UNIT 4 3 Dimensional Analysis January 9 2015










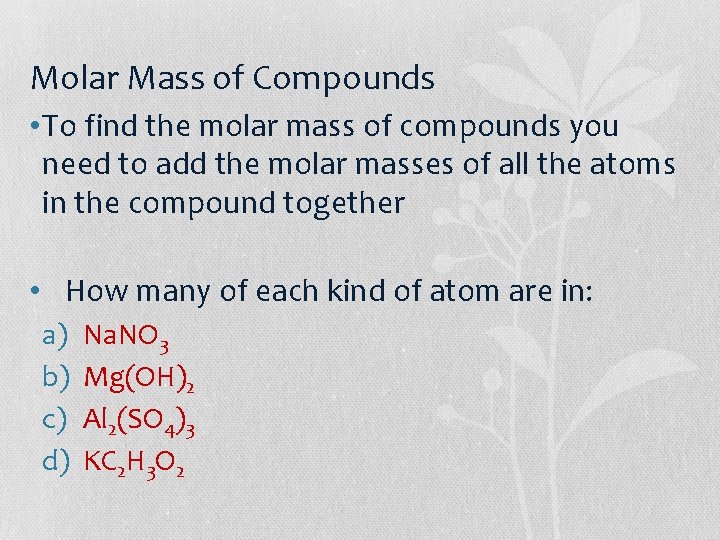
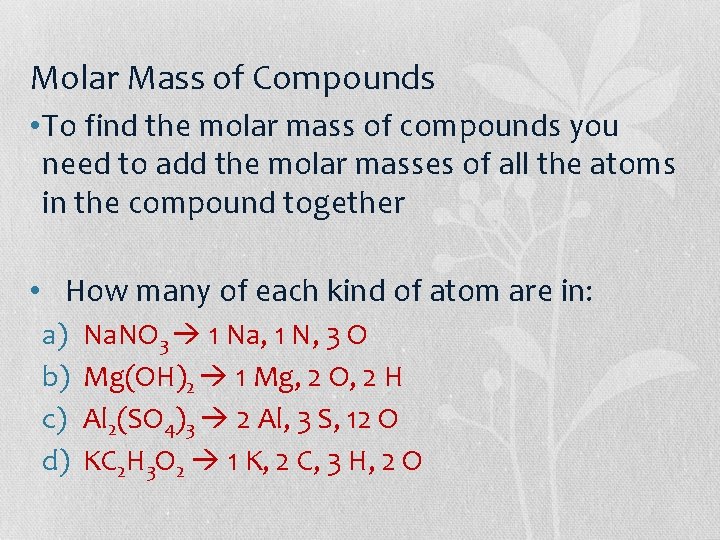





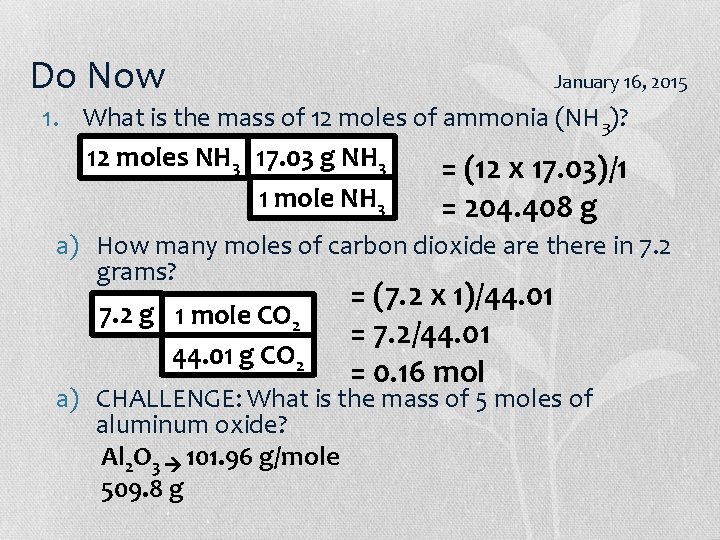



























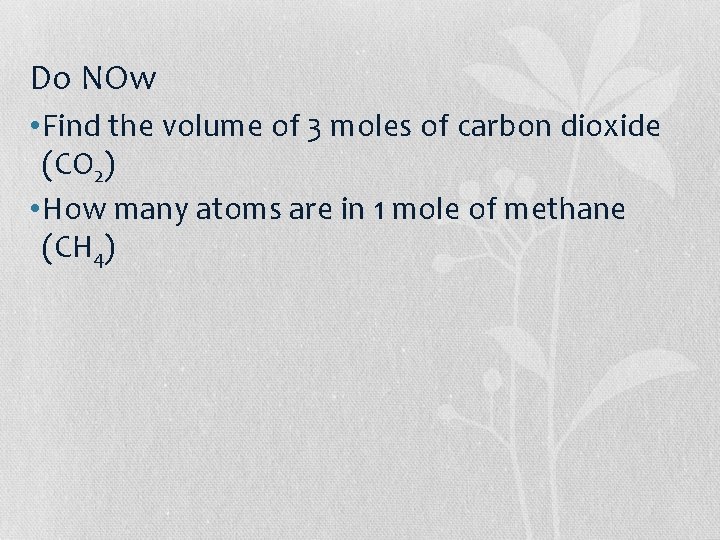
















- Slides: 138

UNIT 4 - 3 Dimensional Analysis
January 9, 2015 – 96 minutes

Do Now January 9, 2015 • What occurs when an atom of magnesium and an atom of oxygen become a molecule of magnesium oxide? a) b) c) d) A chemical bond is formed and energy is released A chemical bond is formed and energy is absorbed A chemical bond is broken and energy is released A chemical bond is broken and energy is absorbed

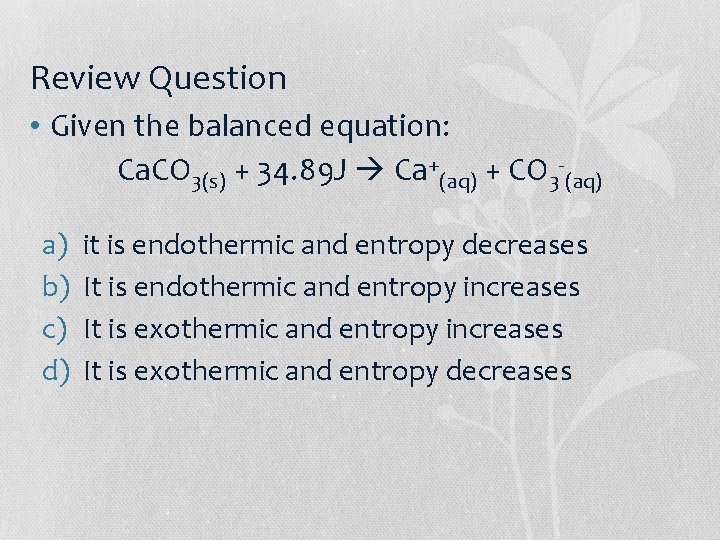
Review Question • Given the balanced equation: Ca. CO 3(s) + 34. 89 J Ca+(aq) + CO 3 -(aq) a) b) c) d) it is endothermic and entropy decreases It is endothermic and entropy increases It is exothermic and entropy decreases

Objective • I can use dimensional analysis to determine the amount, mass, or volume of a substance produced or required in a chemical reaction.

Agenda 1. Do Now, Objective 2. How Many Pennies Do You Have? 3. Dimensional Analysis Guided Notes & Practice 4. Weekly Quiz

Homework – HW #3 • Complete the HW #3 sheet to practice converting grams, moles, and liters to other units of measurement using dimensional analysis

How Many Pennies Do You Have? ? ? • Today you will need to work with your group to determine how many pennies are in a plastic bag. You may do anything EXCEPT – count the pennies or open the bag. Materials - 1 bag filled with pennies - 1 single penny - 1 electronic balance Go!

How Many Pennies Do You Have? ? ? • Write down a procedure for how to figure out how many pennies you have on your graph paper. How to Find the Number of Pennies in a Bag

Congratulations - You Just Did Dimensional Analysis!!

Wait… What? • Dimensional analysis is a method for converting something from one unit of measurement to another. For example: • 60 seconds = 1 minute • 60 minutes = 1 hour • 24 hours = 1 day

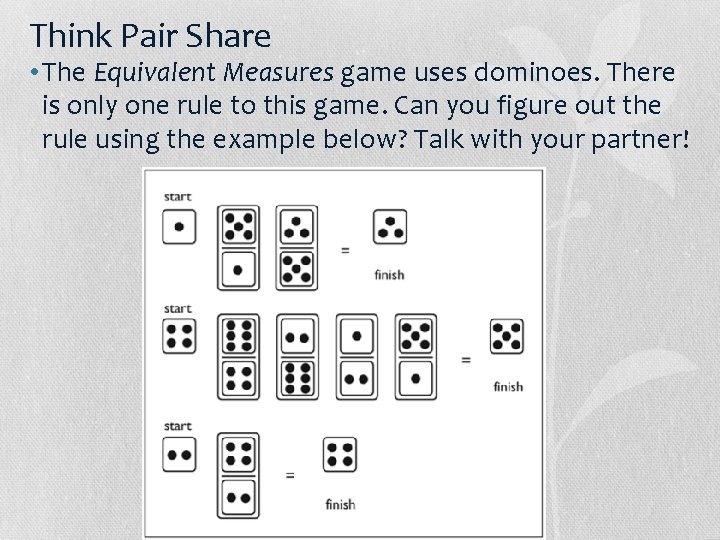
Think Pair Share • The Equivalent Measures game uses dominoes. There is only one rule to this game. Can you figure out the rule using the example below? Talk with your partner!

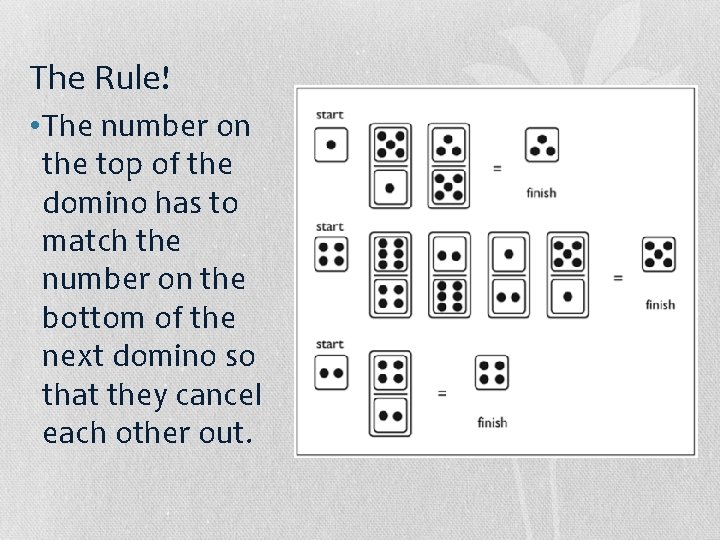
The Rule! • The number on the top of the domino has to match the number on the bottom of the next domino so that they cancel each other out.

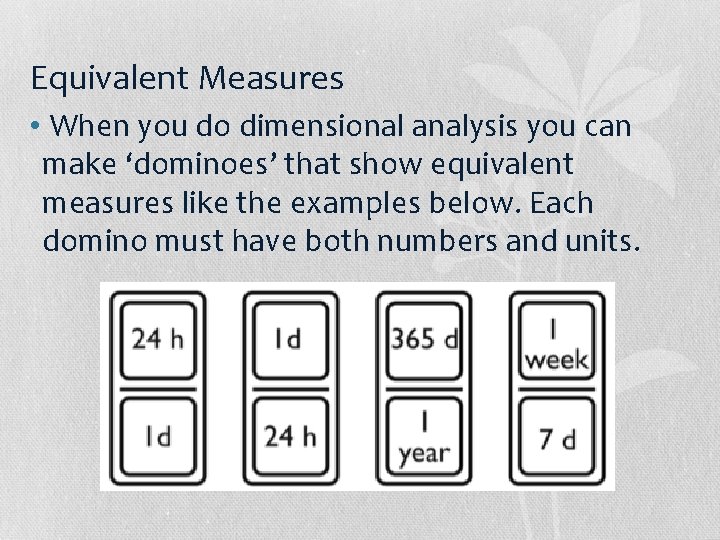
Equivalent Measures • When you do dimensional analysis you can make ‘dominoes’ that show equivalent measures like the examples below. Each domino must have both numbers and units.

Quick Write • Write down 2 more dominoes that show equivalent measures. These could be for any type of measurement you want – time, length, mass, volume, etc.

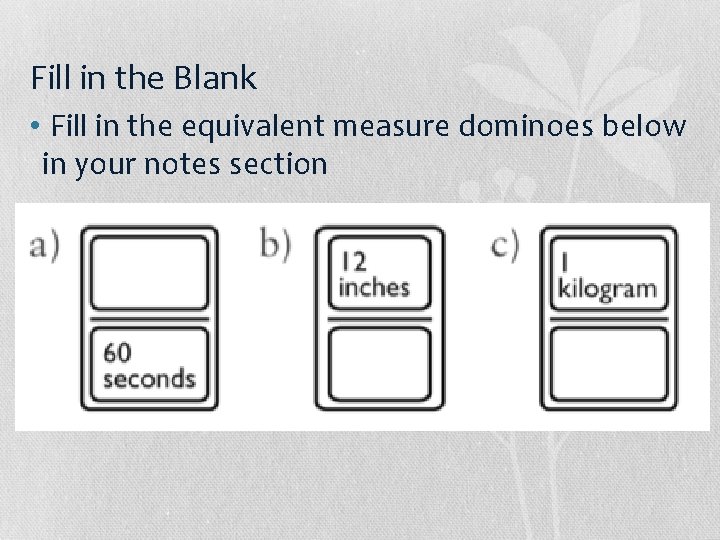
Fill in the Blank • Fill in the equivalent measure dominoes below in your notes section

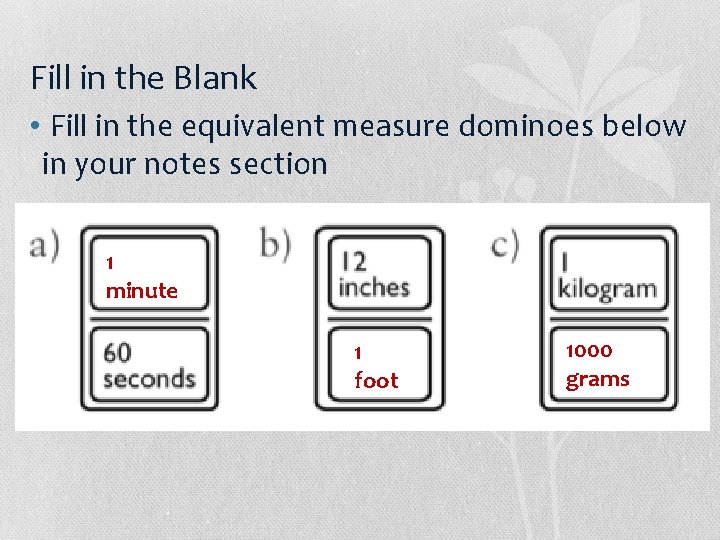
Fill in the Blank • Fill in the equivalent measure dominoes below in your notes section 1 minute 1 foot 1000 grams

Solving Problems with Equivalent Measures • When you solve problems using these ‘dominoes’ you change the unit, NOT the number. To change from one domino to another, the unit in the bottom of the second domino must be the same as the unit in the top of the first domino. The numbers in the dominos can be different.

The Mole! - Atoms and molecules are like pennies. All particles of one kind have the same average mass. - If you know the mass of one particle, you can figure out the mass of a whole bunch of particles BUT… - Atoms are really small, so why talk about the mass of one single atom? - Scientists talk about the mass of a counting group called ‘the mole’

The Mole (mol) • 1 Dozen = 12 • 1 Mole = 6. 02 x 1023 Age ain’t nothin’ but a number, but a mole IS JUST A NUMBER.

