Chapter 12 Stoichiometry 1 Stoichiometry Calculation of quantities





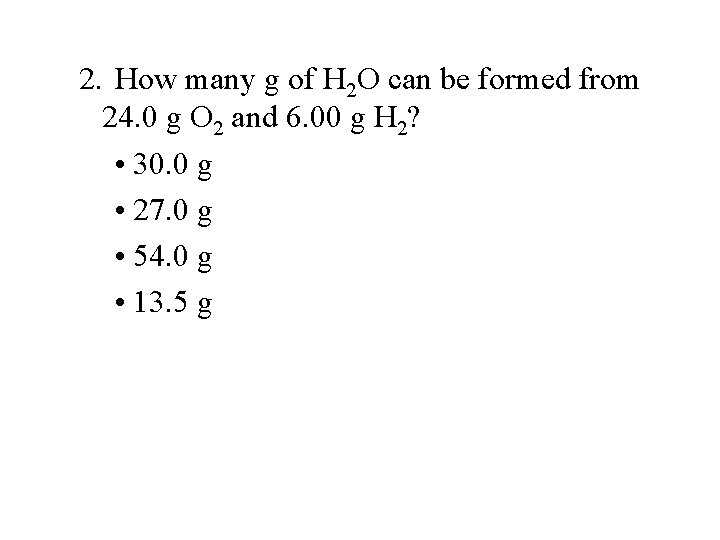
- Slides: 17

Chapter 12 Stoichiometry 1

Stoichiometry • Calculation of quantities in chem rxns. 2

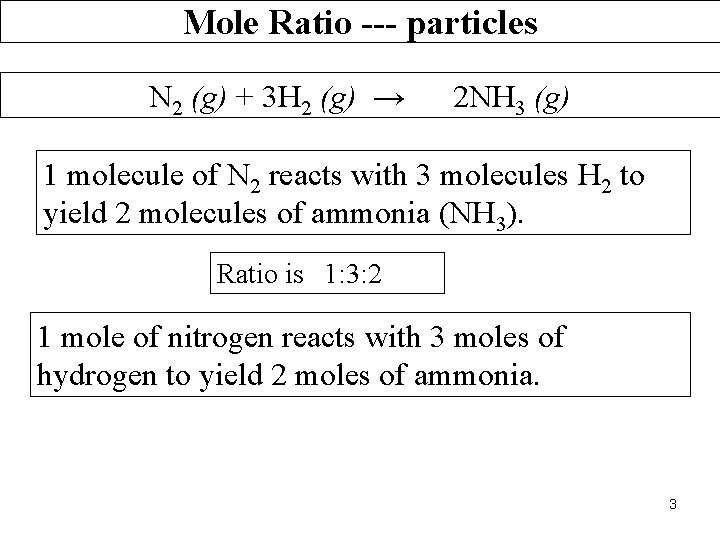
Mole Ratio --- particles N 2 (g) + 3 H 2 (g) → 2 NH 3 (g) 1 molecule of N 2 reacts with 3 molecules H 2 to yield 2 molecules of ammonia (NH 3). Ratio is 1: 3: 2 1 mole of nitrogen reacts with 3 moles of hydrogen to yield 2 moles of ammonia. 3

