Thermodynamics Standard 7 Chemistry Ms Siddall Standard 7





































- Slides: 49

Thermodynamics Standard 7 Chemistry. Ms. Siddall.

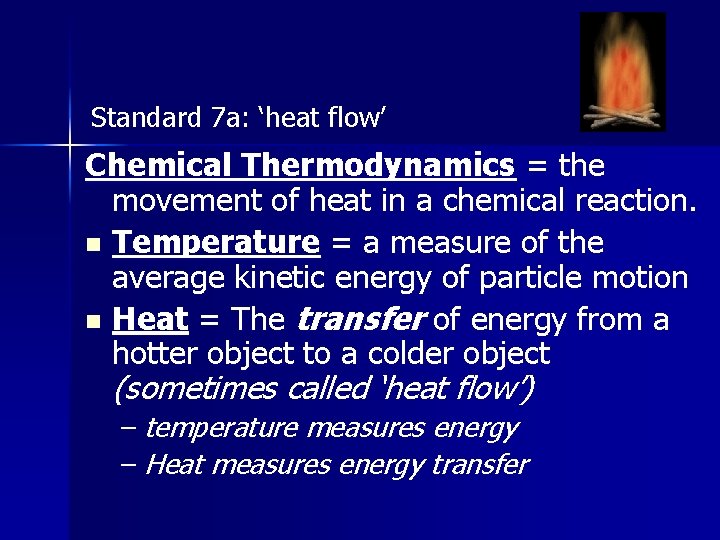
Standard 7 a: ‘heat flow’ Chemical Thermodynamics = the movement of heat in a chemical reaction. n Temperature = a measure of the average kinetic energy of particle motion n Heat = The transfer of energy from a hotter object to a colder object (sometimes called ‘heat flow’) – temperature measures energy – Heat measures energy transfer

Summary 1 n Describe the difference between heat and temperature

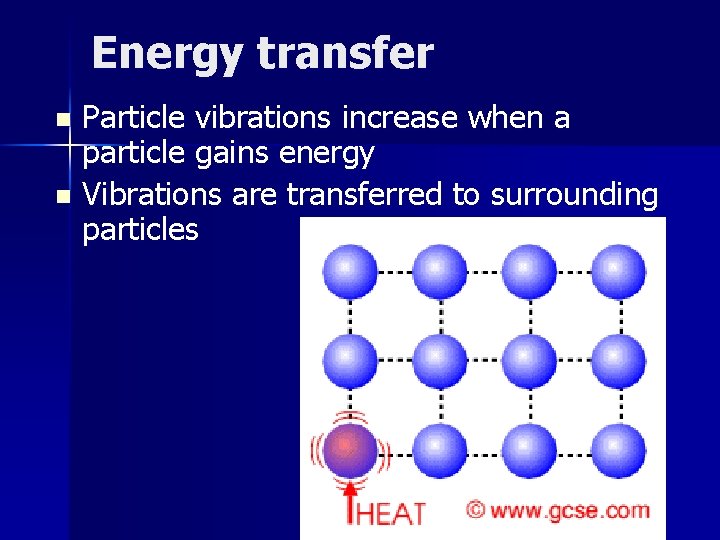
Energy transfer Particle vibrations increase when a particle gains energy n Vibrations are transferred to surrounding particles n

Summary 2 Describe how energy is transferred between atoms.

Identifying heat transfer: n System: experiences a change n Surroundings: causes a change ne. x. hot coffee (system) cools because it transfers heat to the air, the cup, the table & the whole universe! (surroundings)

Summary 3 Consider an ice cube dropped into a glass of warm water. n Ice cube = system n Water = surroundings 1. Does heat flow into the system or out of the system? 2. What is gaining energy (system or surroundings)?

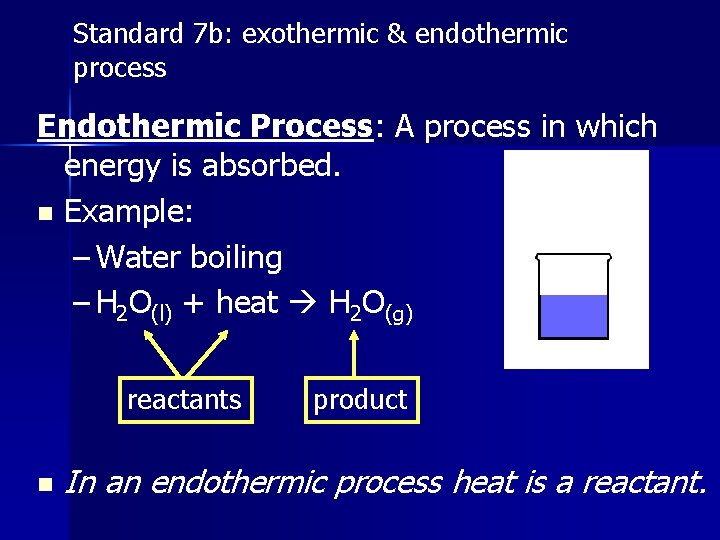
Standard 7 b: exothermic & endothermic process Endothermic Process: A process in which energy is absorbed. n Example: – Water boiling – H 2 O(l) + heat H 2 O(g) reactants n product In an endothermic process heat is a reactant.

