Stoichiometry Moles Unit of measurement that specifies the






- Slides: 21

Stoichiometry

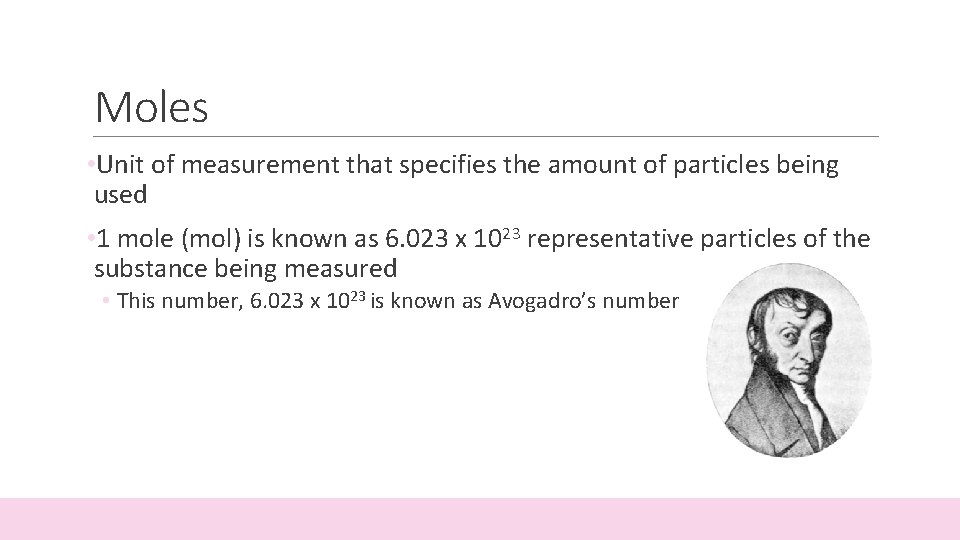
Moles • Unit of measurement that specifies the amount of particles being used • 1 mole (mol) is known as 6. 023 x 1023 representative particles of the substance being measured • This number, 6. 023 x 1023 is known as Avogadro’s number

Representative Particle • The term representative particle refers to the species present in a substance, usually • atoms • molecules • formula units The representative particle of most elements is the atom.

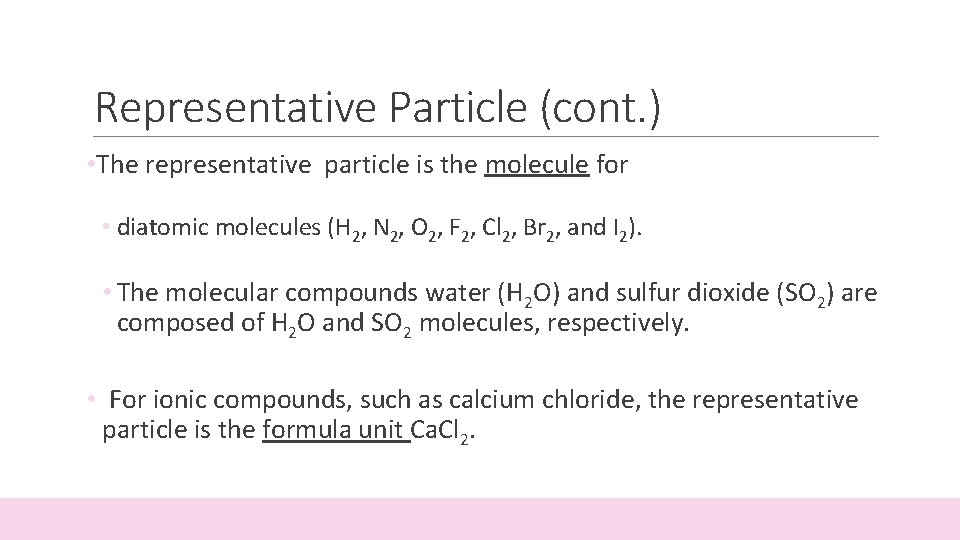
Representative Particle (cont. ) • The representative particle is the molecule for • diatomic molecules (H 2, N 2, O 2, F 2, Cl 2, Br 2, and I 2). • The molecular compounds water (H 2 O) and sulfur dioxide (SO 2) are composed of H 2 O and SO 2 molecules, respectively. • For ionic compounds, such as calcium chloride, the representative particle is the formula unit Ca. Cl 2.