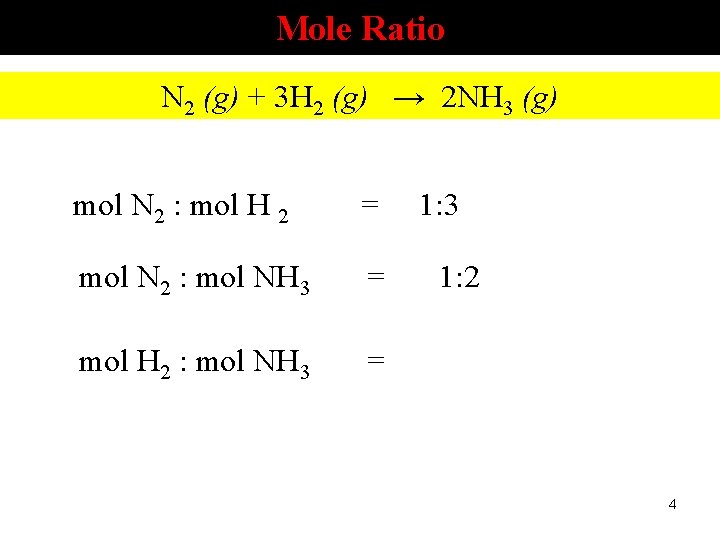
Mole Ratio N 2 (g) + 3 H 2 (g) → 2 NH 3 (g) mol N 2 : mol H 2 = mol N 2 : mol NH 3 = mol H 2 : mol NH 3 = 1: 3 1: 2 4

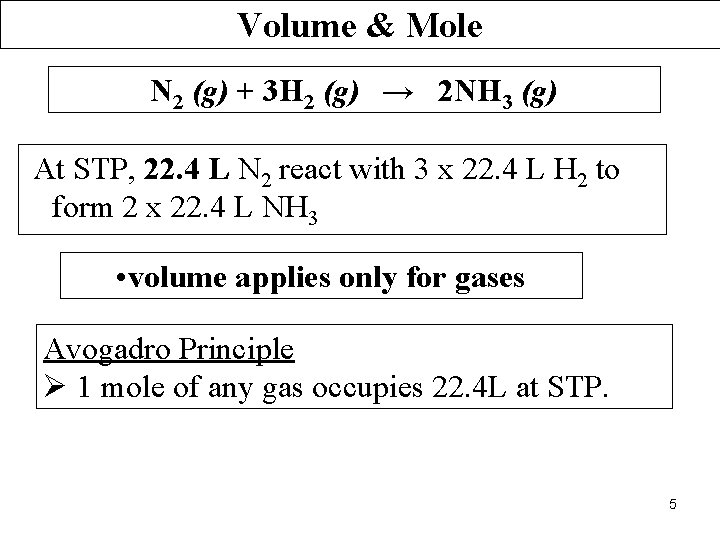
Volume & Mole N 2 (g) + 3 H 2 (g) → 2 NH 3 (g) At STP, 22. 4 L N 2 react with 3 x 22. 4 L H 2 to form 2 x 22. 4 L NH 3 • volume applies only for gases Avogadro Principle Ø 1 mole of any gas occupies 22. 4 L at STP. 5

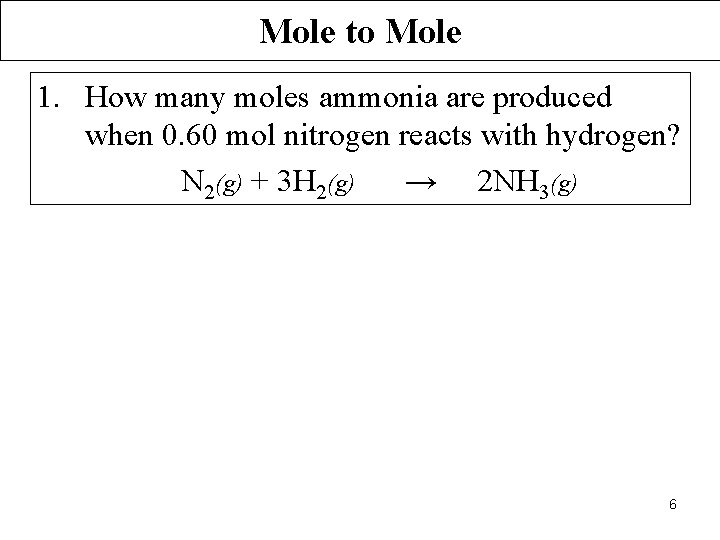
Mole to Mole 1. How many moles ammonia are produced when 0. 60 mol nitrogen reacts with hydrogen? N 2(g) + 3 H 2(g) → 2 NH 3(g) 6