Summary 4 n In an endothermic process which has more energy; reactants or products?

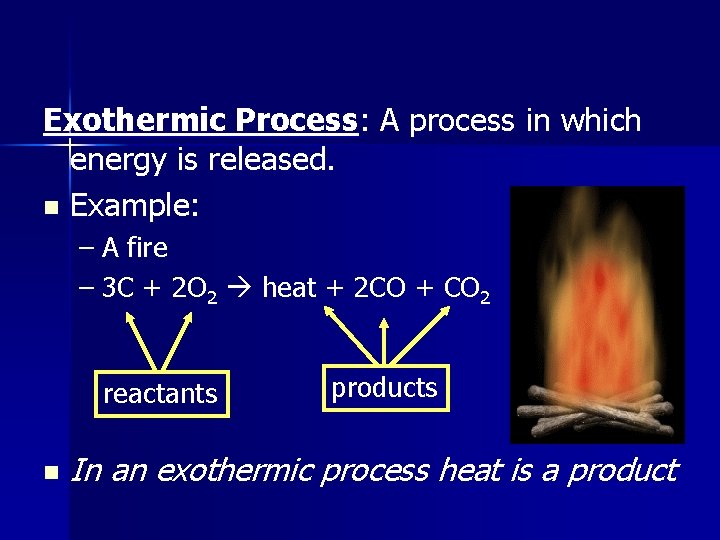
Exothermic Process: A process in which energy is released. n Example: – A fire – 3 C + 2 O 2 heat + 2 CO + CO 2 reactants n products In an exothermic process heat is a product

Summary 5 n In an exothermic process which has more energy, reactants or products?

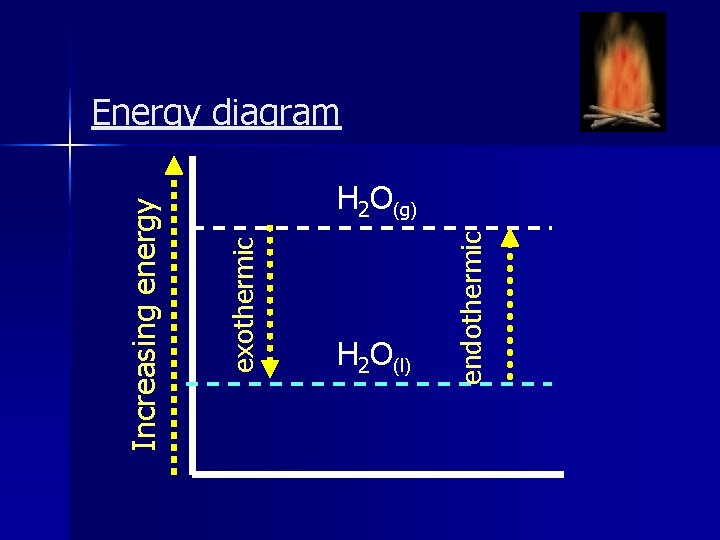
exothermic Increasing energy H 2 O(l) endothermic Energy diagram H 2 O(g)

Summary 6 n Draw an energy diagram for the campfire reaction. – Show reactants and products. – Draw only one arrow from reactants to products and label the arrow (endothermic or exothermic)

Transition State energy diagram activation energy = energy needed to form transition state (activated complex) Transition state energy reactants products Energy released when products form Total energy released during reaction

Transition State: An intermediate state that can occur during a reaction n Also called an ‘activated complex’ n An exothermic reaction is not always spontaneous because energy is needed to form a transition state. n e. x. a spark is needed to start a fire

Summary 7 n Draw a transition state energy diagram for an endothermic reaction

Measuring heat flow. n Energy is measured in joules (J) or calories (cal) n Example: 334 J of energy are needed to melt 1 g of ice. n 1 calorie (c) = 4. 18 J n 1 food calorie (C) = 1000 calories = 4180 J

Summary 8 n If your body burns about 2, 000 food calories a day, approximately how many joules of energy is that?