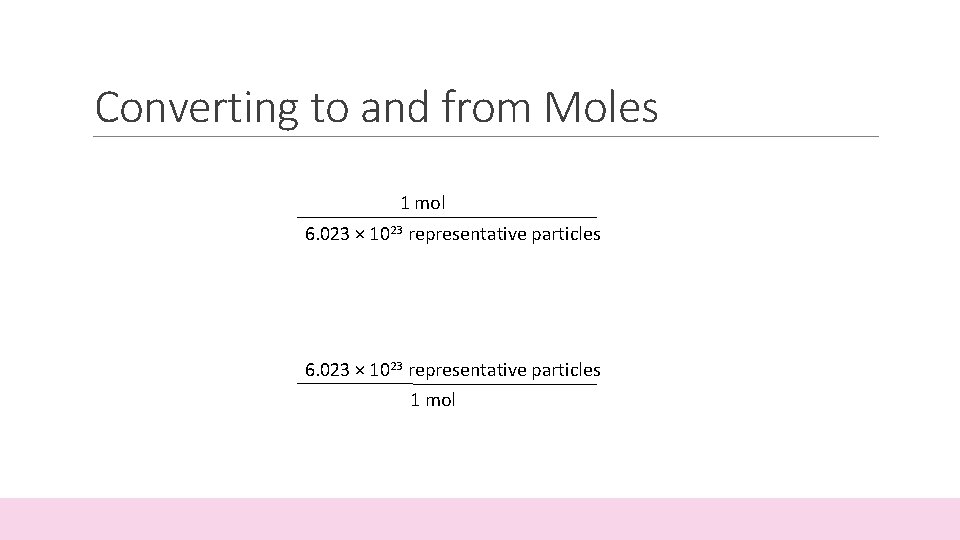
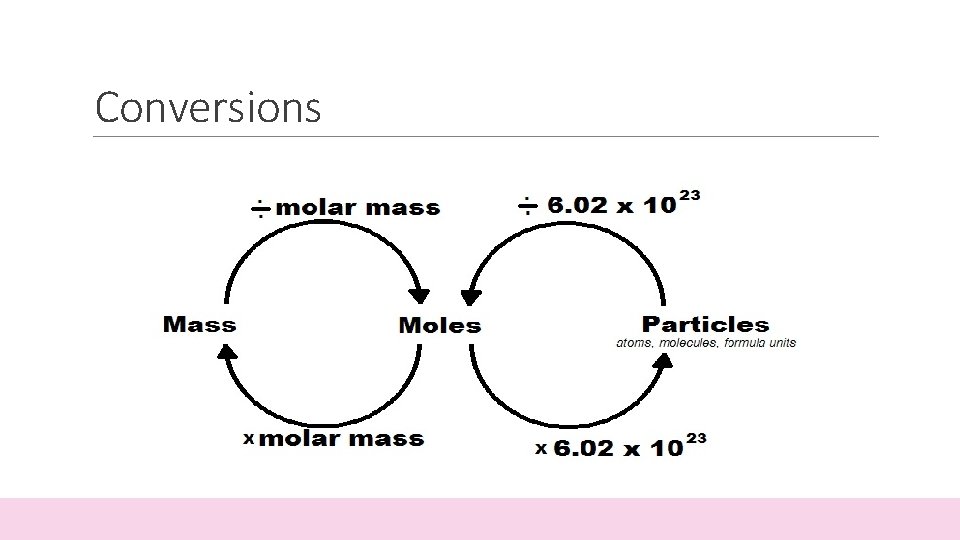
Converting to and from Moles • The mole allows chemists to count the number of representative particles in a substance. • A mole of any substance contains Avogadro’s number of representative particles, or 6. 023 × 1023 representative particles.