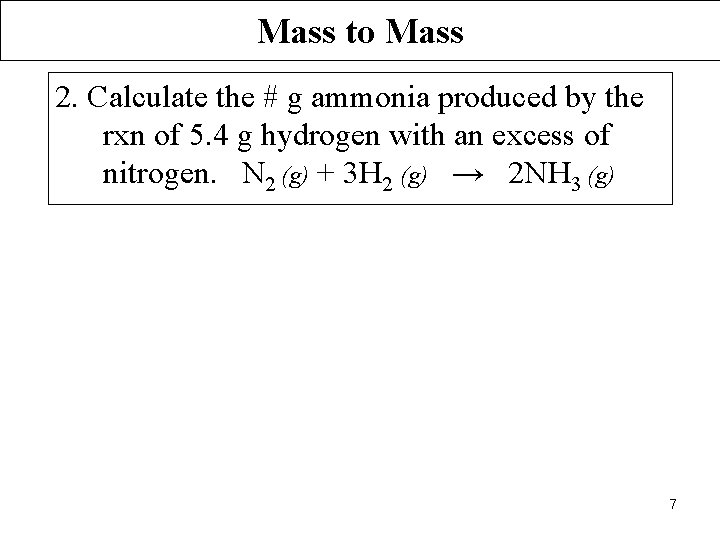
Mass to Mass 2. Calculate the # g ammonia produced by the rxn of 5. 4 g hydrogen with an excess of nitrogen. N 2 (g) + 3 H 2 (g) → 2 NH 3 (g) 7

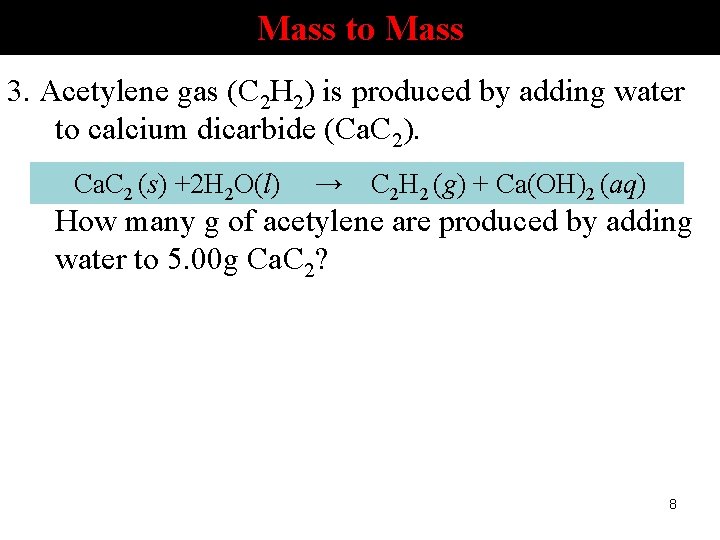
Mass to Mass 3. Acetylene gas (C 2 H 2) is produced by adding water to calcium dicarbide (Ca. C 2). Ca. C 2 (s) +2 H 2 O(l) → C 2 H 2 (g) + Ca(OH)2 (aq) How many g of acetylene are produced by adding water to 5. 00 g Ca. C 2? 8

