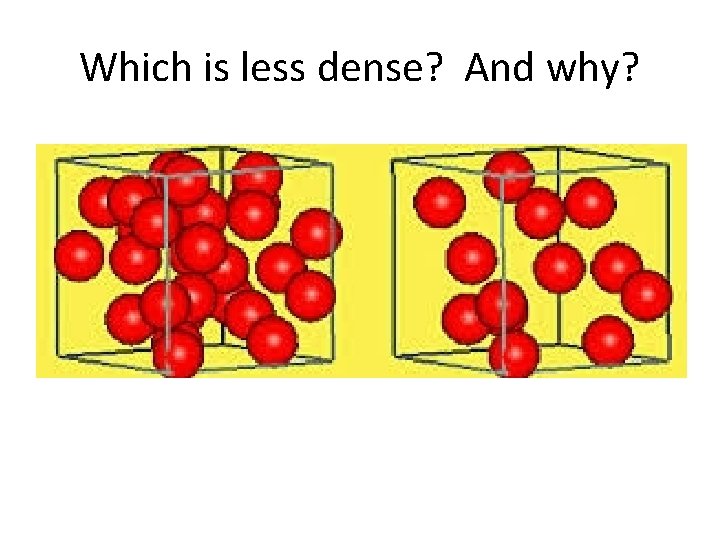
Which is less dense? And why?

Which layer is the most dense? Why?

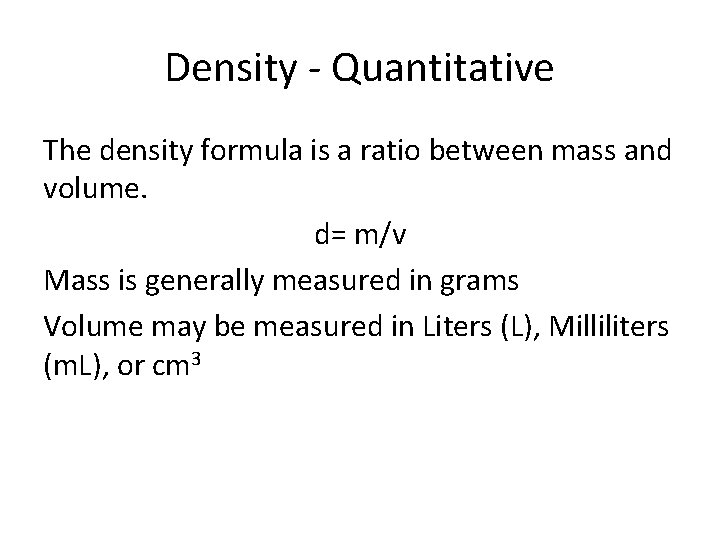
Density - Quantitative The density formula is a ratio between mass and volume. d= m/v Mass is generally measured in grams Volume may be measured in Liters (L), Milliliters (m. L), or cm 3

Example problem A cube has a mass of 2. 33 g and a volume of 1. 45 m. L. What is the density of the cube? To determine the density of the cube, you plug the numbers in the formula according to the quantity it is measuring. m = 2. 33 g V = 1. 45 ml

1. Write down formula: d = m/v 2. Identify what the given quantities are from the problem m = 2. 33 g v = 1. 45 ml 3. Plug the quantities into the formula d = 2. 33 g / 1. 45 m. L 4. Solve for the unknown d = 1. 61 g/m. L Notice: neither unit canceled with the other

Try on your own What is the density of a sphere with a volume of 43 m. L and a mass of 34. 2 g? Hint: what are the two numbers in the problem a measurement of?

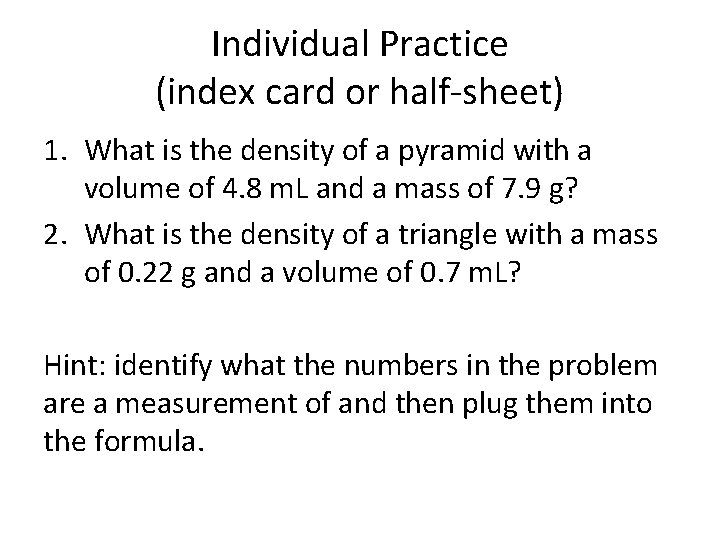
Individual Practice (index card or half-sheet) 1. What is the density of a pyramid with a volume of 4. 8 m. L and a mass of 7. 9 g? 2. What is the density of a triangle with a mass of 0. 22 g and a volume of 0. 7 m. L? Hint: identify what the numbers in the problem are a measurement of and then plug them into the formula.

