Quantities in Chemistry The Relationship Between Mole and
Quantities in Chemistry The Relationship Between Mole and Molar Mass
Mole Review • A Mole is a unit of measurement in chemistry. It represents 6. 02 x 1023 of an entity. One mole of sodium is 6. 02 x 1023 atoms of sodium. • The symbol for mole is “n”. The unit for mole is “mol” • 6. 02 x 1023 is also known as Avogadro’s number.
How do we measure out 1 mol? • Do you count out 6. 02 x 1023 atoms in the lab? • Do you use special equipment? • Can you measure it as a volume? • Can you measure it as a mass?
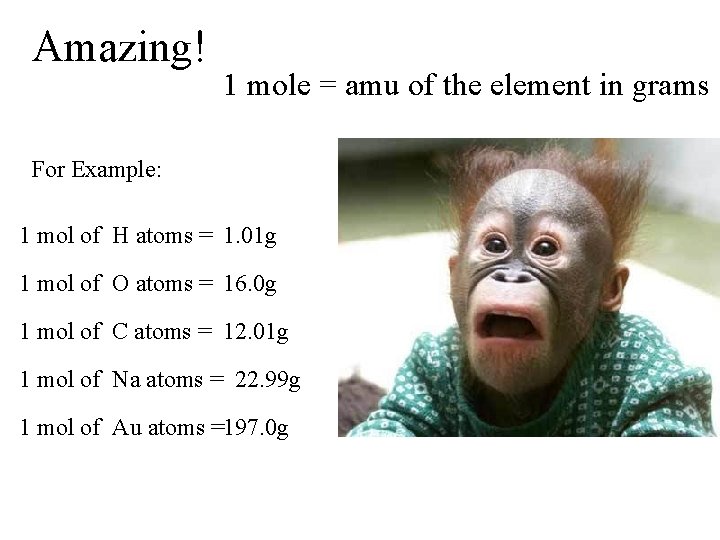
Amazing! 1 mole = amu of the element in grams For Example: 1 mol of H atoms = 1. 01 g 1 mol of O atoms = 16. 0 g 1 mol of C atoms = 12. 01 g 1 mol of Na atoms = 22. 99 g 1 mol of Au atoms =197. 0 g
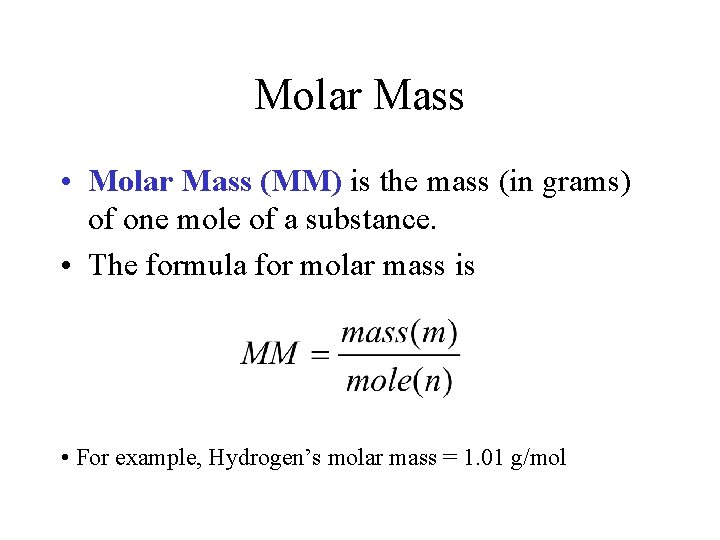
