STOICHIOMETRY Mole to Mole The calculation of quantities






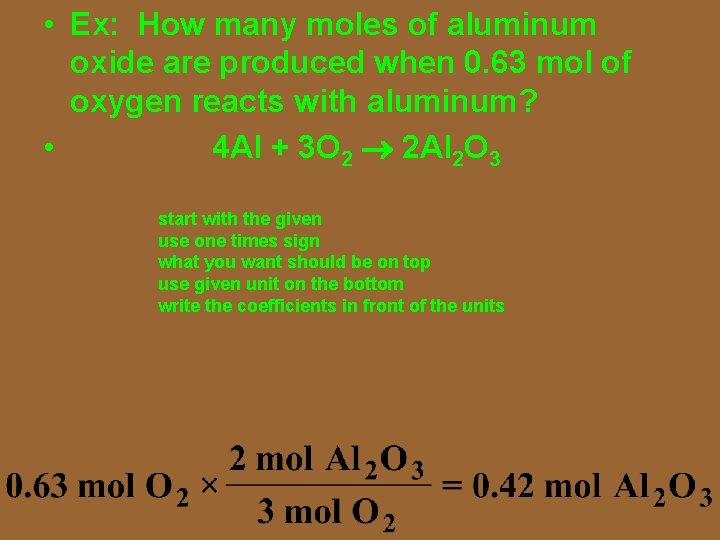



- Slides: 10

STOICHIOMETRY Mole to Mole

• The calculation of quantities in chemical equations is called stoichiometry. • In every chemical reaction the mass and atoms stay the same. • The coefficients represent the numbers of moles or atoms and molecules.

• In the reaction 2 CO + O 2 2 CO 2, the ratio of moles of oxygen used to moles of CO 2 produced is 1: 2 • A correct interpretation of the balanced equation 2 Al(s) + 3 Pb(NO 3)2(aq) 2 Al(NO 3)3(aq) + 3 Pb(s) is … 2 moles Al + 3 moles Pb(NO 3)2 moles Al(NO 3)3 + 3 moles Pb 2

• In C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O • 9 moles of reactants chemically change into 11 moles of product.

• To convert from moles to moles: • • • start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units

• Ex: How many moles of aluminum oxide are produced when 0. 64 mol of aluminum reacts with oxygen? • 4 Al + 3 O 2 2 Al 2 O 3 start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units
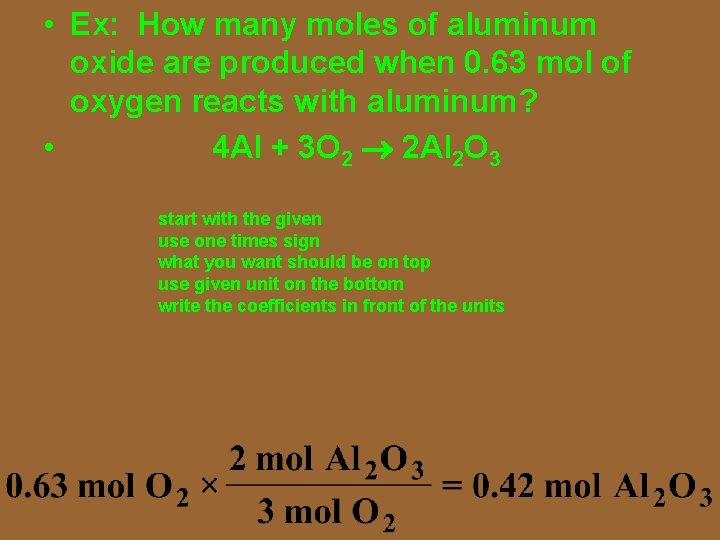
• Ex: How many moles of aluminum oxide are produced when 0. 63 mol of oxygen reacts with aluminum? • 4 Al + 3 O 2 2 Al 2 O 3 start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units

• Ex: If 3. 0 moles of HCl are consumed in the reaction below; how many moles of Fe. Cl 3 are produced? • 6 HCl + Fe 2 O 3 2 Fe. Cl 3 + 3 H 2 O start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units

• Ex: Given the balanced equation • 16 HCl + 2 KMn. O 4 2 KCl + 2 Mn. Cl 2 + 5 Cl 2 + 8 H 2 O if 1. 0 mol of KMn. O 4 reacts, how many moles of H 2 O are produced? start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units

• EX: Calculate the number of moles of Al 2 O 3 that are produced when 0. 60 mol of Fe is produced in the following reaction. • 2 Al + 3 Fe. O 3 Fe + Al 2 O 3 start with the given use one times sign what you want should be on top use given unit on the bottom write the coefficients in front of the units
 Grams to gram
Grams to gram Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus + oxygen
Phosphorus + oxygen Stoichiometry mole-mole
Stoichiometry mole-mole Angular quantities
Angular quantities The calculation of quantities in chemical equations
The calculation of quantities in chemical equations The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step Nitrogen trioxide molar mass
Nitrogen trioxide molar mass Stoichiometry mole island diagram
Stoichiometry mole island diagram Mole tunnel stoichiometry worksheet answers
Mole tunnel stoichiometry worksheet answers