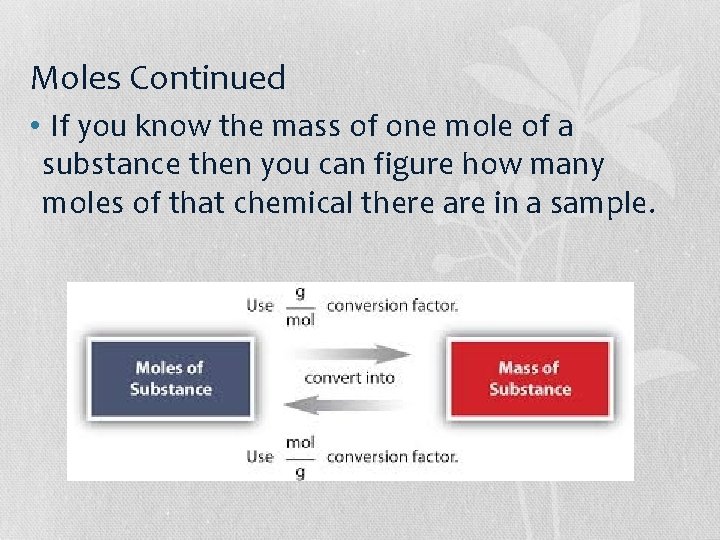
Moles Continued • If you know the mass of one mole of a substance then you can figure how many moles of that chemical there are in a sample.

Molar Mass • The atomic mass of an element is equal to the mass of one mole (6. 02 x 1023 atoms) of that element aka g/mol 1 mole of chlorine atoms has a mass of 35. 45 g

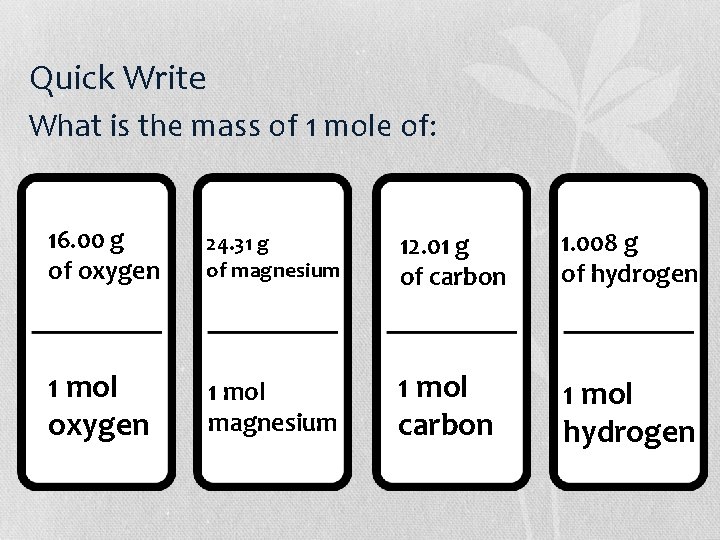
Quick Write • Answer each of these questions by drawing a domino for the answer. • What is the mass of 1 mole of: 1. Oxygen 2. Magnesium 3. Carbon 4. hydrogen

Quick Write What is the mass of 1 mole of: 16. 00 g of oxygen 24. 31 g of magnesium 12. 01 g of carbon 1. 008 g of hydrogen 1 mol oxygen 1 mol magnesium 1 mol carbon 1 mol hydrogen
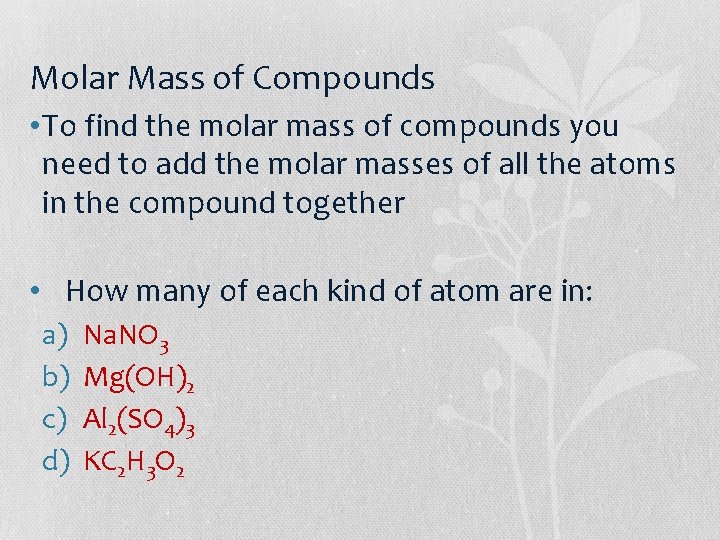
Molar Mass of Compounds • To find the molar mass of compounds you need to add the molar masses of all the atoms in the compound together • How many of each kind of atom are in: a) b) c) d) Na. NO 3 Mg(OH)2 Al 2(SO 4)3 KC 2 H 3 O 2
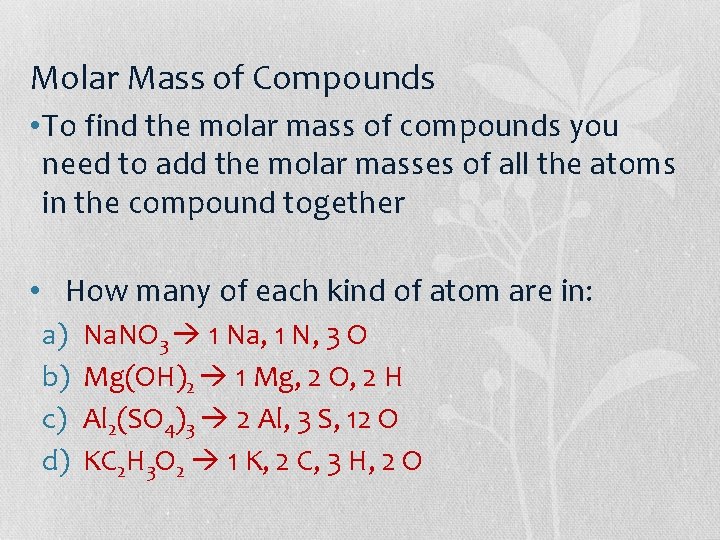
Molar Mass of Compounds • To find the molar mass of compounds you need to add the molar masses of all the atoms in the compound together • How many of each kind of atom are in: a) b) c) d) Na. NO 3 1 Na, 1 N, 3 O Mg(OH)2 1 Mg, 2 O, 2 H Al 2(SO 4)3 2 Al, 3 S, 12 O KC 2 H 3 O 2 1 K, 2 C, 3 H, 2 O

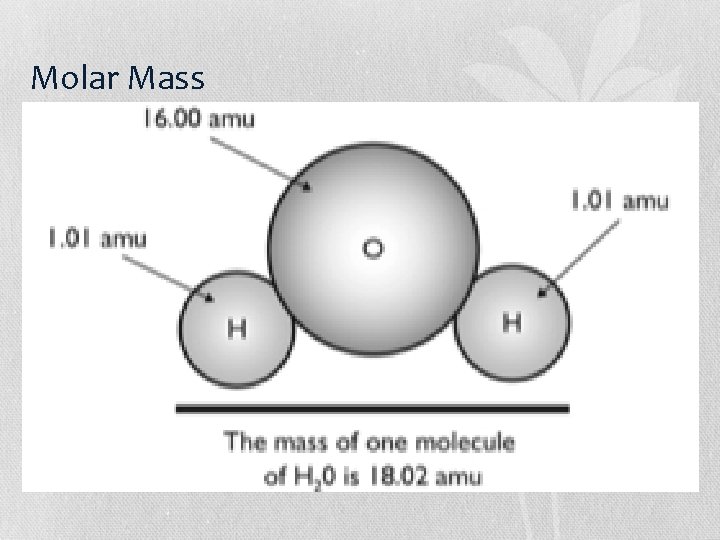
Molar Mass of Compounds • To figure out the mass of one mole of a molecule, you add up the masses of the atoms inside it. • Example: what is the mass of one mole of N 2 O? 1. N = 14. 01 g/mol 2 N’s * 14. 01 g/mol = 28. 02 g/mol 2. O = 16. 00 g/mol 1 O * 16. 00 g/mol = 16. 00 g/mol 3. 28. 02 g/mol + 16. 00 g/mol = 44. 02 g/mol 1 mol of N 2 O has a mass of 44. 02 g/mol

Molar Mass

Quick Write • What is the mass of one mole of a) b) c) d) CO 2 Na. HCO 3 Na. Cl Al. Br 3

Quick Write • What is the mass of one mole of a) b) c) d) CO 2 44. 01 g/mol Na. HCO 3 84. 007 g/mol Na. Cl 58. 44 g/mol Al. Br 3 266. 69 g/mol

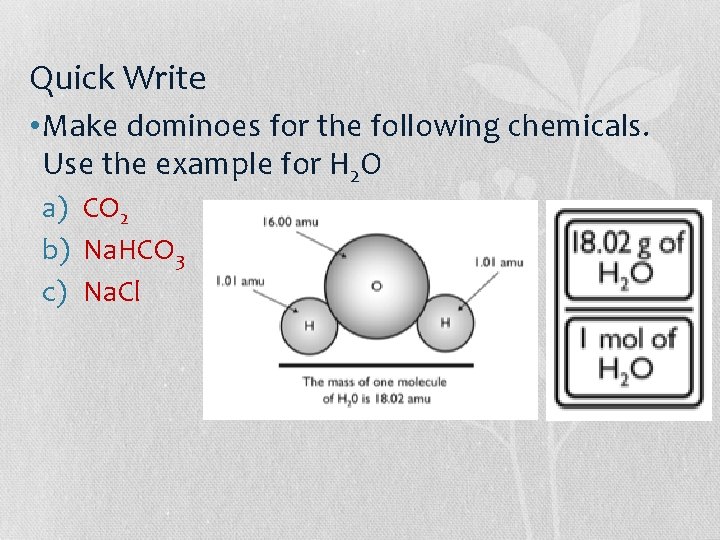
Quick Write • Make dominoes for the following chemicals. Use the example for H 2 O a) CO 2 b) Na. HCO 3 c) Na. Cl

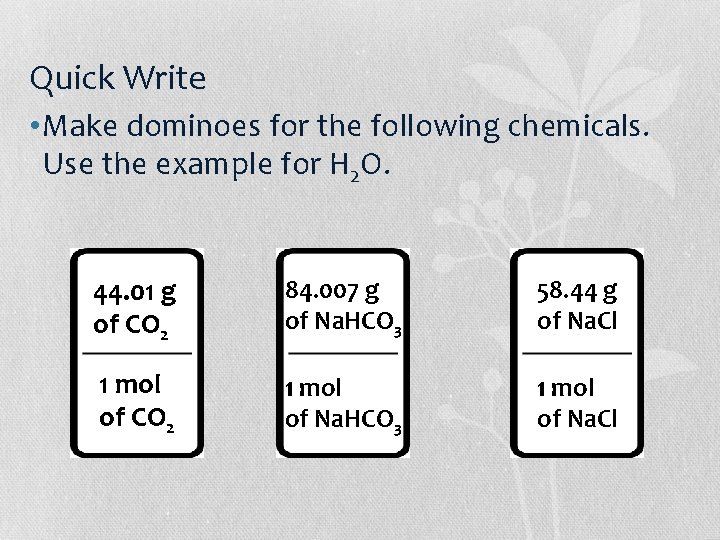
Quick Write • Make dominoes for the following chemicals. Use the example for H 2 O. 44. 01 g of CO 2 84. 007 g of Na. HCO 3 58. 44 g of Na. Cl 1 mol of CO 2 1 mol of Na. HCO 3 1 mol of Na. Cl

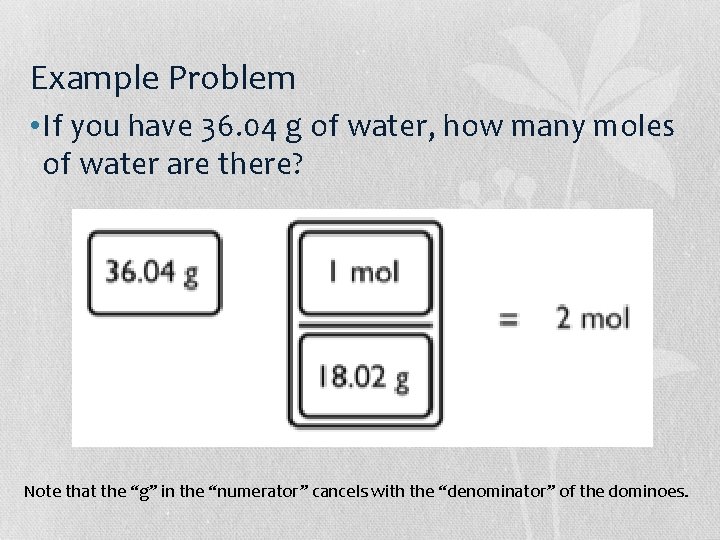
Example Problem • If you have 36. 04 g of water, how many moles of water are there? Note that the “g” in the “numerator” cancels with the “denominator” of the dominoes.

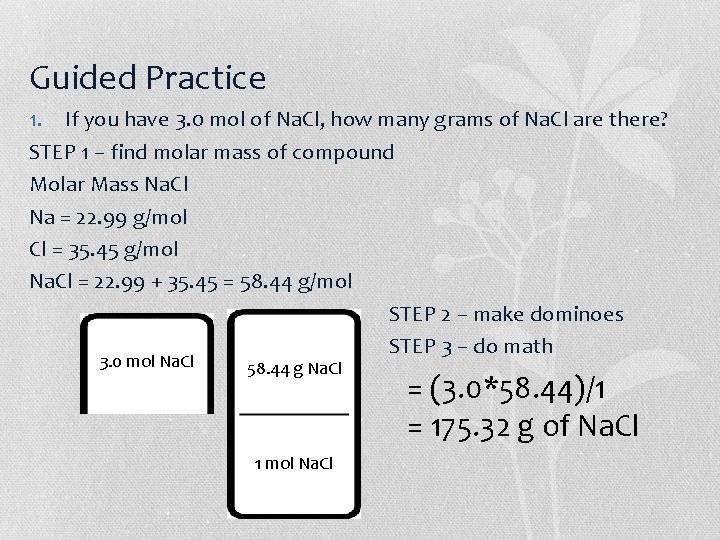
Guided Practice 1. If you have 3. 0 mol of Na. Cl, how many grams of Na. Cl are there? STEP 1 – find molar mass of compound Molar Mass Na. Cl Na = 22. 99 g/mol Cl = 35. 45 g/mol Na. Cl = 22. 99 + 35. 45 = 58. 44 g/mol STEP 2 – make dominoes STEP 3 – do math 3. 0 mol Na. Cl 58. 44 g Na. Cl 1 mol Na. Cl = (3. 0*58. 44)/1 = 175. 32 g of Na. Cl

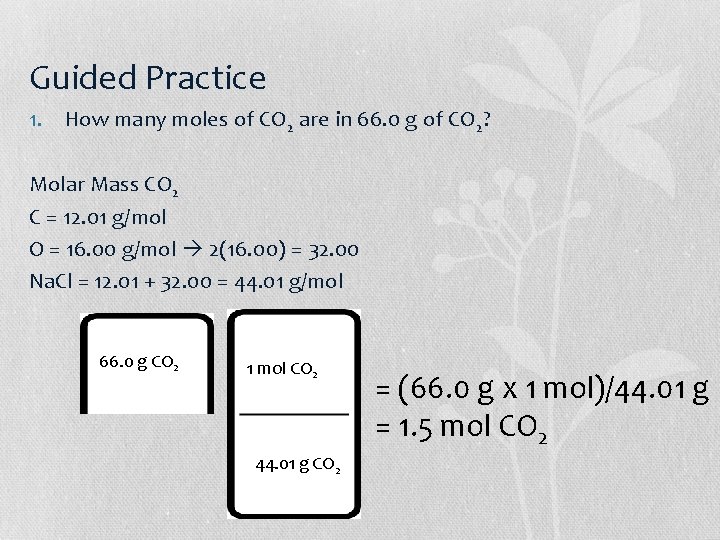
Guided Practice 1. How many moles of CO 2 are in 66. 0 g of CO 2? Molar Mass CO 2 C = 12. 01 g/mol O = 16. 00 g/mol 2(16. 00) = 32. 00 Na. Cl = 12. 01 + 32. 00 = 44. 01 g/mol 66. 0 g CO 2 1 mol CO 2 44. 01 g CO 2 = (66. 0 g x 1 mol)/44. 01 g = 1. 5 mol CO 2