Molar Mass • Molar Mass (MM) is the mass (in grams) of one mole of a substance. • The formula for molar mass is • For example, Hydrogen’s molar mass = 1. 01 g/mol
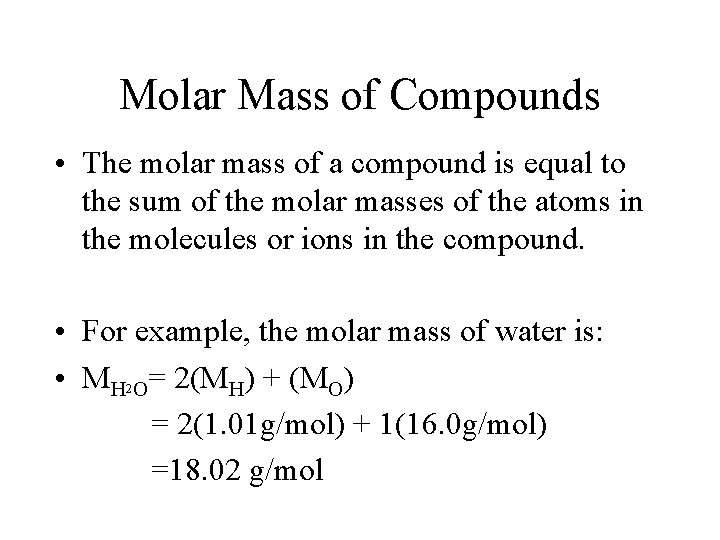
Molar Mass of Compounds • The molar mass of a compound is equal to the sum of the molar masses of the atoms in the molecules or ions in the compound. • For example, the molar mass of water is: • MH 2 O= 2(MH) + (MO) = 2(1. 01 g/mol) + 1(16. 0 g/mol) =18. 02 g/mol
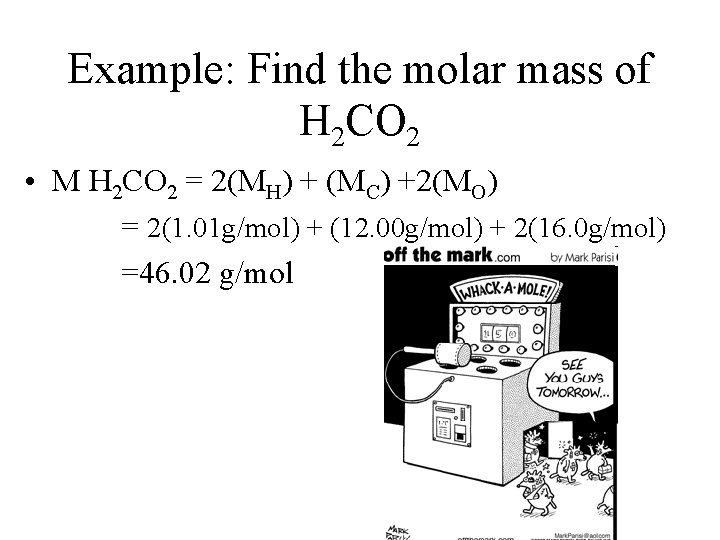
Example: Find the molar mass of H 2 CO 2 • M H 2 CO 2 = 2(MH) + (MC) +2(MO) = 2(1. 01 g/mol) + (12. 00 g/mol) + 2(16. 0 g/mol) =46. 02 g/mol
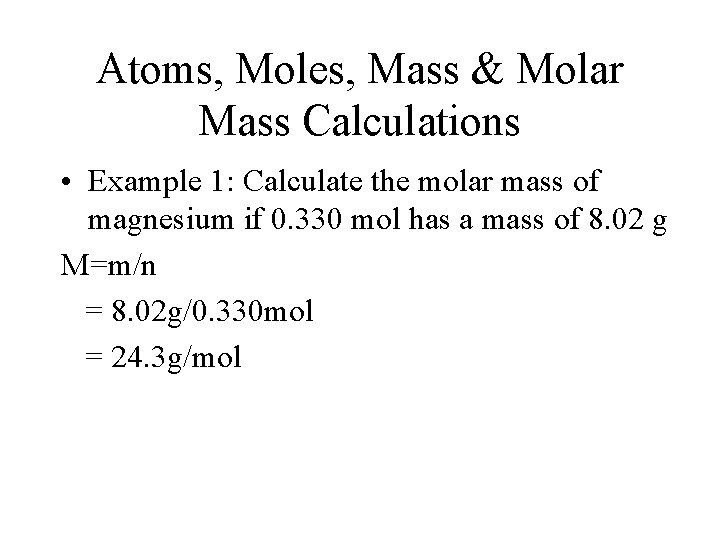
Atoms, Moles, Mass & Molar Mass Calculations • Example 1: Calculate the molar mass of magnesium if 0. 330 mol has a mass of 8. 02 g M=m/n = 8. 02 g/0. 330 mol = 24. 3 g/mol