Converting to and from Moles 1 mol 6. 023 × 1023 representative particles 1 mol

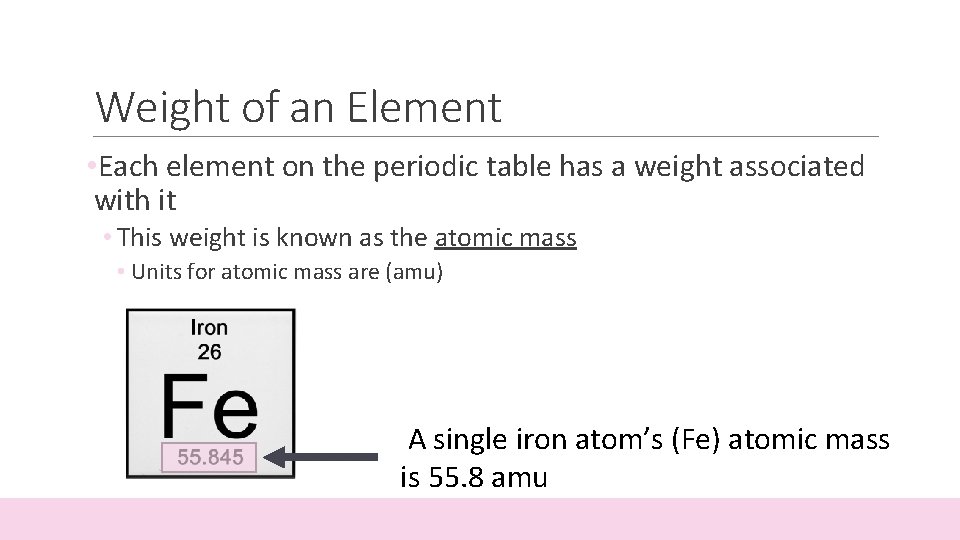
Weight of an Element • Each element on the periodic table has a weight associated with it • This weight is known as the atomic mass • Units for atomic mass are (amu) A single iron atom’s (Fe) atomic mass is 55. 8 amu