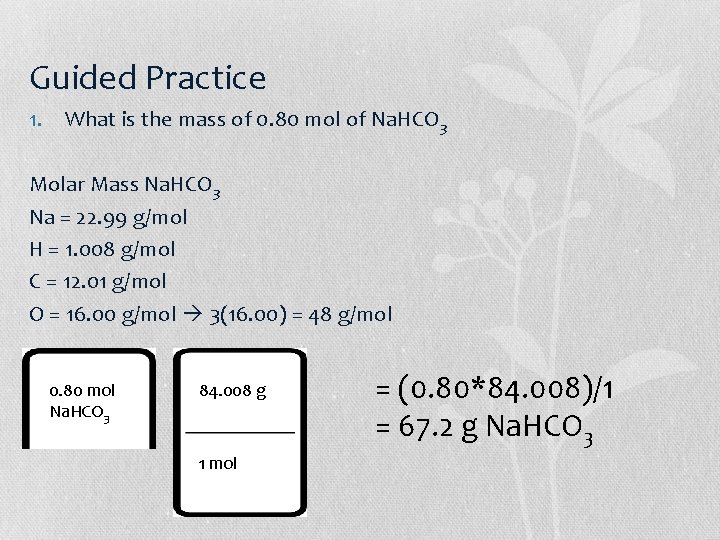
Guided Practice 1. What is the mass of 0. 80 mol of Na. HCO 3 Molar Mass Na. HCO 3 Na = 22. 99 g/mol H = 1. 008 g/mol C = 12. 01 g/mol O = 16. 00 g/mol 3(16. 00) = 48 g/mol 0. 80 mol Na. HCO 3 84. 008 g 1 mol = (0. 80*84. 008)/1 = 67. 2 g Na. HCO 3

Quiz • Weekly Quiz

Day 2 • January 15 th, 16 th • 96 minutes

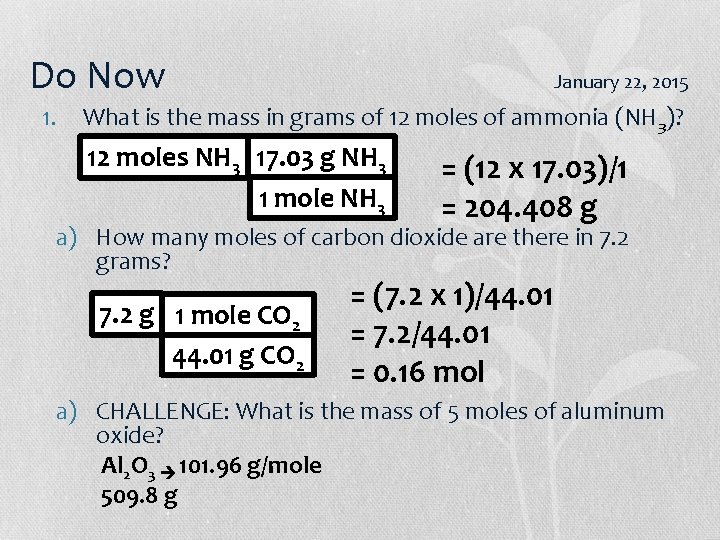
Do Now January 20/22, 2015 1. What is the mass of 12 moles of ammonia (NH 3)? 2. How many grams of carbon dioxide are there in 7. 2 moles? 3. CHALLENGE: What is the mass of 5 moles of aluminum oxide?
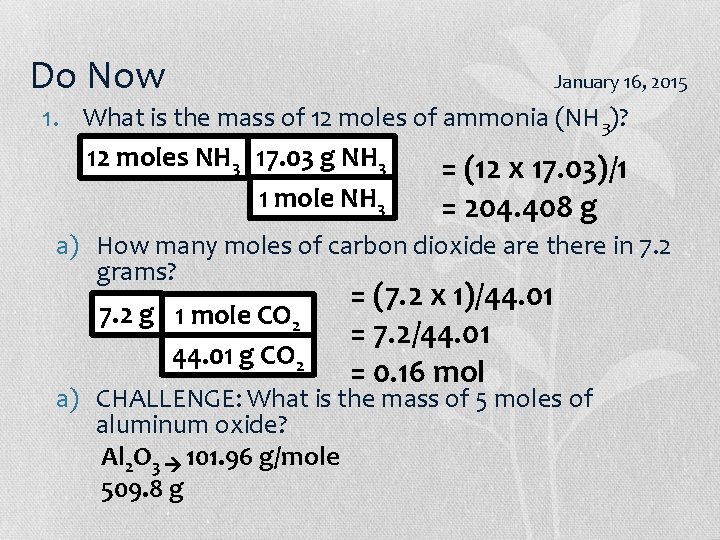
Do Now January 16, 2015 1. What is the mass of 12 moles of ammonia (NH 3)? 12 moles NH 3 17. 03 g NH 3 = (12 x 17. 03)/1 1 mole NH 3 = 204. 408 g a) How many moles of carbon dioxide are there in 7. 2 grams? 7. 2 g 1 mole CO 2 44. 01 g CO 2 = (7. 2 x 1)/44. 01 = 7. 2/44. 01 = 0. 16 mol a) CHALLENGE: What is the mass of 5 moles of aluminum oxide? Al 2 O 3 101. 96 g/mole 509. 8 g

Objective • I can use dimensional analysis to determine the amount, mass, or volume of a substance produced or required in a chemical reaction.

Homework #4 • Due Wednesday, January 21 st or Monday, January 26 • SKIP Questions: 1 a, 2 b, 2 c

Agenda 1. Do Now, Objective 2. Stoichiometry 1: Moles Grams Review 3. Stoichiometry 2: Coefficients in Balanced Equations 4. Independent Practice 5. Stoichiometry 3: Volume of 1 Mole of a Gas 6. Multi-Step Problems 7. Exit Ticket

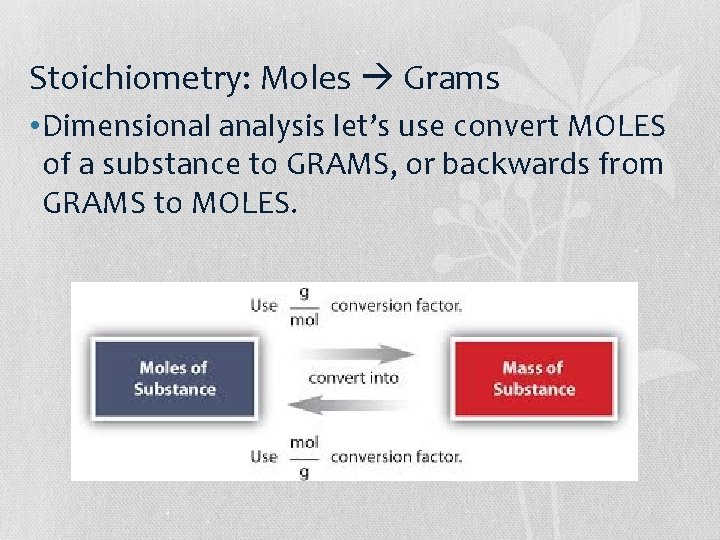
Stoichiometry: Moles Grams • Dimensional analysis let’s use convert MOLES of a substance to GRAMS, or backwards from GRAMS to MOLES.

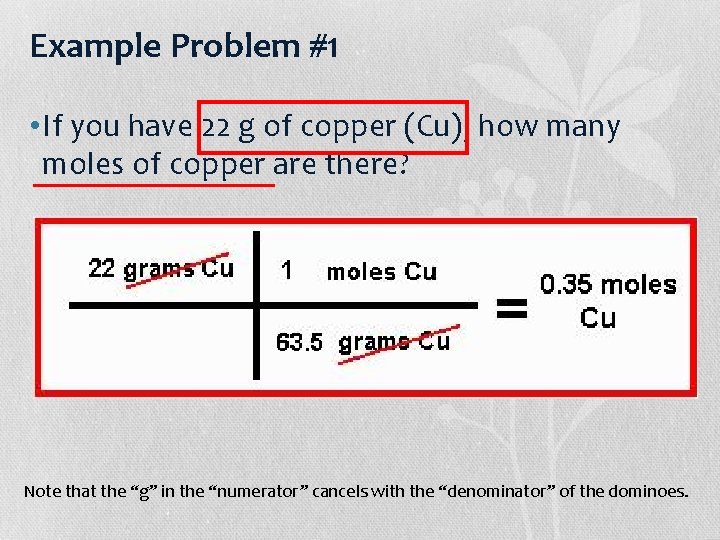
Example Problem #1 • If you have 22 g of copper (Cu), how many moles of copper are there? Note that the “g” in the “numerator” cancels with the “denominator” of the dominoes.

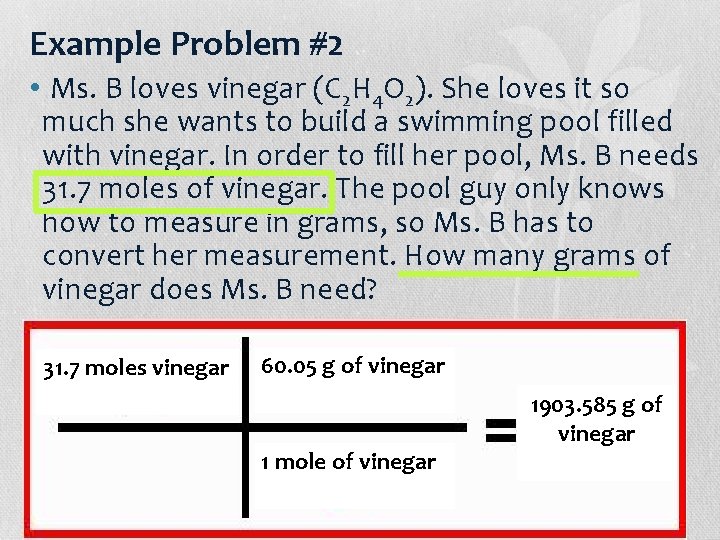
Example Problem #2 • Ms. B loves vinegar (C 2 H 4 O 2). She loves it so much she wants to build a swimming pool filled with vinegar. In order to fill her pool, Ms. B needs 31. 7 moles of vinegar. The pool guy only knows how to measure in grams, so Ms. B has to convert her measurement. How many grams of vinegar does Ms. B need? 31. 7 moles vinegar Molar 60. 05 of g of. Vinegar vinegar (C H O ) Mass 2 4 2 • C = 12. 01 g/mol 2*12. 01 = 24. 02 • H = 1. 008 g/mol 1. 008*4 = 4. 032 1 mole of vinegar • O = 16. 00 g/mol 16. 00*2 = 32 • 24. 02+4. 032+32 = 60. 05 g/mol 1903. 585 g of vinegar

Independent Practice Problems • Solve the problem below with a friend. When you have found the answer, look around the room for the paper with the correct answer. Then, look underneath the paper to get your next question. Make sure you write down the question and the answer for each problem. How many moles of carbon are in 18. 0 g of graphite pencil leads (graphite is C)

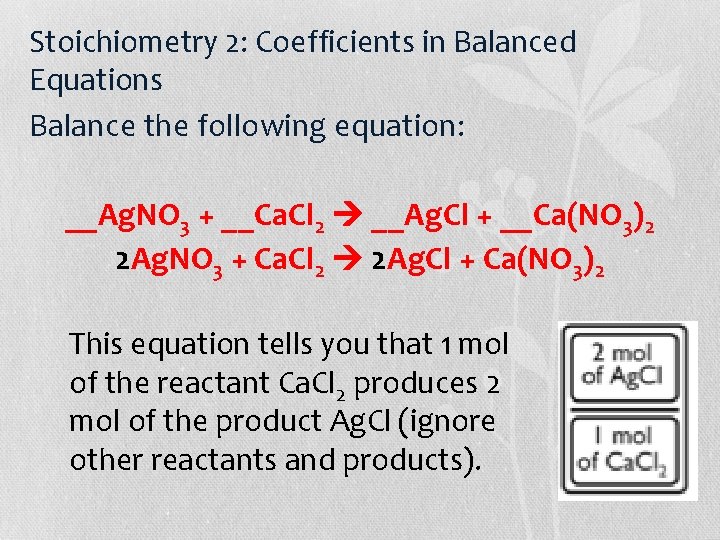
Stoichiometry 2: Coefficients in Balanced Equations • A second kind of equivalent measure domino comes from balanced chemical equations. You can make dominoes out of any two substances in a balanced chemical equation.

Stoichiometry 2: Coefficients in Balanced Equations Balance the following equation: __Ag. NO 3 + __Ca. Cl 2 __Ag. Cl + __Ca(NO 3)2 2 Ag. NO 3 + Ca. Cl 2 2 Ag. Cl + Ca(NO 3)2 This equation tells you that 1 mol of the reactant Ca. Cl 2 produces 2 mol of the product Ag. Cl (ignore other reactants and products).

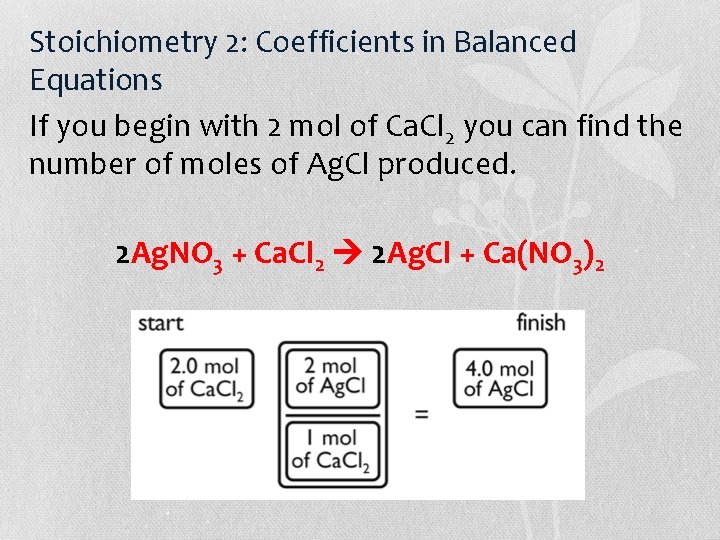
Stoichiometry 2: Coefficients in Balanced Equations If you begin with 2 mol of Ca. Cl 2 you can find the number of moles of Ag. Cl produced. 2 Ag. NO 3 + Ca. Cl 2 2 Ag. Cl + Ca(NO 3)2