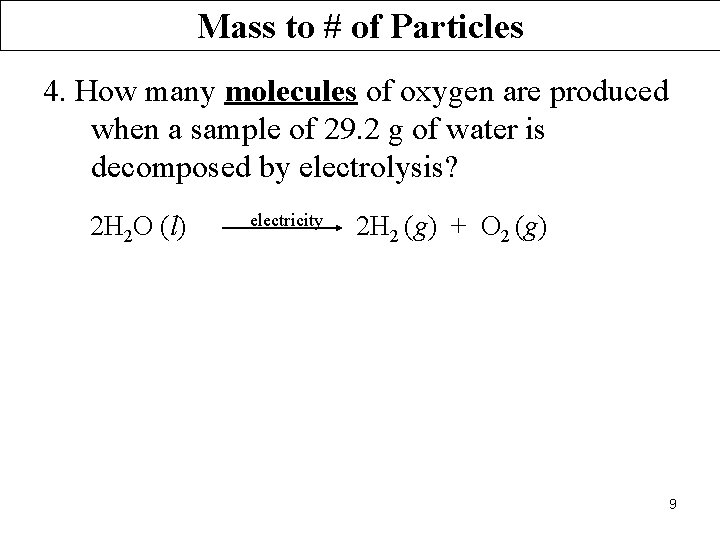
Mass to # of Particles 4. How many molecules of oxygen are produced when a sample of 29. 2 g of water is decomposed by electrolysis? 2 H 2 O (l) electricity 2 H 2 (g) + O 2 (g) 9

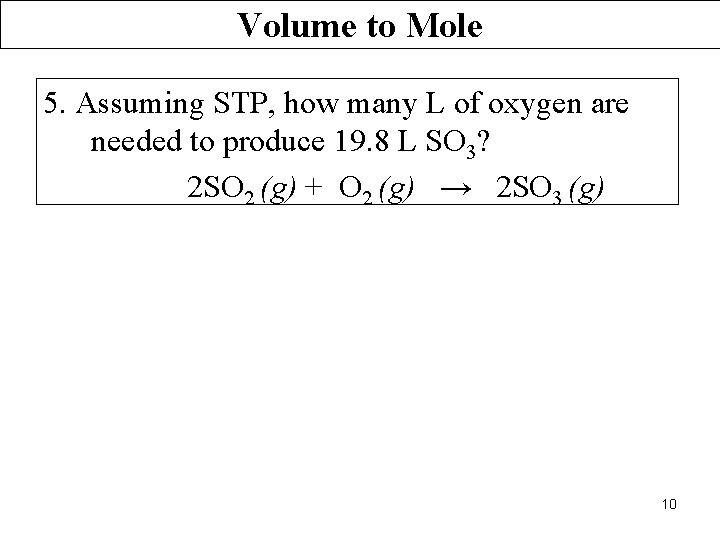
Volume to Mole 5. Assuming STP, how many L of oxygen are needed to produce 19. 8 L SO 3? 2 SO 2 (g) + O 2 (g) → 2 SO 3 (g) 10

Mole & Volume 6. The eqn for the combustion of carbon monoxide is 2 CO (g) + O 2 (g) → 2 CO 2 (g) How many L of oxygen are required to burn 3. 86 L of carbon monoxide at STP? 11

CST example 1 Mg 3 N 2(s) + 6 H 2 O (l) → 2 NH 3(aq) + 3 Mg(OH)2(s) If 54. 0 g of water are mixed with excess magnesium nitride, then how many g of ammonia are produced? A 1. 00 B 17. 0 C 51. 0 D 153 12

CST problem 2 Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2 In this rxn, how many g of Fe 2 O 3 are required to completely react with 84 g of CO? A 64 g B 80 g C 160 g D 1400 g 13