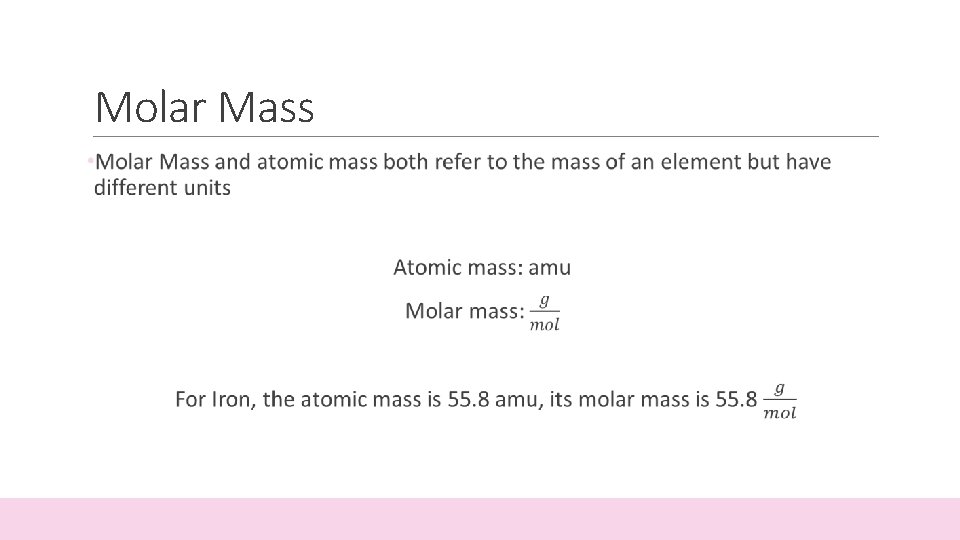
Molar Mass

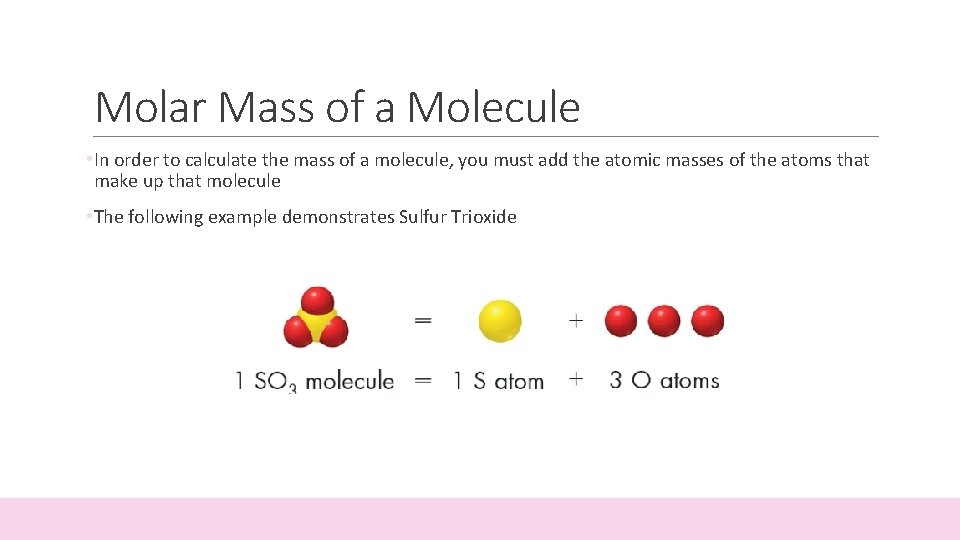
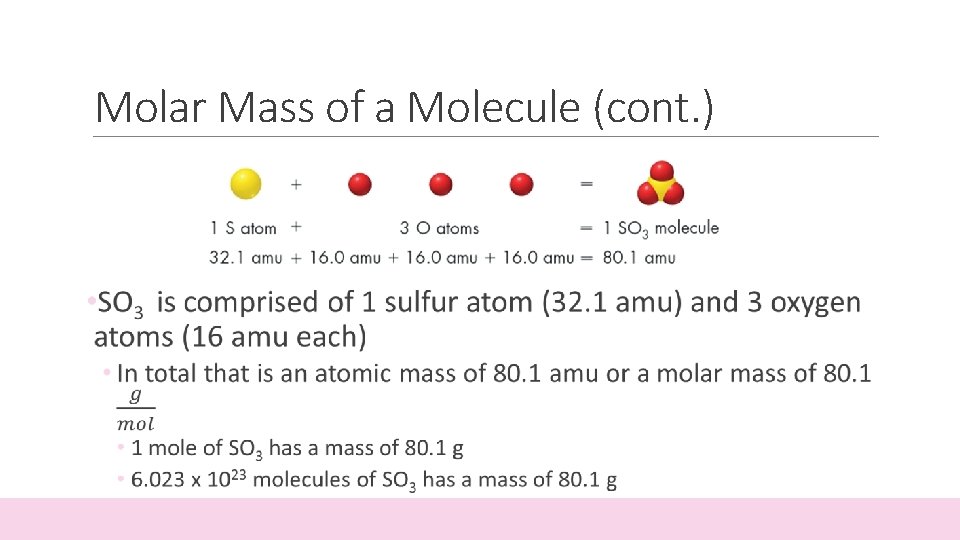
Molar Mass of a Molecule • In order to calculate the mass of a molecule, you must add the atomic masses of the atoms that make up that molecule • The following example demonstrates Sulfur Trioxide

Molar Mass of a Molecule (cont. )