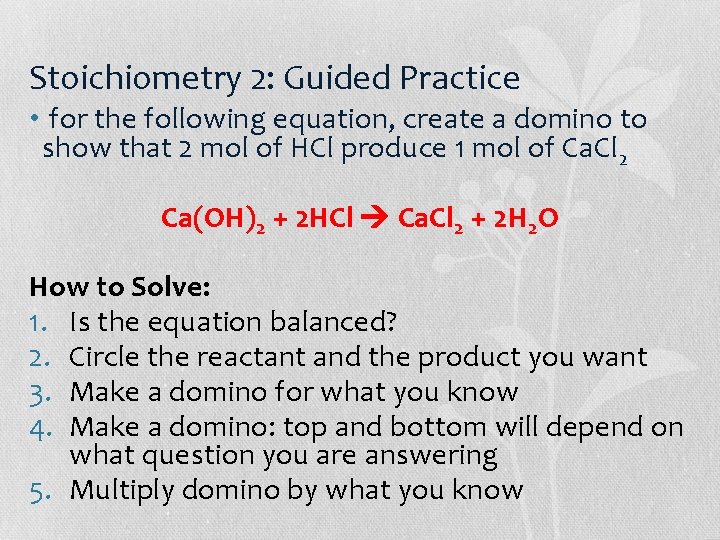
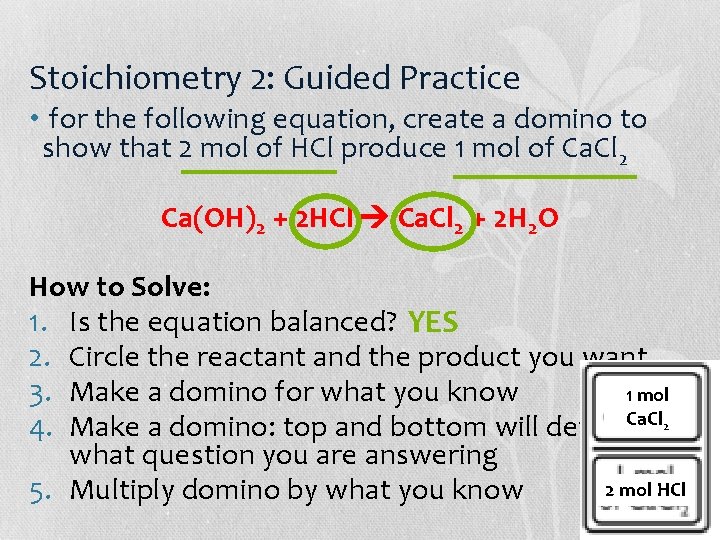
Stoichiometry 2: Guided Practice • for the following equation, create a domino to show that 2 mol of HCl produce 1 mol of Ca. Cl 2 Ca(OH)2 + 2 HCl Ca. Cl 2 + 2 H 2 O How to Solve: 1. Is the equation balanced? 2. Circle the reactant and the product you want 3. Make a domino for what you know 4. Make a domino: top and bottom will depend on what question you are answering 5. Multiply domino by what you know

Stoichiometry 2: Guided Practice • for the following equation, create a domino to show that 2 mol of HCl produce 1 mol of Ca. Cl 2 Ca(OH)2 + 2 HCl Ca. Cl 2 + 2 H 2 O How to Solve: 1. Is the equation balanced? YES 2. Circle the reactant and the product you want 3. Make a domino for what you know 1 mol Ca. Cl 2 4. Make a domino: top and bottom will depend on what question you are answering 2 mol HCl 5. Multiply domino by what you know

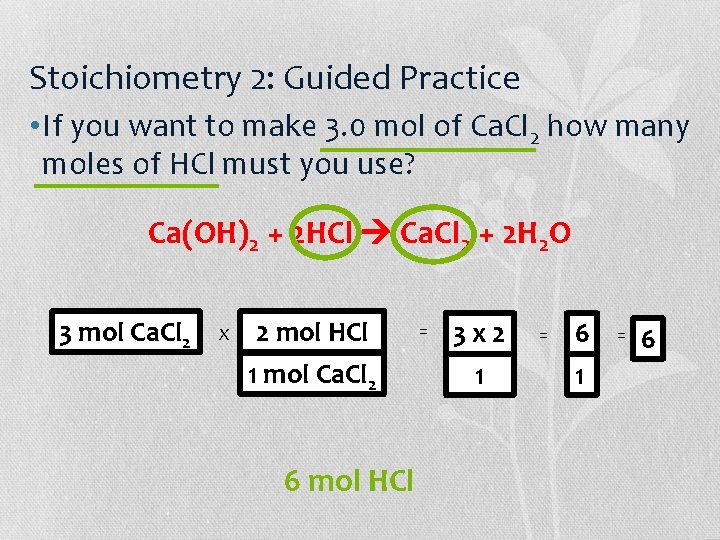
Stoichiometry 2: Guided Practice • If you want to make 3. 0 mol of Ca. Cl 2 how many moles of HCl must you use? Ca(OH)2 + 2 HCl Ca. Cl 2 + 2 H 2 O How to Solve: 1. Is the equation balanced? 2. Circle the reactant and the product you want 3. Make a domino for what you know 4. Make a domino: top and bottom will depend on what question you are answering 5. Multiply domino by what you know

Stoichiometry 2: Guided Practice • If you want to make 3. 0 mol of Ca. Cl 2 how many moles of HCl must you use? Ca(OH)2 + 2 HCl Ca. Cl 2 + 2 H 2 O 3 mol Ca. Cl 2 X 2 mol HCl 1 mol Ca. Cl 2 6 mol HCl = 3 x 2 1 = 6

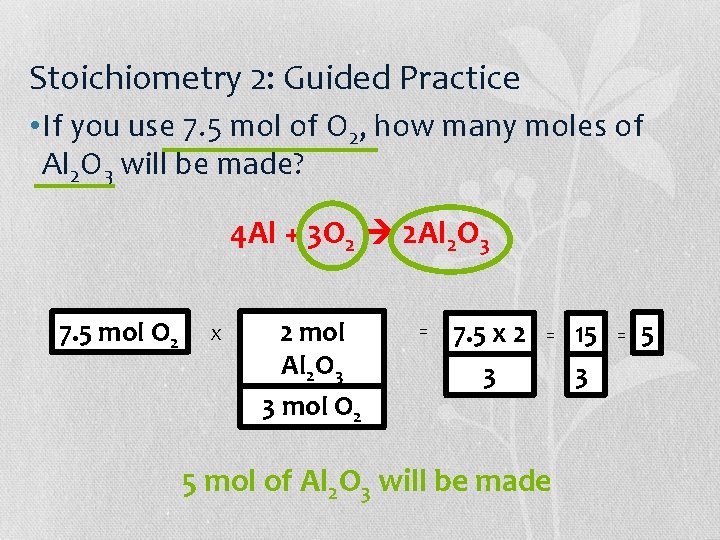
Stoichiometry 2: Guided Practice • If you use 7. 5 mol of O 2, how many moles of Al 2 O 3 will be made? 4 Al + 3 O 2 2 Al 2 O 3 7. 5 mol O 2 X 2 mol Al 2 O 3 3 mol O 2 = 7. 5 x 2 3 = 5 mol of Al 2 O 3 will be made 15 3 = 5

Stoichiometry 2: Independent Practice 1 - 3 • Answer the independent practice questions in your notes. NOT all of the equations are balanced, so you may have to balance them first, before you can answer the questions.

Do Now January 22, 2015 1. What is the mass in grams of 12 moles of ammonia (NH 3)? 12 moles NH 3 17. 03 g NH 3 1 mole NH 3 = (12 x 17. 03)/1 = 204. 408 g a) How many moles of carbon dioxide are there in 7. 2 grams? 7. 2 g 1 mole CO 2 44. 01 g CO 2 = (7. 2 x 1)/44. 01 = 7. 2/44. 01 = 0. 16 mol a) CHALLENGE: What is the mass of 5 moles of aluminum oxide? Al 2 O 3 101. 96 g/mole 509. 8 g

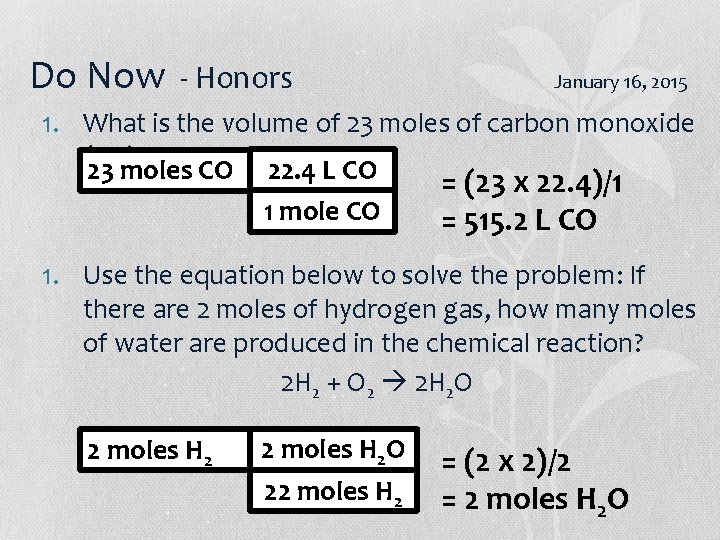
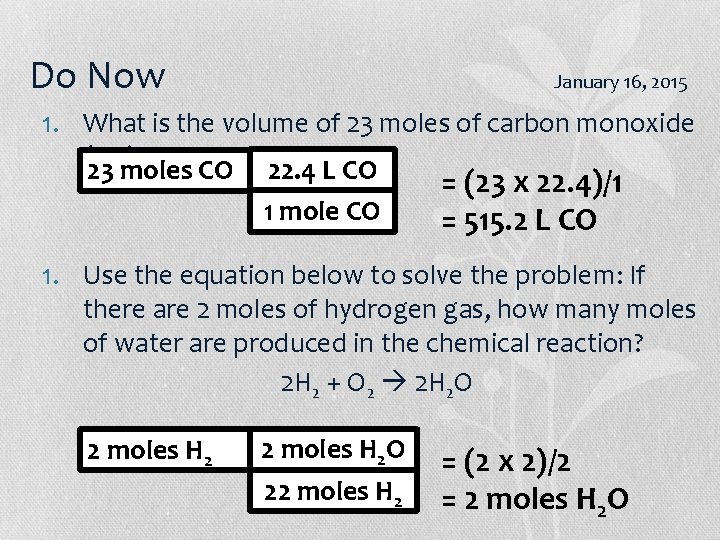
Do Now - Honors January 16, 2015 1. What is the volume of 23 moles of carbon monoxide (CO)? 23 moles CO 22. 4 L CO 1 mole CO = (23 x 22. 4)/1 = 515. 2 L CO 1. Use the equation below to solve the problem: If there are 2 moles of hydrogen gas, how many moles of water are produced in the chemical reaction? 2 H 2 + O 2 2 H 2 O 2 moles H 2 O 22 moles H 2 = (2 x 2)/2 = 2 moles H 2 O

Objective • I can use dimensional analysis to determine the amount, mass, or volume of a substance produced or required in a chemical reaction in multi-step problems.

Homework #4 • Due Friday, January 23 or Monday, January 26

Agenda 1. 2. 3. 4. 5. 6. Do Now, Objective Stoichiometry Review Stoichiometry 3: Moles to Volume Multi-Step Problems Independent Practice Weekly Quiz

Stoichiometry Review • Stoichiometry #1 – moles to grams; grams to moles • Stoichiometry #2 – moles to moles How to Solve Stoichiometry Problems 1. Balance the equation! 2. Circle what you know, underline what you want 3. Find the molar mass of the compounds in the problem 4. Set up your table, make sure all units cancel out except for the units you want in your answer 5. Multiply across the top and the bottom 6. Divide the top by the bottom 7. Write your answer including the correct units!

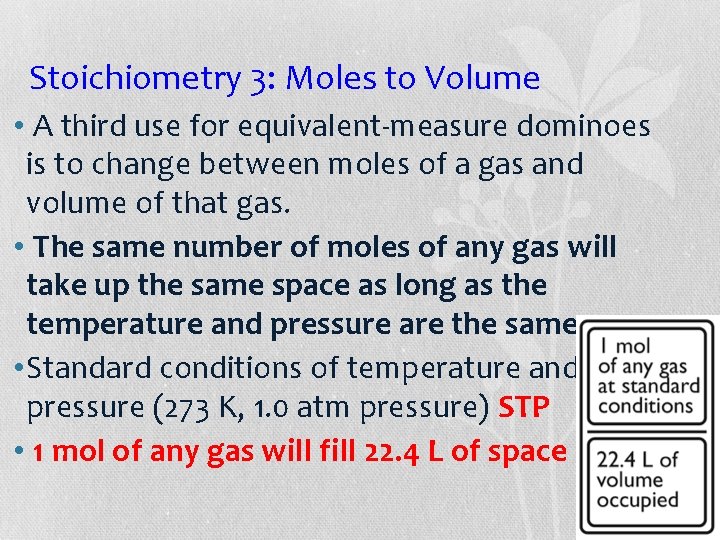
Stoichiometry 3: Moles to Volume • A third use for equivalent-measure dominoes is to change between moles of a gas and volume of that gas. • The same number of moles of any gas will take up the same space as long as the temperature and pressure are the same. • Standard conditions of temperature and pressure (273 K, 1. 0 atm pressure) STP • 1 mol of any gas will fill 22. 4 L of space

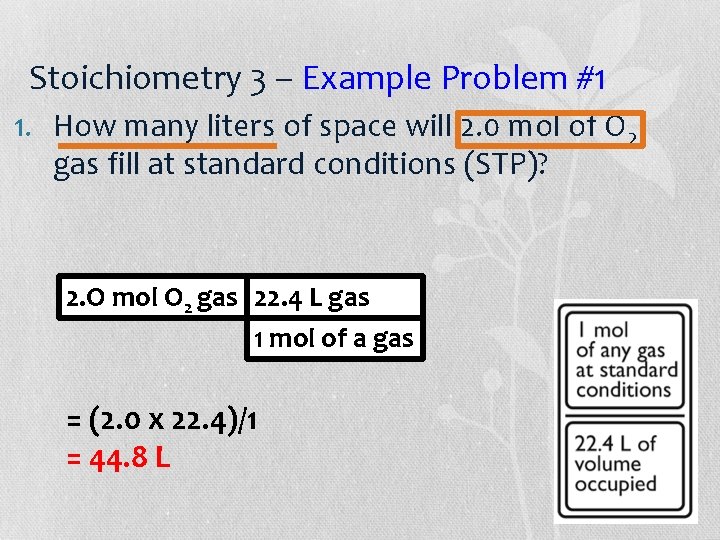
Stoichiometry 3 – Example Problem #1 1. How many liters of space will 2. 0 mol of O 2 gas fill at standard conditions (STP)? 2. O mol O 2 gas 22. 4 L gas 1 mol of a gas = (2. 0 x 22. 4)/1 = 44. 8 L

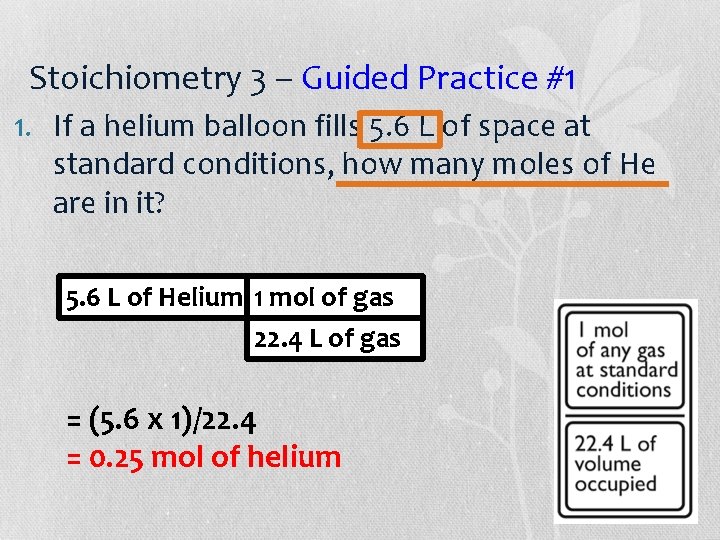
Stoichiometry 3 – Guided Practice #1 1. If a helium balloon fills 5. 6 L of space at standard conditions, how many moles of He are in it? 5. 6 L of Helium 1 mol of gas 22. 4 L of gas = (5. 6 x 1)/22. 4 = 0. 25 mol of helium

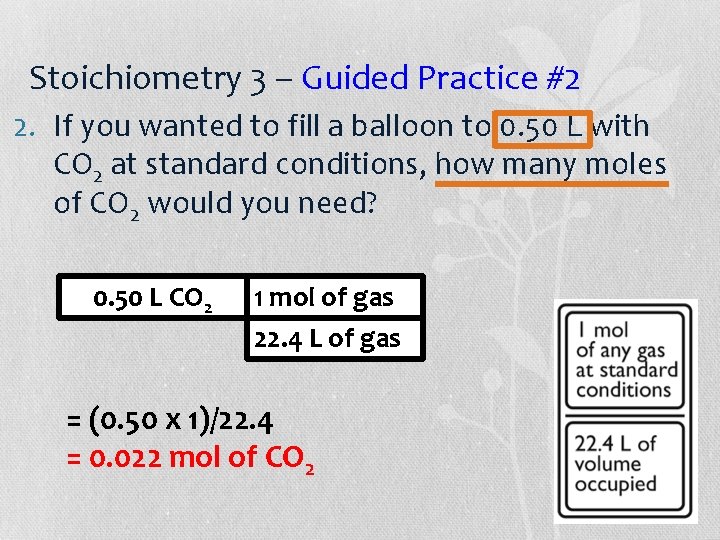
Stoichiometry 3 – Guided Practice #2 2. If you wanted to fill a balloon to 0. 50 L with CO 2 at standard conditions, how many moles of CO 2 would you need? 0. 50 L CO 2 1 mol of gas 22. 4 L of gas = (0. 50 x 1)/22. 4 = 0. 022 mol of CO 2

Stoichiometry 3 – Independent Practice • Take 10 minutes to answer questions 1 - 5 on your independent practice. Check your work at the front of the room, and raise your hand when you are finished to get it signed off on by Ms. Bergman

Do Now January 16, 2015 1. What is the volume of 23 moles of carbon monoxide (CO)? 23 moles CO 22. 4 L CO 1 mole CO = (23 x 22. 4)/1 = 515. 2 L CO 1. Use the equation below to solve the problem: If there are 2 moles of hydrogen gas, how many moles of water are produced in the chemical reaction? 2 H 2 + O 2 2 H 2 O 2 moles H 2 O 22 moles H 2 = (2 x 2)/2 = 2 moles H 2 O

Objective • I can use dimensional analysis to determine the amount, mass, or volume of a substance produced or required in a chemical reaction in multi-step problems.