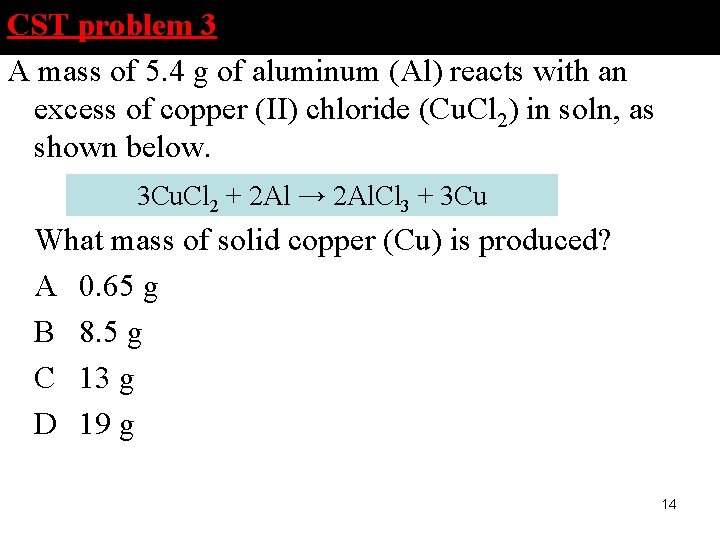
CST problem 3 A mass of 5. 4 g of aluminum (Al) reacts with an excess of copper (II) chloride (Cu. Cl 2) in soln, as shown below. 3 Cu. Cl 2 + 2 Al → 2 Al. Cl 3 + 3 Cu What mass of solid copper (Cu) is produced? A 0. 65 g B 8. 5 g C 13 g D 19 g 14

The End 15
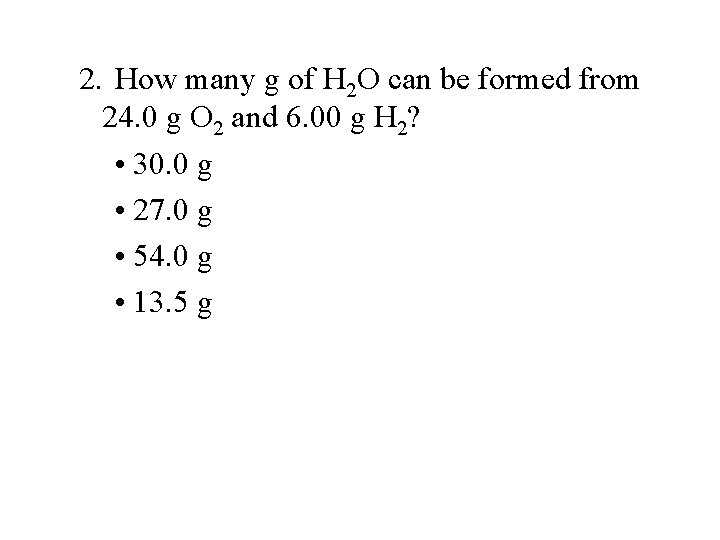
2. How many g of H 2 O can be formed from 24. 0 g O 2 and 6. 00 g H 2? • 30. 0 g • 27. 0 g • 54. 0 g • 13. 5 g

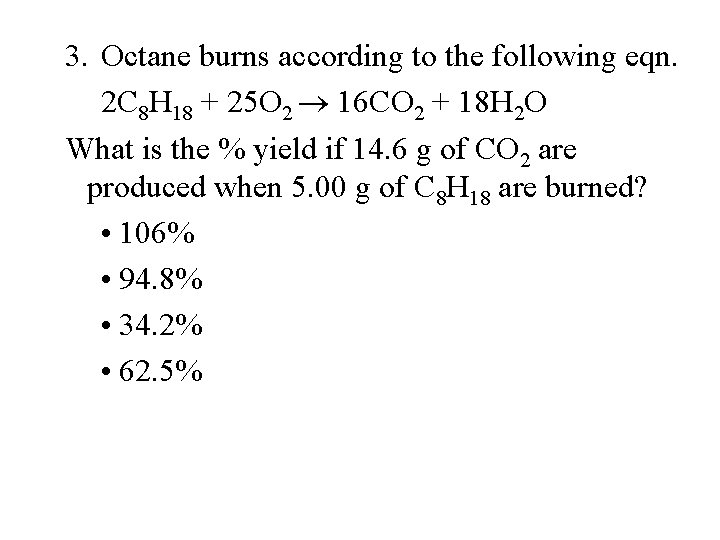
3. Octane burns according to the following eqn. 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O What is the % yield if 14. 6 g of CO 2 are produced when 5. 00 g of C 8 H 18 are burned? • 106% • 94. 8% • 34. 2% • 62. 5%