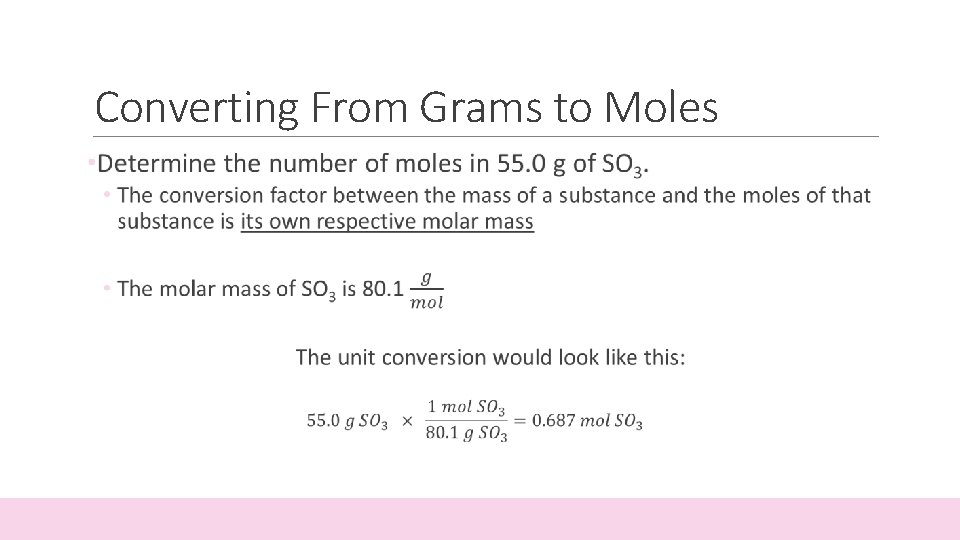
Converting From Grams to Moles

Conversions

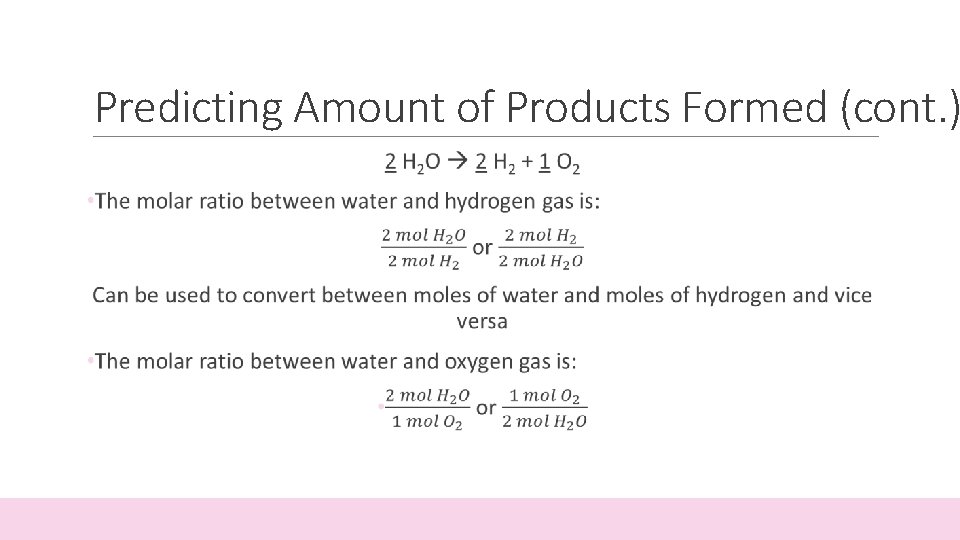
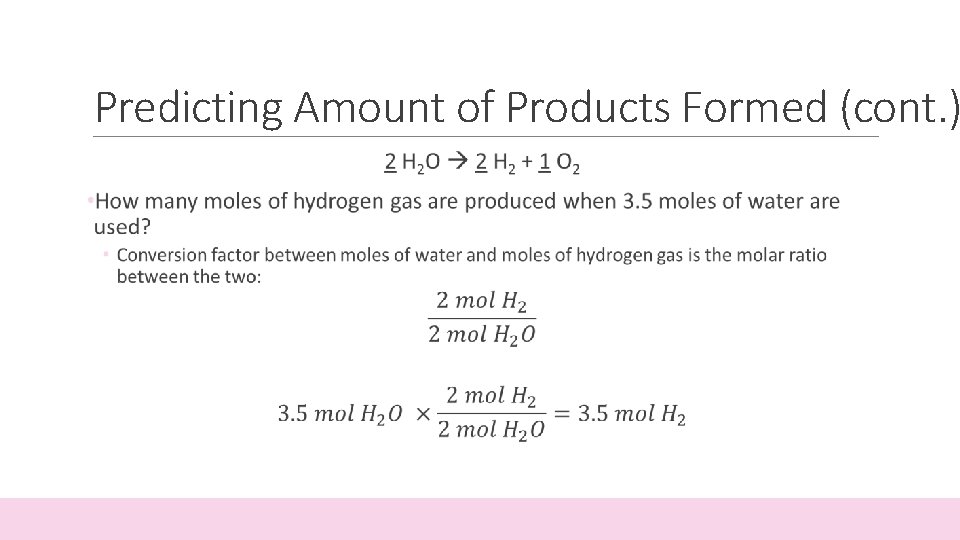
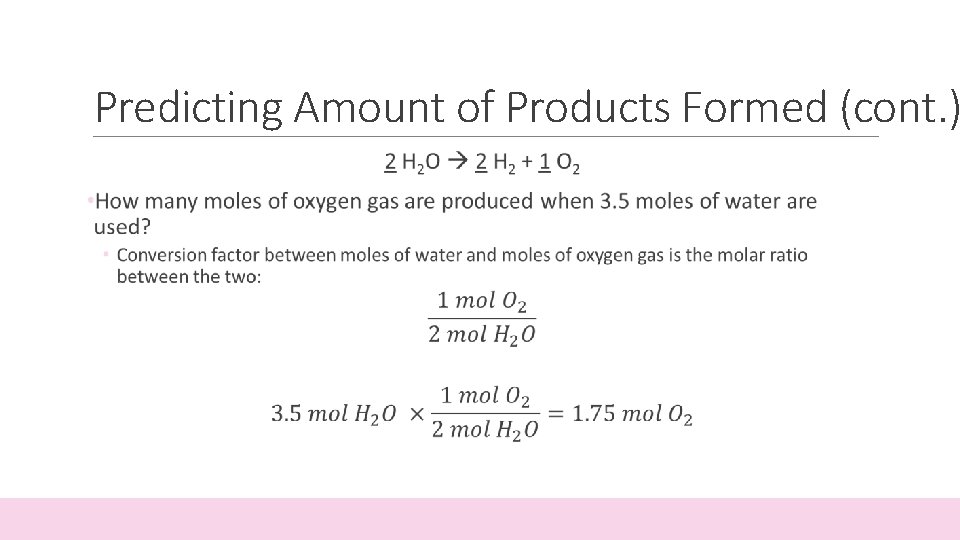
Predicting Amount of Products Formed • The coefficients in a balanced equation give the relative amounts (in moles) of reactants and products • Mole Ratio: the ratio between the amounts (in moles) between one starting material and one product 2 H 2 O 2 H 2 + 1 O 2 • For every 2 moles of water (H 2 O) put into the reaction, you get 2 moles of hydrogen (H 2) and 1 mole of oxygen (O 2)