Homework #4 • Due Friday, January 23 TODAY!

Agenda 1. 2. 3. 4. Do Now, Objective Multi-Step Problems Independent Practice Weekly Quiz

Multi-Step Stoichiometry Problems • Some stoichiometry problems require you to do multiple unit conversions (aka go from grams of one substance to moles of another substance) • Types of Multi-step problems • • Moles to Mass to Volume

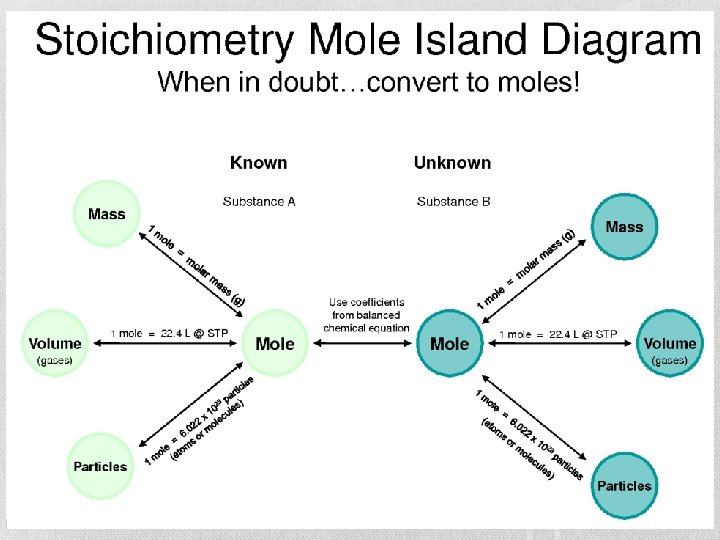
Mole Island • You’ll always be back…


Multi-Step Problems – Example #1: Moles to Mass • Use the equation below to answer the problem: 1. 5 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? 1. Balance the equation __KCl. O 3 __KCl + __O 2 2 KCl. O 3 2 KCl + 3 O 2

Multi-Step Problems – Example #1: Moles to Mass • Use the equation below to answer the problem: 1. 5 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? 1. Balance the equation 2. Circle what you know, underline what you want 3. Circle what you need in the equation 2 KCl. O 3 2 KCl + 3 O 2

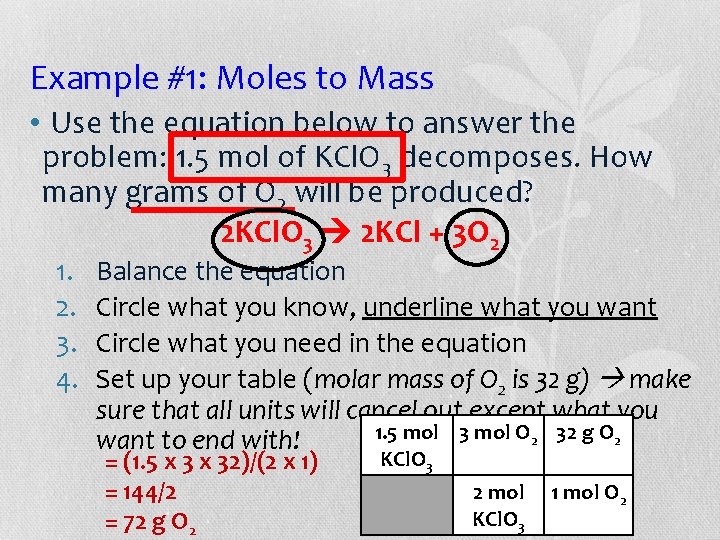
Example #1: Moles to Mass • Use the equation below to answer the problem: 1. 5 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? 2 KCl. O 3 2 KCl + 3 O 2 1. 2. 3. 4. Balance the equation Circle what you know, underline what you want Circle what you need in the equation Set up your table (molar mass of O 2 is 32 g) make sure that all units will cancel out except what you 1. 5 mol 3 mol O 2 32 g O 2 want to end with! = (1. 5 x 32)/(2 x 1) = 144/2 = 72 g O 2 KCl. O 3 2 mol KCl. O 3 1 mol O 2

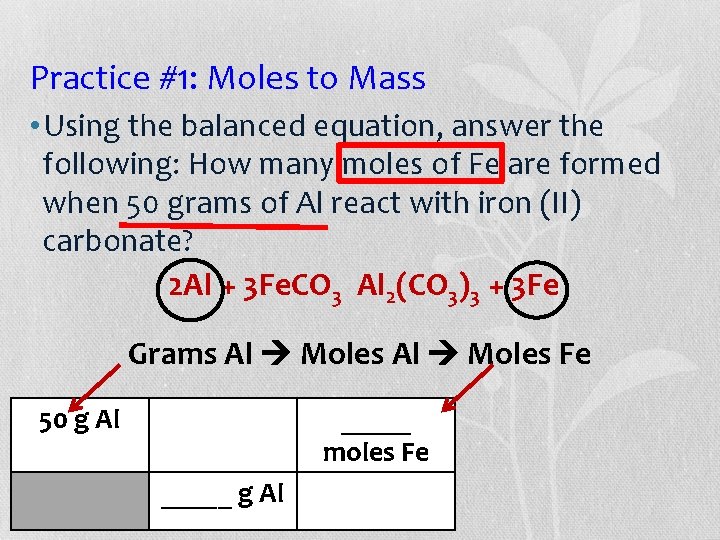
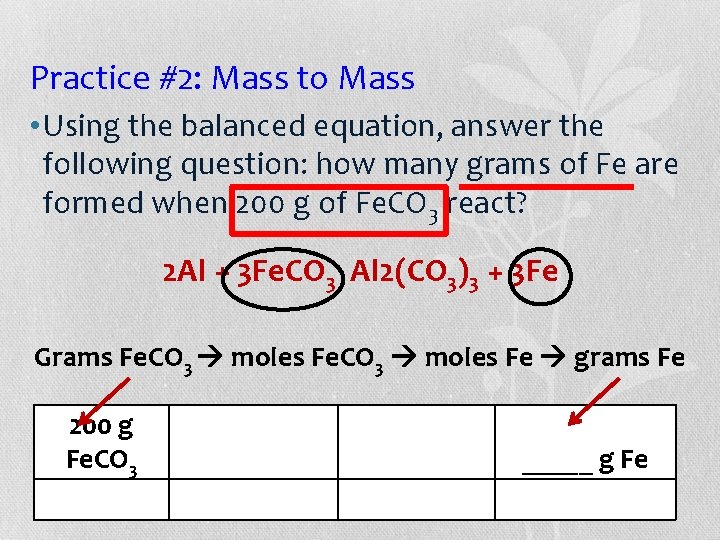
Practice #1: Moles to Mass • Using the balanced equation, answer the following: How many moles of Fe are formed when 50 grams of Al react with iron (II) carbonate? 2 Al + 3 Fe. CO 3 Al 2(CO 3)3 + 3 Fe Grams Al Moles Fe 50 g Al _____ moles Fe _____ g Al

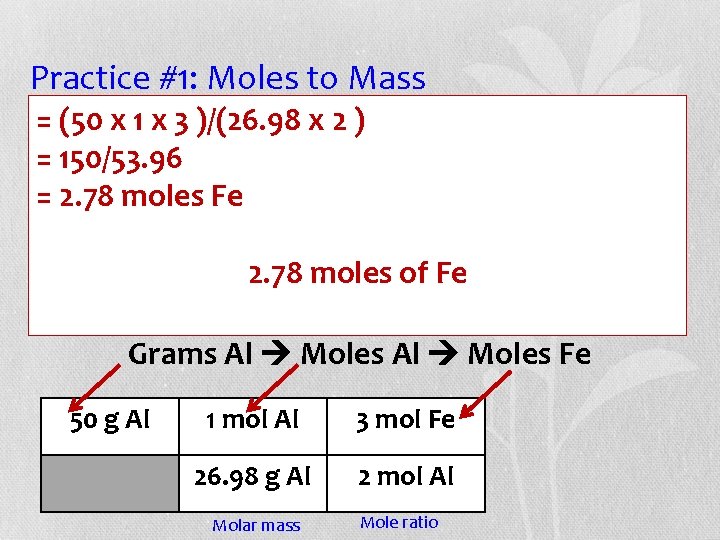
Practice #1: Moles to Mass (50 x the 1 x 3 balanced )/(26. 98 xequation, 2) • =Using answer the =following: 150/53. 96 How many moles of Fe are formed =when 2. 78 moles Fe of Al react with iron (II) 50 grams carbonate? 2. 78 moles of Fe + 3 Fe 2 Al + 3 Fe. CO 3 Al 2(CO 3)3 Grams Al Moles Fe 50 g Al 1 mol Al 3 mol Fe 26. 98 g Al 2 mol Al Molar mass Mole ratio

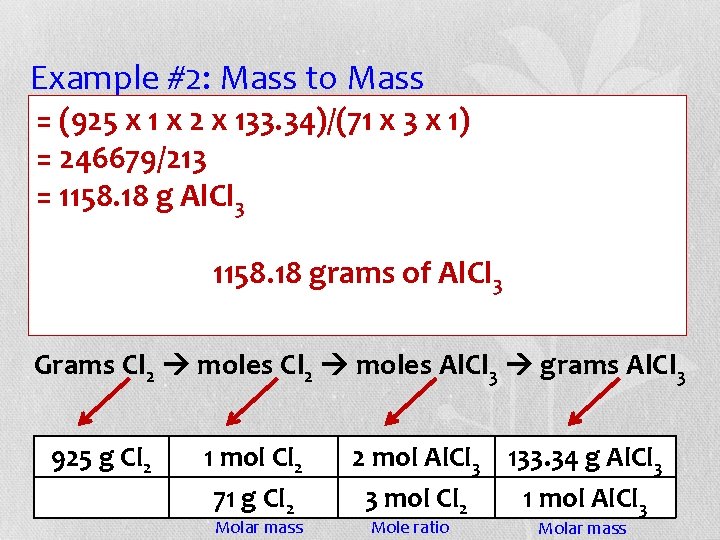
Example #2: Mass to Mass (925 the x 1 equation x 2 x 133. 34)/(71 x 3 determine x 1) • =Use given to how =many 246679/213 grams of aluminum chloride will be =produced 1158. 18 g Al. Cl from 3 925 g of Cl 2. 1158. 18 grams of Al. Cl 3 2 Al. Br 3 + 3 Cl 2 3 Br 2 + 2 Al. Cl 3 Grams Cl 2 moles Al. Cl 3 grams Al. Cl 3 925 g Cl 2 1 mol Cl 2 71 g Cl 2 Molar mass 2 mol Al. Cl 3 133. 34 g Al. Cl 3 3 mol Cl 2 1 mol Al. Cl 3 Mole ratio Molar mass

Practice #2: Mass to Mass • Using the balanced equation, answer the following question: how many grams of Fe are formed when 200 g of Fe. CO 3 react? 2 Al + 3 Fe. CO 3 Al 2(CO 3)3 + 3 Fe Grams Fe. CO 3 moles Fe grams Fe 200 g Fe. CO 3 _____ g Fe

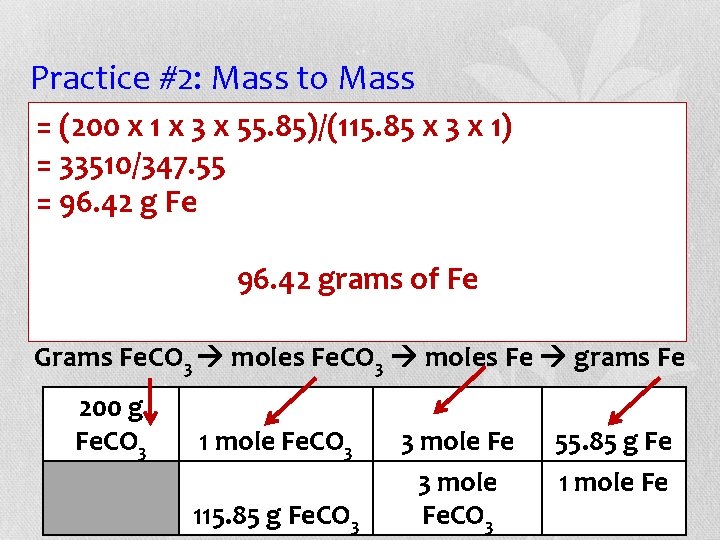
Practice #2: Mass to Mass (200 xthe 1 xbalanced 3 x 55. 85)/(115. 85 x 3 answer x 1) the • =Using equation, =following 33510/347. 55 question: how many grams of Fe are =formed 96. 42 gwhen Fe 200 g of Fe. CO 3 react? 2 Al + 96. 42 3 Fe. COgrams 3 Al 2(CO 3)3 + 3 Fe of Fe Grams Fe. CO 3 moles Fe grams Fe 200 g Fe. CO 3 1 mole Fe. CO 3 115. 85 g Fe. CO 3 3 mole Fe. CO 3 55. 85 g Fe 1 mole Fe