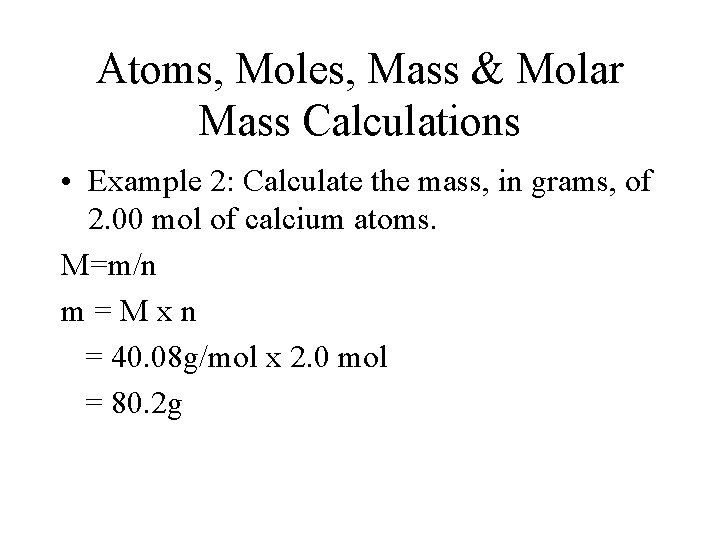
Atoms, Moles, Mass & Molar Mass Calculations • Example 2: Calculate the mass, in grams, of 2. 00 mol of calcium atoms. M=m/n m=Mxn = 40. 08 g/mol x 2. 0 mol = 80. 2 g
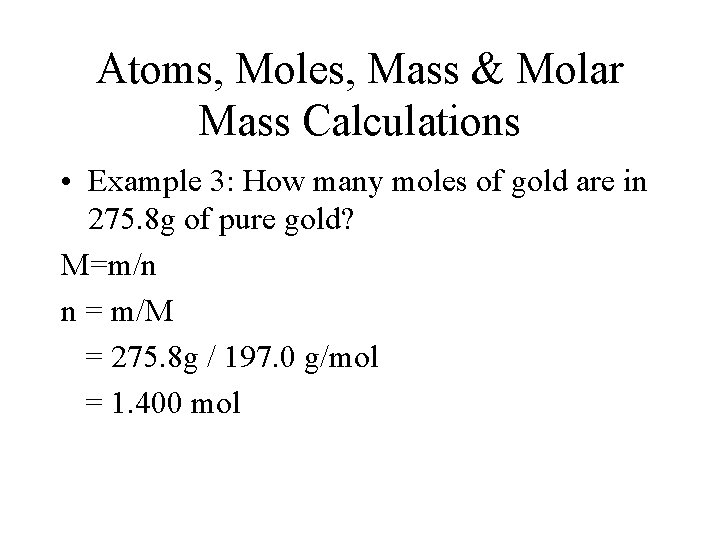
Atoms, Moles, Mass & Molar Mass Calculations • Example 3: How many moles of gold are in 275. 8 g of pure gold? M=m/n n = m/M = 275. 8 g / 197. 0 g/mol = 1. 400 mol
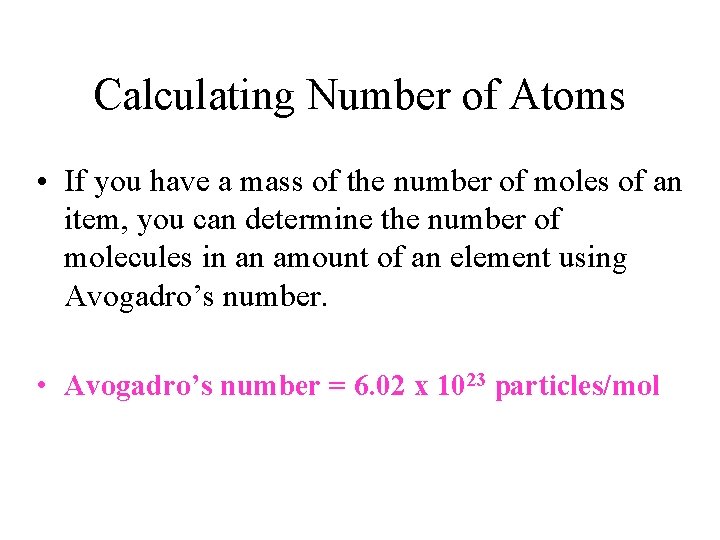
Calculating Number of Atoms • If you have a mass of the number of moles of an item, you can determine the number of molecules in an amount of an element using Avogadro’s number. • Avogadro’s number = 6. 02 x 1023 particles/mol
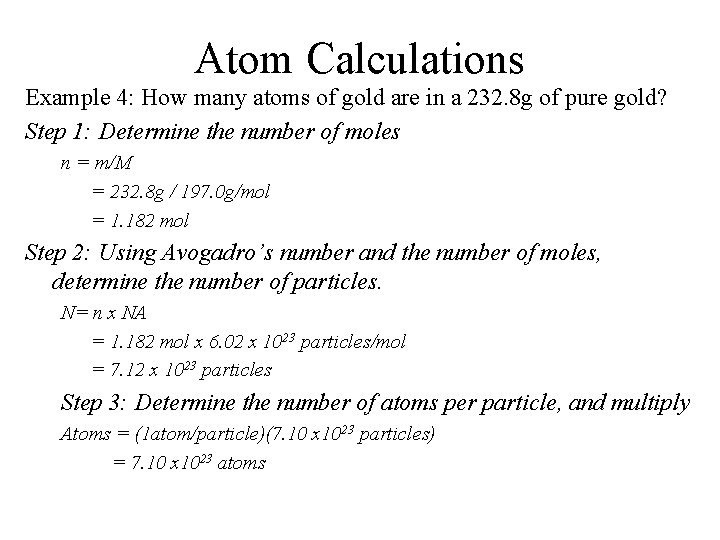
Atom Calculations Example 4: How many atoms of gold are in a 232. 8 g of pure gold? Step 1: Determine the number of moles n = m/M = 232. 