Predicting Amount of Products Formed (cont. )

Predicting Amount of Products Formed (cont. )

Predicting Amount of Products Formed (cont. )

Limiting Reagents • A limiting reagent (or limiting reactant), is the reactant that is first used up in a chemical reaction • Once this reactant is used up, the reaction stops • This reactant limits the amount of product that can form • The excess reagent (or excess reactant), is the unused reactant that is left over once the limiting reagent is used Passage I stole from the AP Chem book:

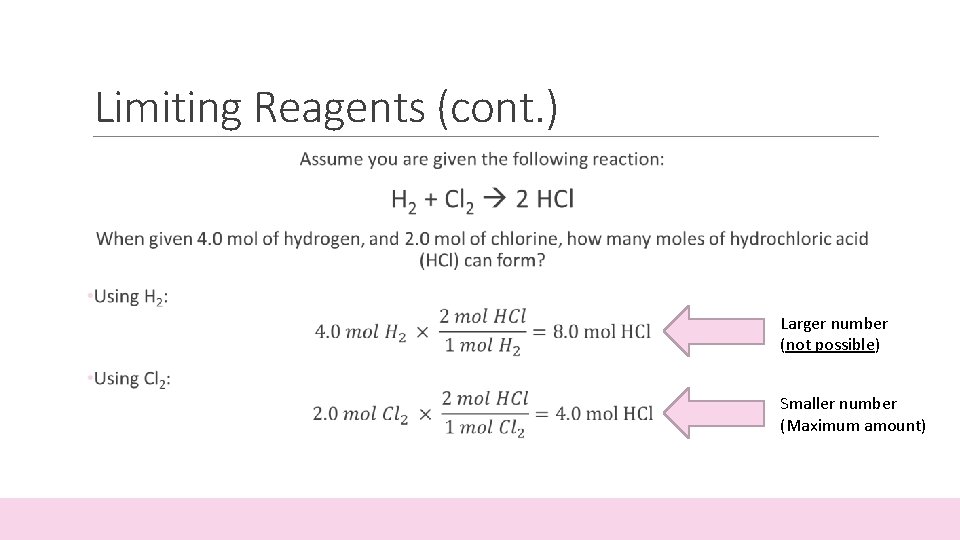
Limiting Reagents (cont. ) Assume you are given the following reaction: H 2 + Cl 2 2 HCl When given 4. 0 mol of hydrogen, and 2. 0 mol of chlorine, how many moles of hydrochloric acid (HCl) can form? • In order to solve this problem: • You must calculate the amount of product formed from each reactant, H 2 and Cl 2 • The limiting reagent will be the reactant (H 2 or Cl 2) that produces the smaller amount of product

Limiting Reagents (cont. ) Larger number (not possible) Smaller number (Maximum amount)