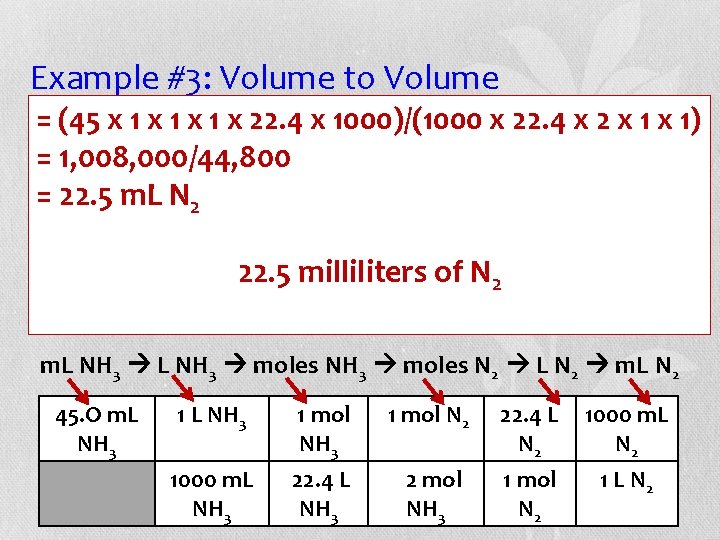
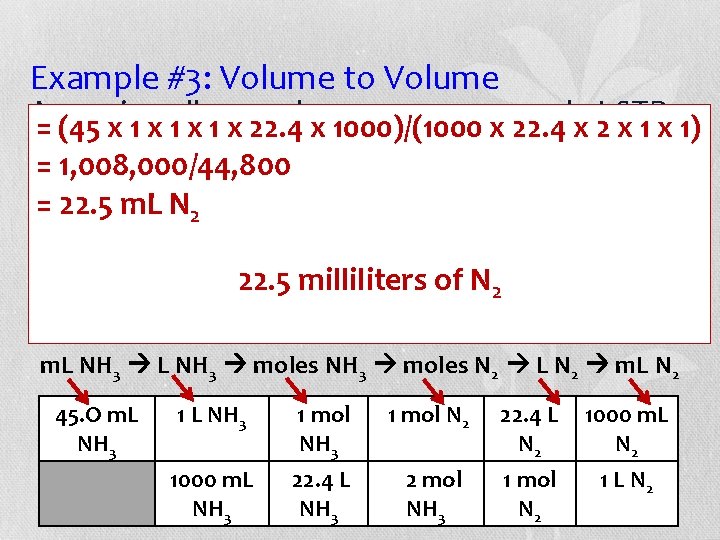
Example #3: Volume to Volume Assuming volumes are measured = (45 x 1 x all 1 x gas 1 x 22. 4 x 1000)/(1000 x 22. 4 xat 2 STP, x 1) how many milliliters of nitrogen gas react to = 1, 008, 000/44, 800 give 45. 0 = 22. 5 m. L N 2 of ammonia gas? Balance the equation. ___H ___N 2 ___NH 22. 5 of N 23 2 +milliliters 3 H 2 + N 2 2 NH 3 m. L NH 3 moles N 2 L N 2 m. L N 2 45. O m. L NH 3 1 mol N 2 22. 4 L N 2 1000 m. L NH 3 22. 4 L NH 3 2 mol NH 3 1 mol N 2 1 L N 2

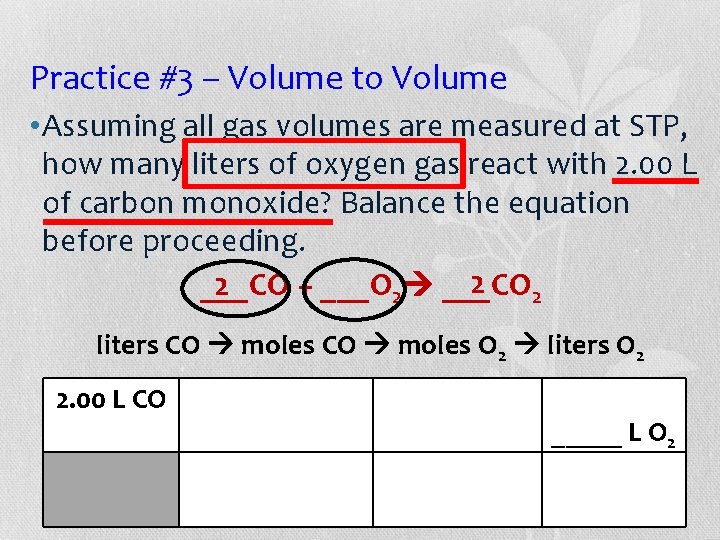
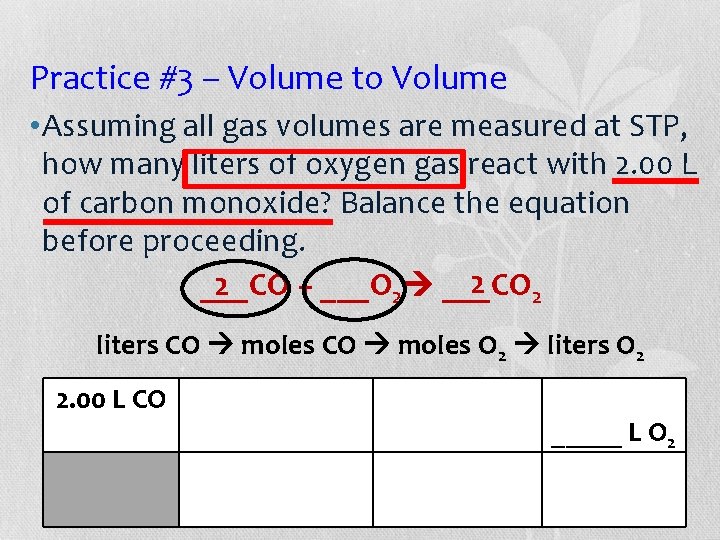
Practice #3 – Volume to Volume • Assuming all gas volumes are measured at STP, how many liters of oxygen gas react with 2. 00 L of carbon monoxide? Balance the equation before proceeding. 2 2 2 ___CO + ___O 2 ___CO liters CO moles O 2 liters O 2 2. 00 L CO _____ L O 2

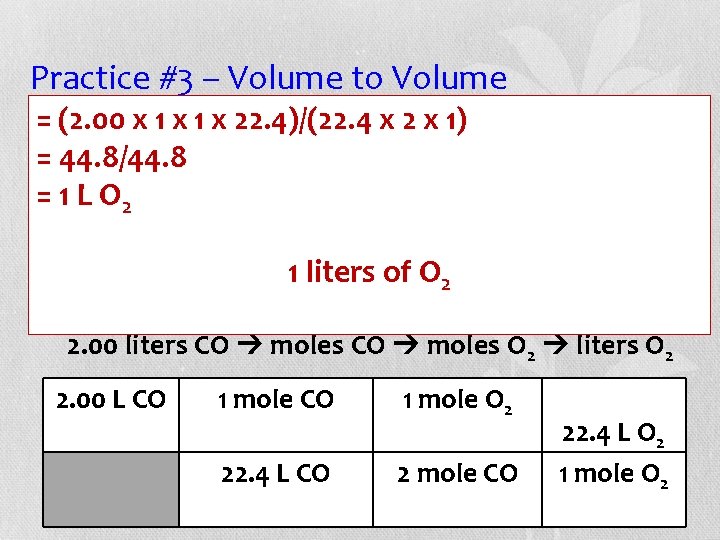
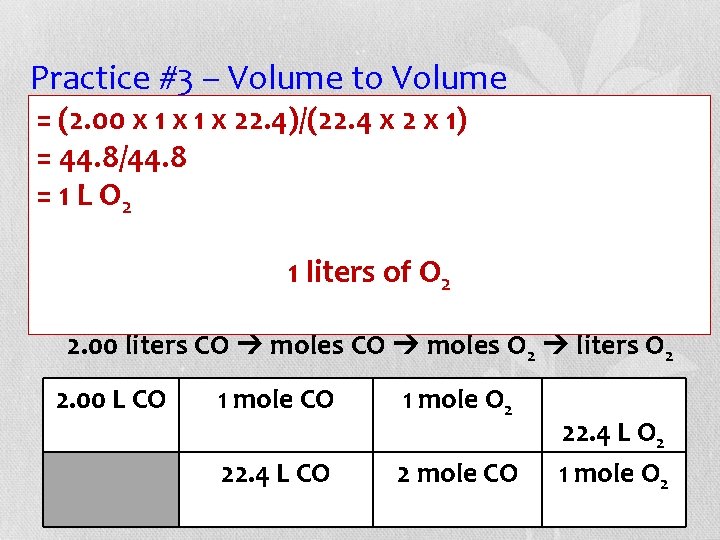
Practice #3 – Volume to Volume (2. 00 x 1 xall 1 xgas 22. 4)/(22. 4 2 x 1) • =Assuming volumesx are measured at STP, =how 44. 8/44. 8 many liters of oxygen gas react with 2. 00 L =of 1 Lcarbon O 2 monoxide? Balance the equation before proceeding. 1+liters O 2___CO 2 2 of ___CO ___O 2 2. 00 liters CO moles O 2 liters O 2 2. 00 L CO 1 mole O 2 22. 4 L CO 2 mole CO 22. 4 L O 2 1 mole O 2

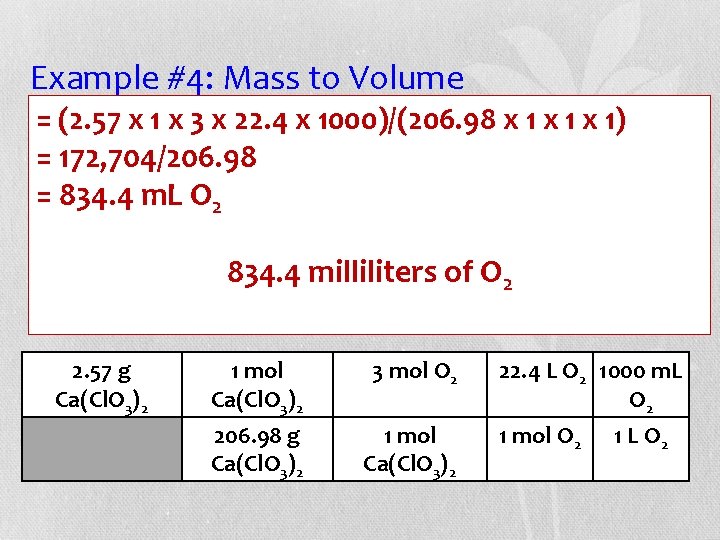
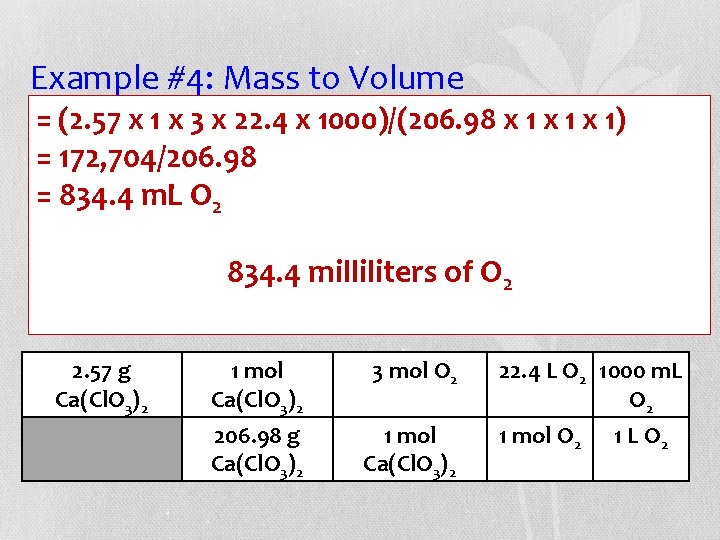
Example #4: Mass to Volume (2. 57 the x 1 xbalanced 3 x 22. 4 xequation, 1000)/(206. 98 x 1 milliliters x 1) • =Given how many =of 172, 704/206. 98 oxygen gas at STP are released from the =decomposition 834. 4 m. L O 2 of 2. 57 g of calcium chlorate (Ca(Cl. O 3)2)? 834. 43)milliliters O 2 Ca(Cl. O + 3 O 2 Ca. Cl 2 of Grams Ca(Cl. O 3)2 moles O 2 L O 2 m. L O 2 2. 57 g Ca(Cl. O 3)2 1 mol Ca(Cl. O 3)2 3 mol O 2 206. 98 g Ca(Cl. O 3)2 1 mol Ca(Cl. O 3)2 22. 4 L O 2 1000 m. L O 2 1 mol O 2 1 L O 2

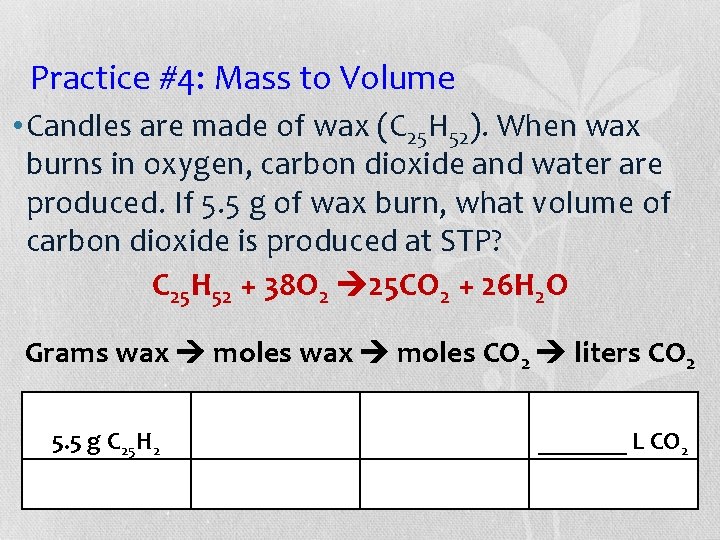
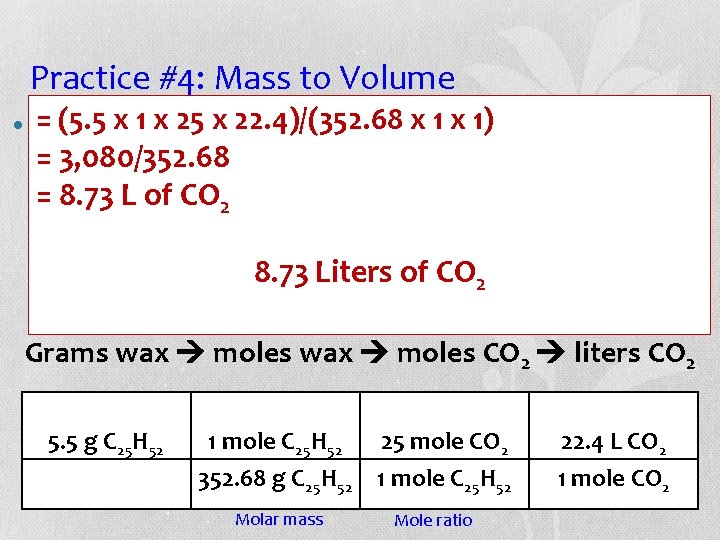
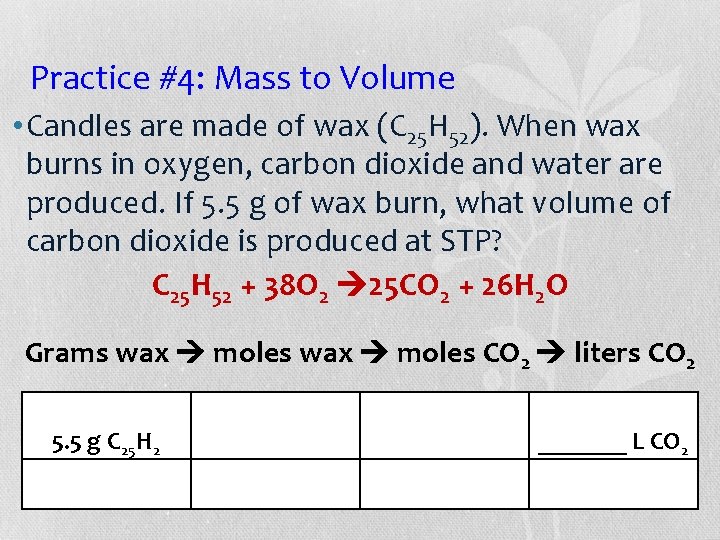
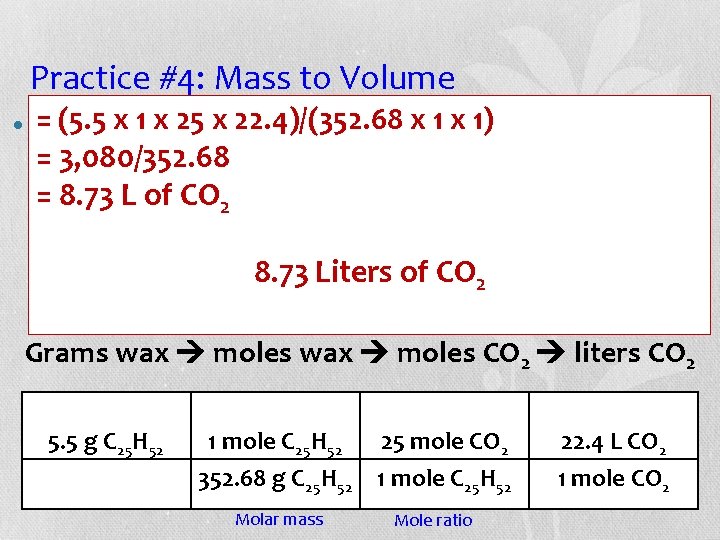
Practice #4: Mass to Volume • Candles are made of wax (C 25 H 52). When wax burns in oxygen, carbon dioxide and water are produced. If 5. 5 g of wax burn, what volume of carbon dioxide is produced at STP? C 25 H 52 + 38 O 2 25 CO 2 + 26 H 2 O Grams wax moles CO 2 liters CO 2 5. 5 g C 25 H 2 _______ L CO 2