8 g / 197. 0 g/mol = 1. 182 mol Step 2: Using Avogadro’s number and the number of moles, determine the number of particles. N= n x NA = 1. 182 mol x 6. 02 x 1023 particles/mol = 7. 12 x 1023 particles Step 3: Determine the number of atoms per particle, and multiply Atoms = (1 atom/particle)(7. 10 x 1023 particles) = 7. 10 x 1023 atoms
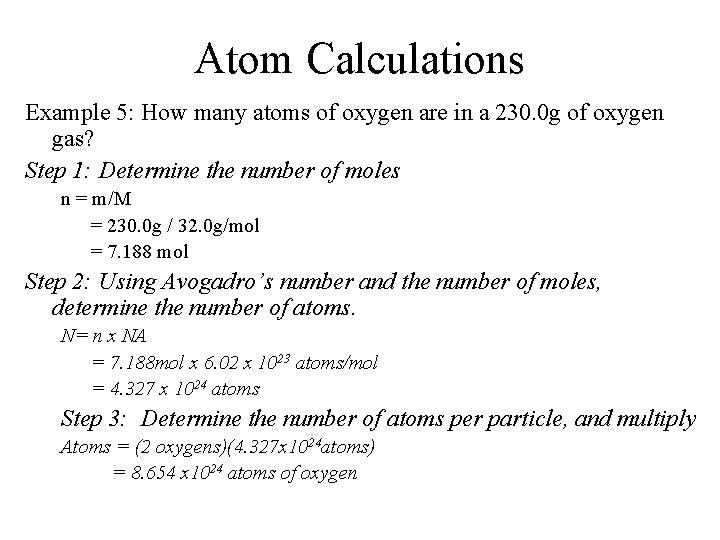
Atom Calculations Example 5: How many atoms of oxygen are in a 230. 0 g of oxygen gas? Step 1: Determine the number of moles n = m/M = 230. 0 g / 32. 0 g/mol = 7. 188 mol Step 2: Using Avogadro’s number and the number of moles, determine the number of atoms. N= n x NA = 7. 188 mol x 6. 02 x 1023 atoms/mol = 4. 327 x 1024 atoms Step 3: Determine the number of atoms per particle, and multiply Atoms = (2 oxygens)(4. 327 x 1024 atoms) = 8. 654 x 1024 atoms of oxygen
- Slides: 13