If the density is 3. 5 g/ml and the volume is 34 ml, then what is the mass of the solution? 1. Formula: d=m/v 2. ID the variables: d = 3. 5 g/ml and v = 34 ml 3. Plug the variables into the formula 3. 5 g/ml = m/ 34 ml 4. Solve for the unknown 3. 5 g/ml * 34 ml = = 119 g
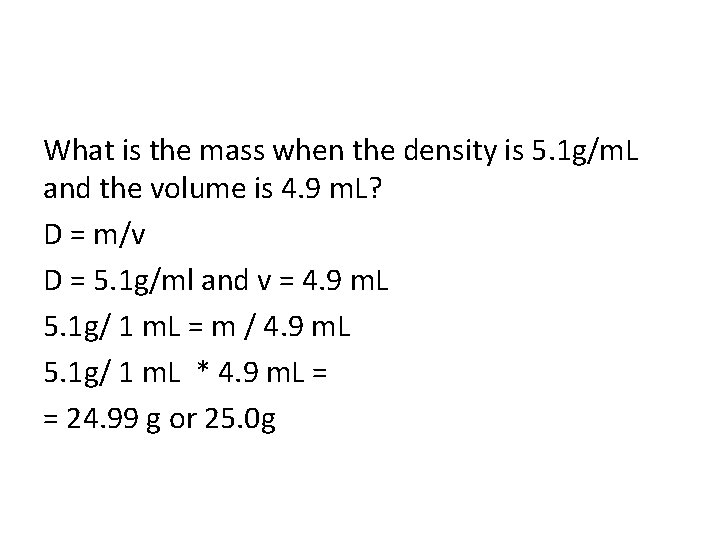
What is the mass when the density is 5. 1 g/m. L and the volume is 4. 9 m. L? D = m/v D = 5. 1 g/ml and v = 4. 9 m. L 5. 1 g/ 1 m. L = m / 4. 9 m. L 5. 1 g/ 1 m. L * 4. 9 m. L = = 24. 99 g or 25. 0 g
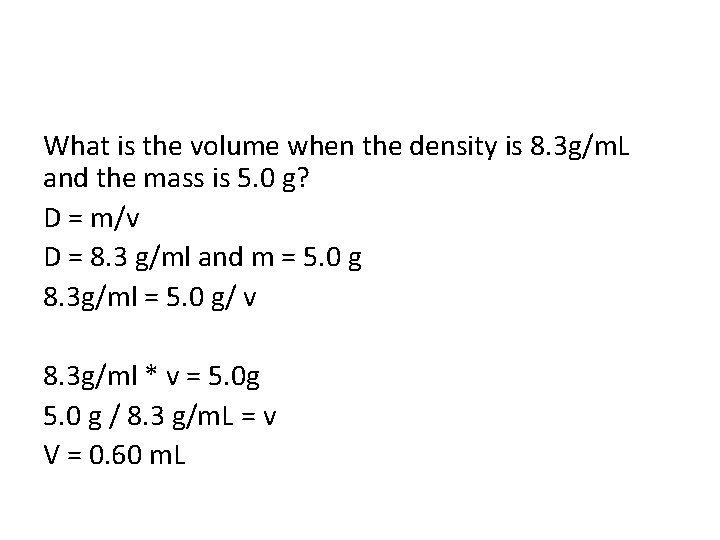
What is the volume when the density is 8. 3 g/m. L and the mass is 5. 0 g? D = m/v D = 8. 3 g/ml and m = 5. 0 g 8. 3 g/ml = 5. 0 g/ v 8. 3 g/ml * v = 5. 0 g 5. 0 g / 8. 3 g/m. L = v V = 0. 60 m. L
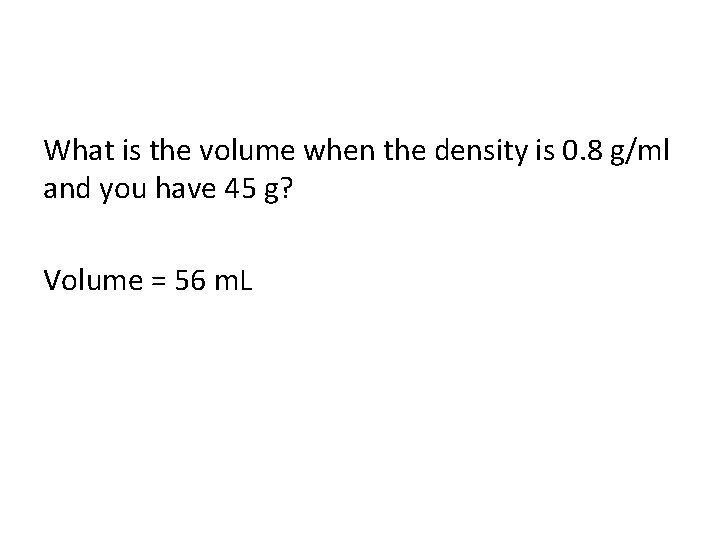
What is the volume when the density is 0. 8 g/ml and you have 45 g? Volume = 56 m. L