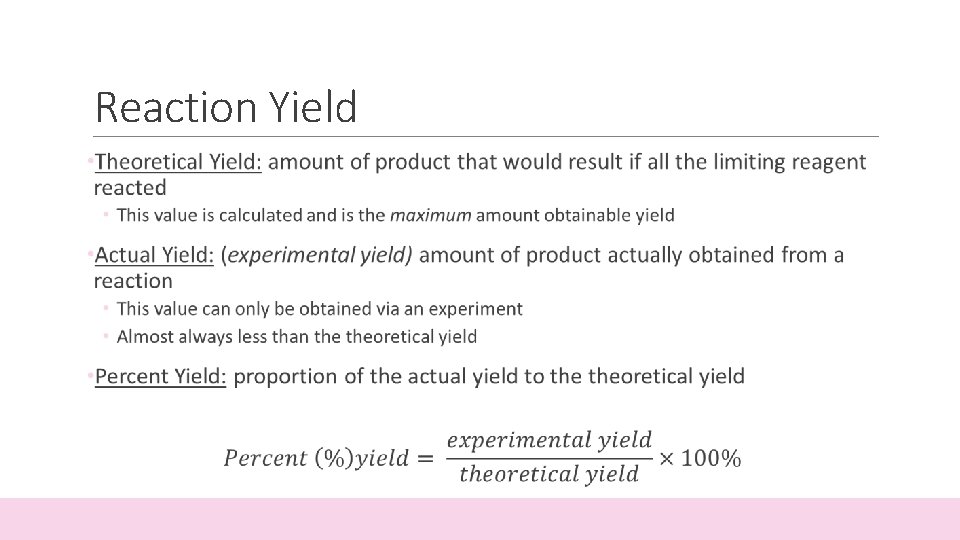
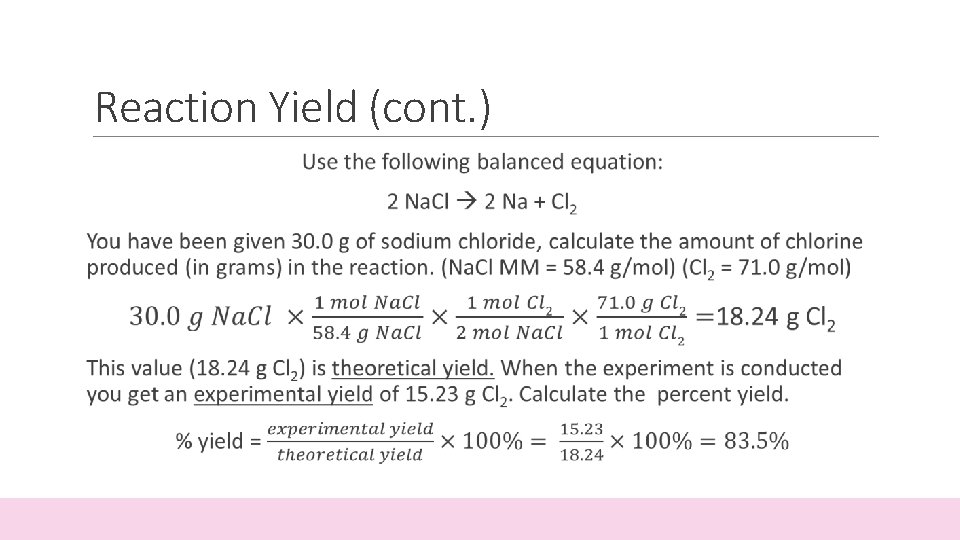
Reaction Yield

Reaction Yield (cont. )
 Converting grams to moles
Converting grams to moles Ideal gas law powerpoint
Ideal gas law powerpoint Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Descriptor privilege level
Descriptor privilege level Coordinate that specifies the north-south position
Coordinate that specifies the north-south position Sorting defination
Sorting defination What specifies the headings paragraphs images
What specifies the headings paragraphs images 8051 instruction
8051 instruction Route sheet specifies
Route sheet specifies Organizational structure specifies the firm's:
Organizational structure specifies the firm's: Unit: stoichiometry “multi-step problems” – ws #3
Unit: stoichiometry “multi-step problems” – ws #3 Mole unit of measurement
Mole unit of measurement Unit 6 review questions
Unit 6 review questions