Practice #4: Mass to Volume = (5. 5 x 1 are x 25 made x 22. 4)/(352. 68 x. H 1 52 x ). 1) When wax • Candles of wax (C 25 = 3, 080/352. 68 burns in oxygen, carbon dioxide and water are = 8. 73 L of. If. CO produced. 5. 5 2 g of wax burn, what volume of carbon dioxide is produced at STP? of CO 2 C 25 H 52 +8. 73 38 OLiters 25 CO 2 2 + 26 H 2 O Grams wax moles CO 2 liters CO 2 5. 5 g C 25 H 52 1 mole C 25 H 52 25 mole CO 2 352. 68 g C 25 H 52 1 mole C 25 H 52 Molar mass Mole ratio 22. 4 L CO 2 1 mole CO 2

Multi-Step Problems – Independent Practice • Take 20 minutes to answer questions 6 – 11 on your independent practice. When you are finished, check your answers at the front, and get it signed off on by Ms. Bergman • If you finish before time ends, you may: • Check your grades and start a grade-tracking sheet • Work on homework #4 • Read answer ‘checking up’ questions from the stoichiometry chemtalk for extra credit (pg. 289) • Help another student

Weekly Quiz • Quiz #2 Covers üThe Mole! üMolar mass üMole ratios üStoichiometry üBalancing reactions

Monday, January 26

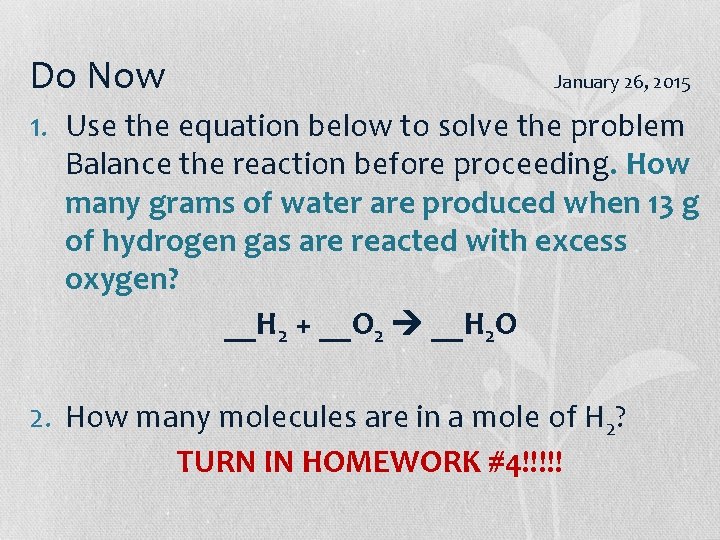
Do Now January 26, 2015 1. Use the equation below to solve the problem Balance the reaction before proceeding. How many grams of water are produced when 13 g of hydrogen gas are reacted with excess oxygen? __H 2 + __O 2 __H 2 O 2. How many molecules are in a mole of H 2? TURN IN HOMEWORK #4!!!!!

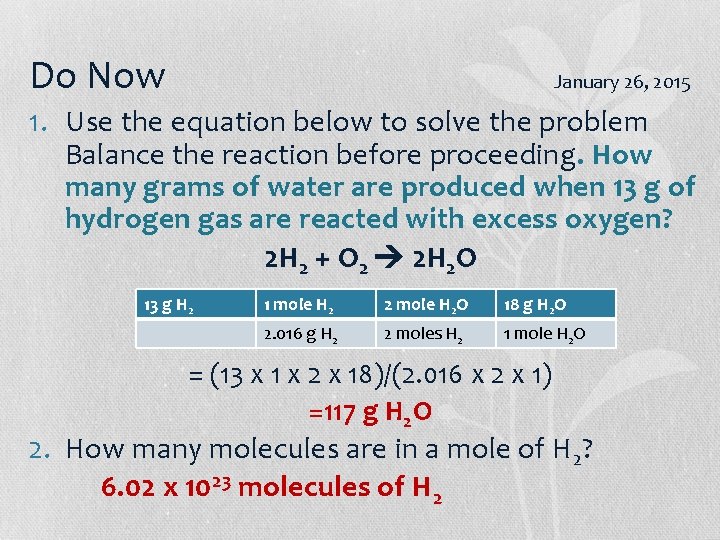
Do Now January 26, 2015 1. Use the equation below to solve the problem Balance the reaction before proceeding. How many grams of water are produced when 13 g of hydrogen gas are reacted with excess oxygen? 2 H 2 + O 2 2 H 2 O 13 g H 2 1 mole H 2 2 mole H 2 O 18 g H 2 O 2. 016 g H 2 2 moles H 2 1 mole H 2 O = (13 x 1 x 2 x 18)/(2. 016 x 2 x 1) =117 g H 2 O 2. How many molecules are in a mole of H 2? 6. 02 x 1023 molecules of H 2

Objective • I can use dimensional analysis and stoichiometry to determine the amount, mass, or volume of a substance produced or required in a chemical reaction in multi-step problems.

Homework #5 • Due Thursday, January 29 or Friday, January 30

Agenda 1. Do Now, Objective (10 min) 2. Stoichiometry Multi-Step Problems (30 min) • Examples • Practice problems 3. Independent Practice (45 min) 4. Exit Ticket (5 min)

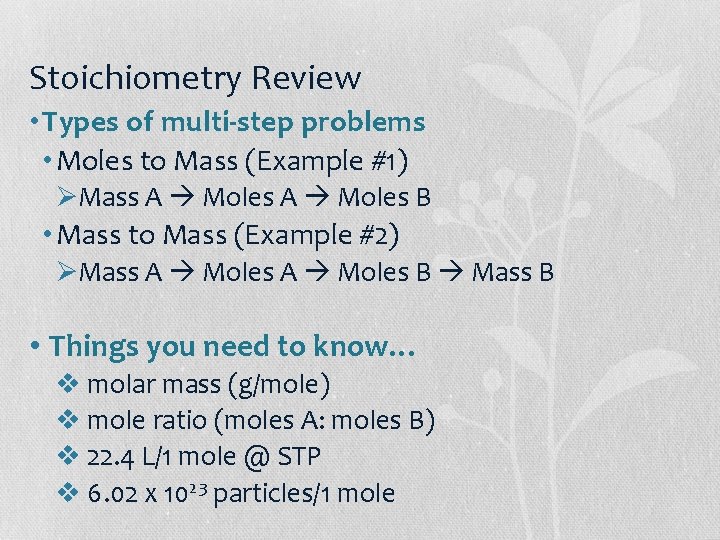
Stoichiometry Review • Types of multi-step problems • Moles to Mass (Example #1) ØMass A Moles B • Mass to Mass (Example #2) ØMass A Moles B Mass B • Things you need to know… v molar mass (g/mole) v mole ratio (moles A: moles B) v 22. 4 L/1 mole @ STP v 6. 02 x 1023 particles/1 mole

Example #3: Volume to Volume Assuming all gas volumes are measured at STP, = (45 x 1 x 1 x 22. 4 x 1000)/(1000 x 22. 4 x 2 x 1) how many milliliters of nitrogen gas react to = 1, 008, 000/44, 800 give 45. 0 m. L of ammonia gas? Balance the = 22. 5 m. L N 2 equation. ___H ___N 2 ___NH 2 +milliliters 22. 5 of N 23 3 H 2 + N 2 2 NH 3 m. L NH 3 moles N 2 L N 2 m. L N 2 45. O m. L NH 3 1 mol N 2 22. 4 L N 2 1000 m. L NH 3 22. 4 L NH 3 2 mol NH 3 1 mol N 2 1 L N 2

Practice #3 – Volume to Volume • Assuming all gas volumes are measured at STP, how many liters of oxygen gas react with 2. 00 L of carbon monoxide? Balance the equation before proceeding. 2 2 2 ___CO + ___O 2 ___CO liters CO moles O 2 liters O 2 2. 00 L CO _____ L O 2

Practice #3 – Volume to Volume (2. 00 x 1 xall 1 xgas 22. 4)/(22. 4 2 x 1) • =Assuming volumesx are measured at STP, =how 44. 8/44. 8 many liters of oxygen gas react with 2. 00 L =of 1 Lcarbon O 2 monoxide? Balance the equation before proceeding. 1+liters O 2___CO 2 2 of ___CO ___O 2 2. 00 liters CO moles O 2 liters O 2 2. 00 L CO 1 mole O 2 22. 4 L CO 2 mole CO 22. 4 L O 2 1 mole O 2

Example #4: Mass to Volume (2. 57 the x 1 xbalanced 3 x 22. 4 xequation, 1000)/(206. 98 x 1 milliliters x 1) • =Given how many =of 172, 704/206. 98 oxygen gas at STP are released from the =decomposition 834. 4 m. L O 2 of 2. 57 g of calcium chlorate (Ca(Cl. O 3)2)? 834. 43)milliliters O 2 Ca(Cl. O + 3 O 2 Ca. Cl 2 of Grams Ca(Cl. O 3)2 moles O 2 L O 2 m. L O 2 2. 57 g Ca(Cl. O 3)2 1 mol Ca(Cl. O 3)2 3 mol O 2 206. 98 g Ca(Cl. O 3)2 1 mol Ca(Cl. O 3)2 22. 4 L O 2 1000 m. L O 2 1 mol O 2 1 L O 2

Practice #4: Mass to Volume • Candles are made of wax (C 25 H 52). When wax burns in oxygen, carbon dioxide and water are produced. If 5. 5 g of wax burn, what volume of carbon dioxide is produced at STP? C 25 H 52 + 38 O 2 25 CO 2 + 26 H 2 O Grams wax moles CO 2 liters CO 2 5. 5 g C 25 H 2 _______ L CO 2

Practice #4: Mass to Volume = (5. 5 x 1 are x 25 made x 22. 4)/(352. 68 x. H 1 52 x ). 1) When wax • Candles of wax (C 25 = 3, 080/352. 68 burns in oxygen, carbon dioxide and water are = 8. 73 L of. If. CO produced. 5. 5 2 g of wax burn, what volume of carbon dioxide is produced at STP? of CO 2 C 25 H 52 +8. 73 38 OLiters 25 CO 2 2 + 26 H 2 O Grams wax moles CO 2 liters CO 2 5. 5 g C 25 H 52 1 mole C 25 H 52 25 mole CO 2 352. 68 g C 25 H 52 1 mole C 25 H 52 Molar mass Mole ratio 22. 4 L CO 2 1 mole CO 2

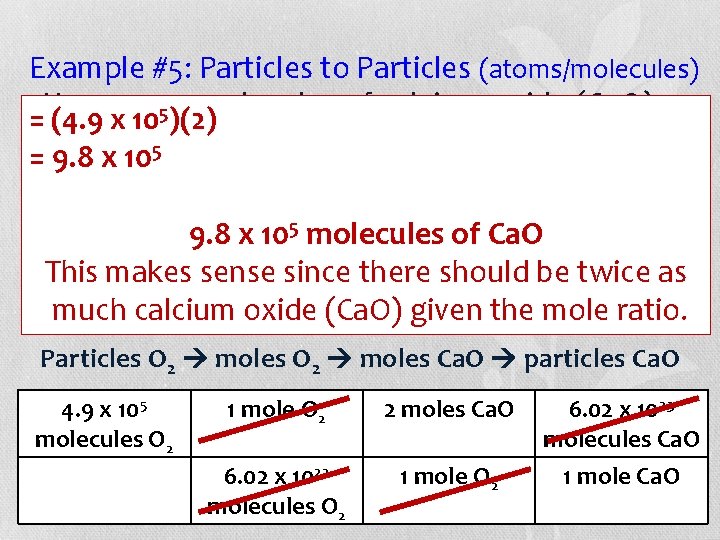
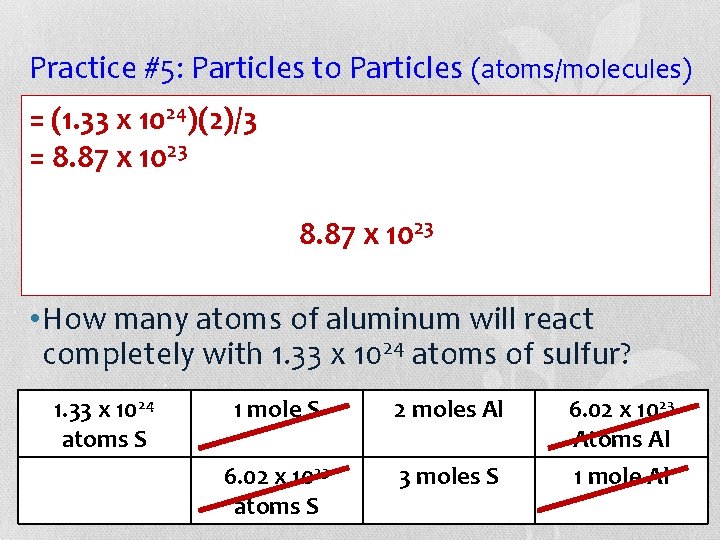
Example #5: Particles to Particles (atoms/molecules) • =How many molecules of calcium oxide (Ca. O) 5 (4. 9 x 10 )(2) 5 molecules of can be produced from 4. 9 x 10 = 9. 8 x 105 oxygen gas (O 2) that react with calcium (Ca) according to chemical 5 molecules 9. 8 this x 10 balanced of Ca. Oequation? This makes sense since there should be twice as 2 Ca(s) + O 2(g) 2 Ca. O(s) much calcium oxide (Ca. O) given the mole ratio. Particles O 2 moles Ca. O particles Ca. O 4. 9 x 105 molecules O 2 1 mole O 2 2 moles Ca. O 6. 02 x 1023 molecules O 2 1 mole O 2 6. 02 x 1023 molecules Ca. O 1 mole Ca. O

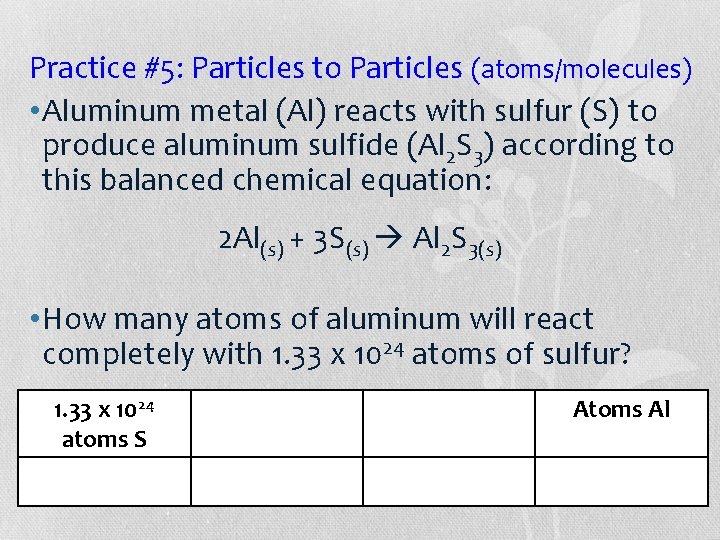
Practice #5: Particles to Particles (atoms/molecules) • Aluminum metal (Al) reacts with sulfur (S) to produce aluminum sulfide (Al 2 S 3) according to this balanced chemical equation: 2 Al(s) + 3 S(s) Al 2 S 3(s) • How many atoms of aluminum will react completely with 1. 33 x 1024 atoms of sulfur? 1. 33 x 1024 Atoms Al atoms S O 2 moles Ca. O particles Ca. O Particles

Practice #5: Particles to Particles (atoms/molecules) • =Aluminum metal (Al) reacts with sulfur (S) to (1. 33 x 1024)(2)/3 sulfide (Al 2 S 3) according to 23 =produce 8. 87 x 10 aluminum this balanced chemical equation: 23 8. 87 x 10 2 Al(s) + 3 S(s) Al 2 S 3(s) • How many atoms of aluminum will react completely with 1. 33 x 1024 atoms of sulfur? 1. 33 x 1024 1 mole S 2 moles Al 6. 02 x 1023 atoms S O 2 moles Ca. O particles Atoms. Ca. O Al Particles 6. 02 x 1023 3 moles S 1 mole Al atoms S

Multi-Step Problems – Independent Practice • Take 20 minutes to answer questions 6 – 11 on your independent practice. When you are finished, check your answers at the front, and get it signed off on by Ms. Bergman

January 28, 2015 (45 minutes; all sections) • Stoichiometry Practice Day

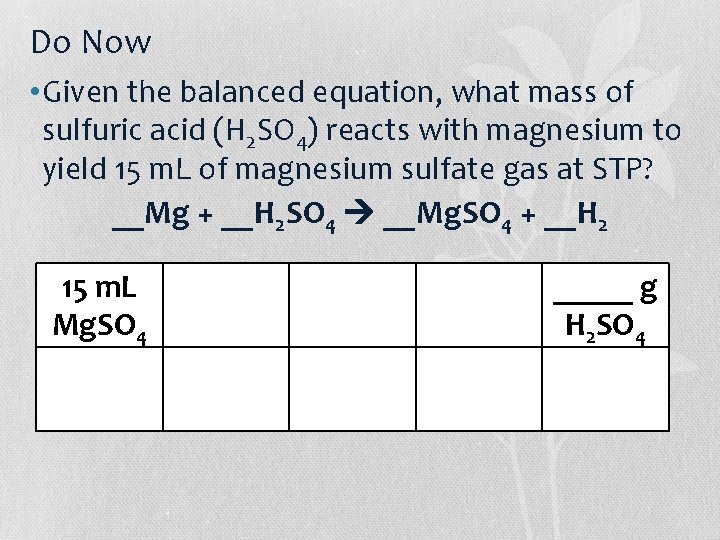
Do Now • Given the balanced equation, what mass of sulfuric acid (H 2 SO 4) reacts with magnesium to yield 15 m. L of magnesium sulfate gas at STP? __Mg + __H 2 SO 4 __Mg. SO 4 + __H 2 15 m. L Mg. SO 4 _____ g H 2 SO 4

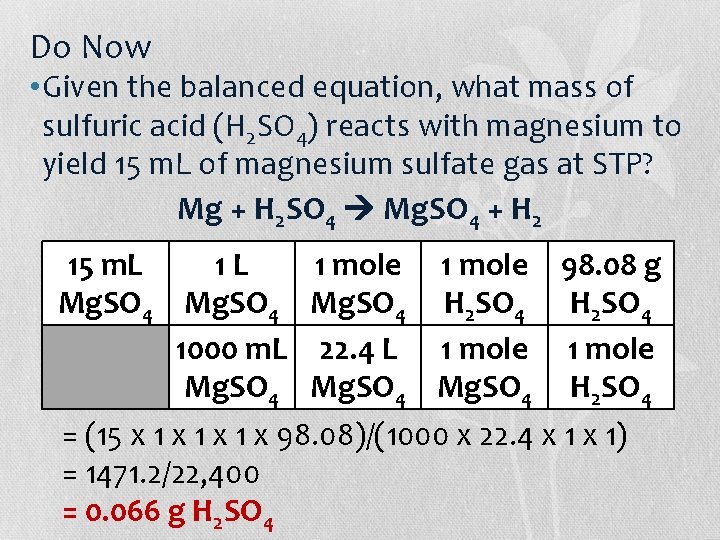
Do Now • Given the balanced equation, what mass of sulfuric acid (H 2 SO 4) reacts with magnesium to yield 15 m. L of magnesium sulfate gas at STP? Mg + H 2 SO 4 Mg. SO 4 + H 2 15 m. L 1 L 1 mole 98. 08 g Mg. SO 4 H 2 SO 4 1000 m. L 22. 4 L 1 mole Mg. SO 4 H 2 SO 4 = (15 x 1 x 1 x 98. 08)/(1000 x 22. 4 x 1) = 1471. 2/22, 400 = 0. 066 g H 2 SO 4

Objective • I can use dimensional analysis and stoichiometry to determine the amount, mass, or volume of a substance produced or used in a chemical reaction with a pop quiz.

Agenda 1. Do Now, Objective (10 minutes) 2. Leveled Stoichiometry Practice (25 minutes) 3. Pop Quiz (10 minutes)

Leveled Stoichiometry Practice • You’ll be using your score on Monday’s exit ticket to choose what level of questions you work on today. • Not Proficient (NP): level 1 practice problems • Partially Proficient (PP): level 2 practice problems • Proficient (P): level 3 practice problems • Advanced (A): level 4 practice problems

Stoichiometry Pop Quiz • Answer the question on the pop quiz and turn it into the bin when you are finished.
Do NOw • Find the volume of 3 moles of carbon dioxide (CO 2) • How many atoms are in 1 mole of methane (CH 4)

Objective • I can use stoichiometry to calculate the amount, volume, and mass of materials required or produced in a chemical reaction in multi-step practice problems.

Agenda 1. Do Now, Objective (7 minutes) 2. What is a Mole Video (15 minutes) 3. Leveled Stoichiometry Problems (50 minutes) 4. Stoichiometry Pop Quiz (period 2/3 only)

What is a mole

Advanced Stoichiometry Problems • Take 25 minutes to answer the advanced stoichiometry problems on your worksheet. These are due at the end of class, and will be graded as classwork (15% of your grade).

Exit Ticket • Answer the exit ticket, turn it into Ms. Bergman, and line up by the door when you are finished.

Independent Practice #1 3. 2 moles CO 2 22. 4 L CO 2 1 mole CO 2 = (3. 2 x 22. 4)/1 = 71. 68 L CO 2

Independent Practice #2 14 L NH 3 1 mole NH 3 22. 4 L NH 3 = (14 x 1)/22. 4 = 0. 625 moles NH 3

Independent Practice #3 3 moles Ne 22. 4 L 1 mole Ne = (3 x 22. 4)/1 = 67. 2 L Ne

Independent Practice #4 2000 L O 2 1 mol O 2 22. 4 L O 2 = (2, 000 x 1)/22. 4 = 89. 3 mol O 2

Independent Practice #5 15 moles CH 4 22. 4 L CH 4 1 mol CH 4 = (15 x 22. 4)/1 = 336 L CH 4

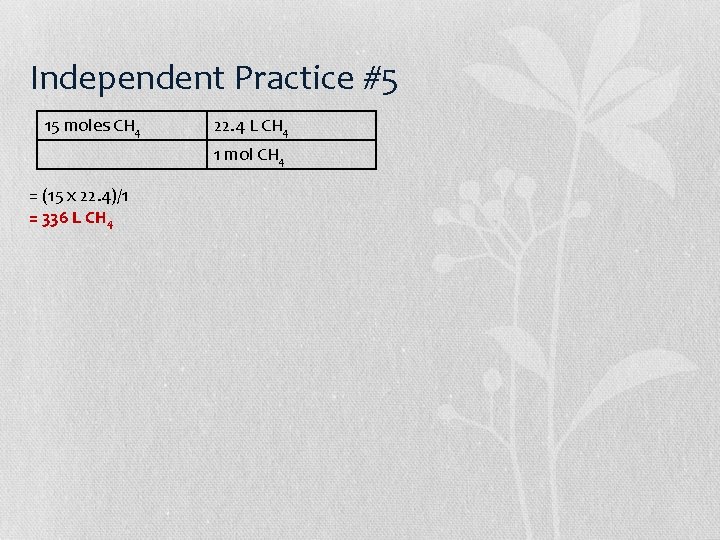
Independent Practice #6 = (4. 50 x 1 x 461. 01)/(149. 89 x 2 x 1) = 2, 074. 545/299. 78 = 6. 92 g Pb. I 2

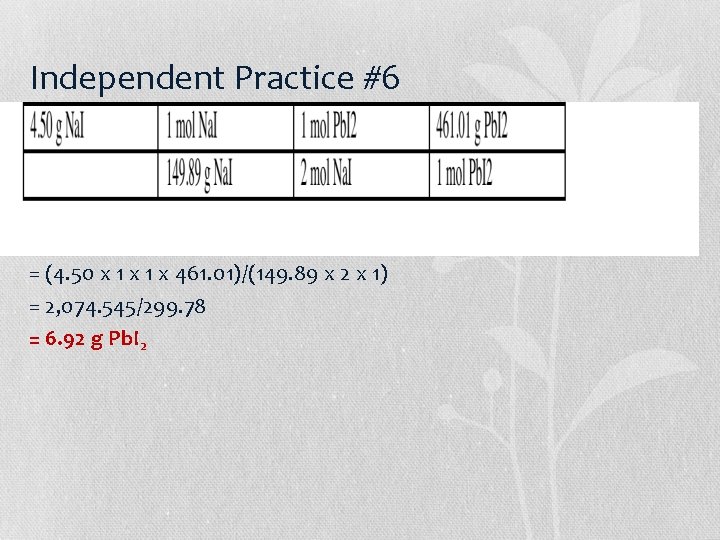
Independent Practice #7 = (4. 00 x 1 x 63. 55)/(159. 61 x 1) = 254. 2/159. 61 = 1. 59 g Cu

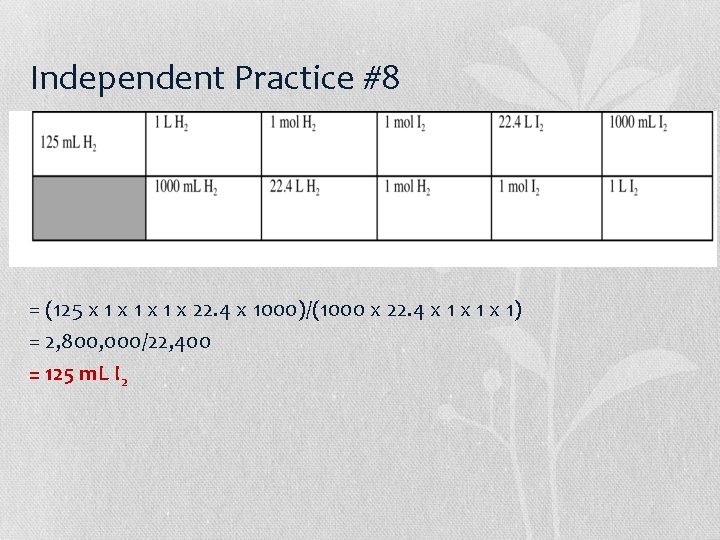
Independent Practice #8 = (125 x 1 x 1 x 22. 4 x 1000)/(1000 x 22. 4 x 1 x 1) = 2, 800, 000/22, 400 = 125 m. L I 2

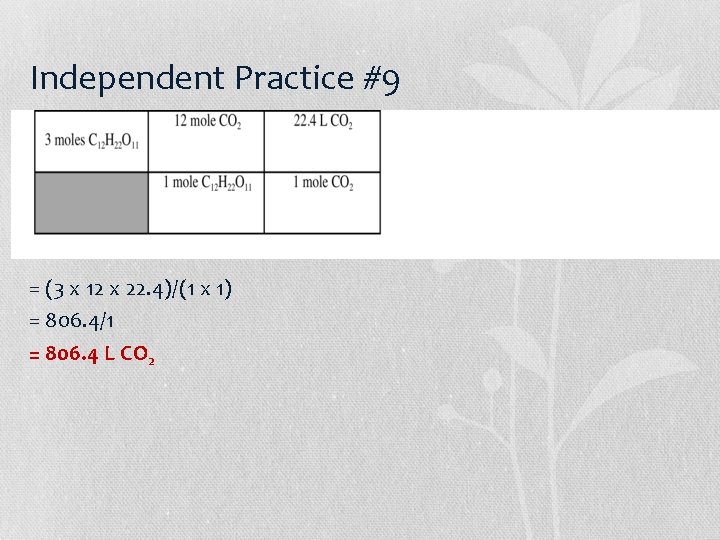
Independent Practice #9 = (3 x 12 x 22. 4)/(1 x 1) = 806. 4/1 = 806. 4 L CO 2

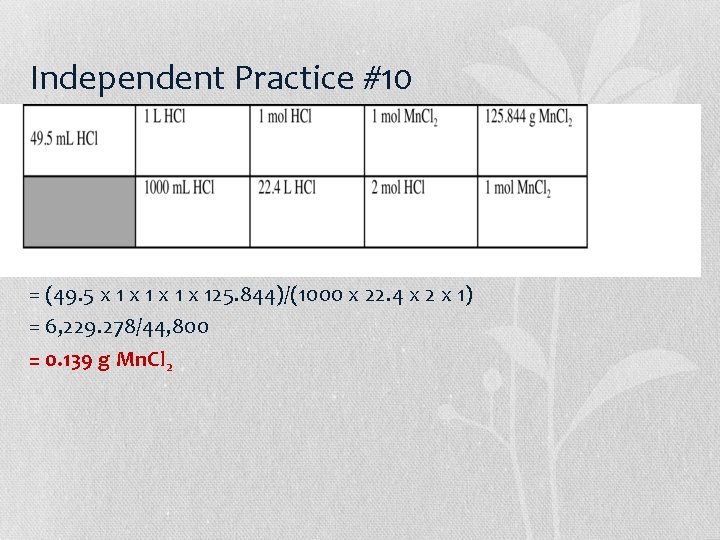
Independent Practice #10 = (49. 5 x 1 x 125. 844)/(1000 x 22. 4 x 2 x 1) = 6, 229. 278/44, 800 = 0. 139 g Mn. Cl 2

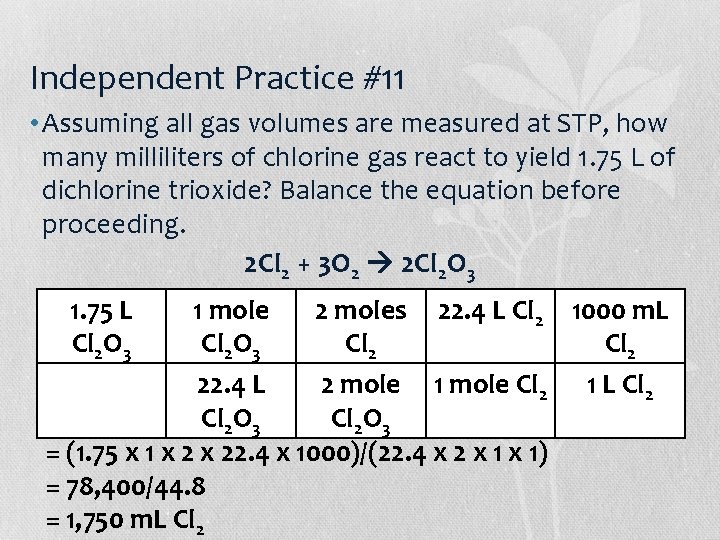
Independent Practice #11 • Assuming all gas volumes are measured at STP, how many milliliters of chlorine gas react to yield 1. 75 L of dichlorine trioxide? Balance the equation before proceeding. 2 Cl 2 + 3 O 2 2 Cl 2 O 3 1. 75 L Cl 2 O 3 1 mole 2 moles 22. 4 L Cl 2 1000 m. L Cl 2 O 3 Cl 2 22. 4 L 2 mole 1 mole Cl 2 1 L Cl 2 O 3 = (1. 75 x 1 x 22. 4 x 1000)/(22. 4 x 2 x 1) = 78, 400/44. 8 = 1, 750 m. L Cl 2

1. 49 mol

Question #2 • What is the mass of 0. 50 mol of aluminum foil?

13. 49 g

Question #3 • What is the mass of 2. 84 g of ammonia gas (NH 3)?

0. 16 mol

Question #4 • How many grams of Ca. CO 3 are there in 10. 0 moles of chalk (Ca. CO 3)

1, 000. 8 g