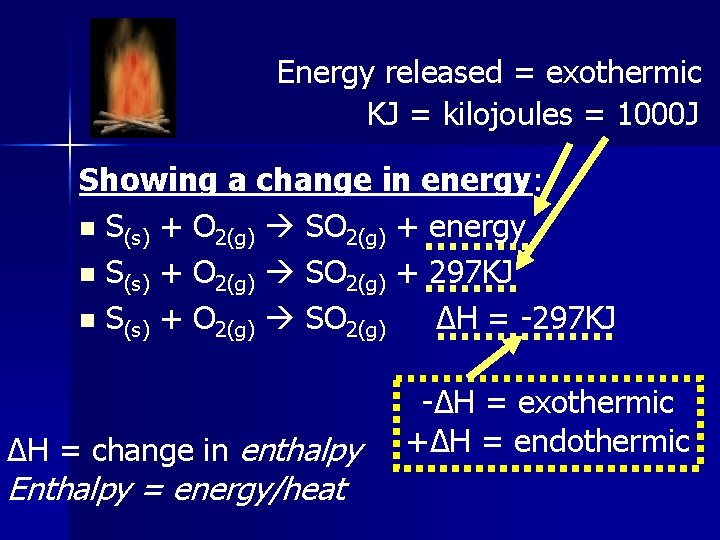
Energy released = exothermic KJ = kilojoules = 1000 J Showing a change in energy: n S(s) + O 2(g) SO 2(g) + energy n S(s) + O 2(g) SO 2(g) + 297 KJ n S(s) + O 2(g) SO 2(g) ∆H = -297 KJ ∆H = change in enthalpy Enthalpy = energy/heat -∆H = exothermic +∆H = endothermic

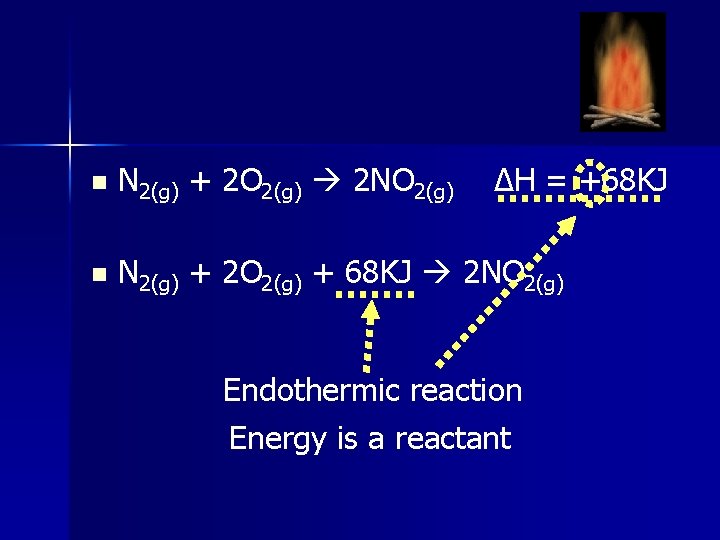
n N 2(g) + 2 O 2(g) 2 NO 2(g) ∆H = +68 KJ n N 2(g) + 2 O 2(g) + 68 KJ 2 NO 2(g) Endothermic reaction Energy is a reactant

Summary 9 Write an equation to show water melting. Use ∆H to show energy. (it takes 5. 9 k. J of energy to melt ice) n Is ∆H negative or positive? Why? n

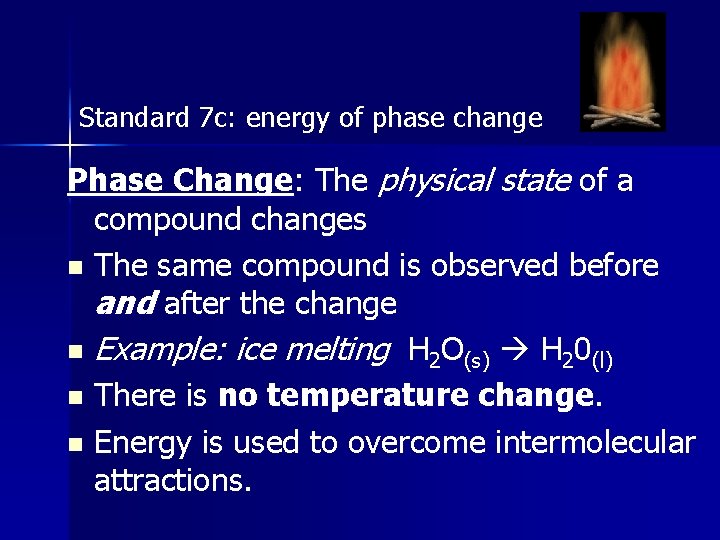
Standard 7 c: energy of phase change Phase Change: The physical state of a compound changes n The same compound is observed before and after the change n Example: ice melting H 2 O(s) H 20(l) n There is no temperature change. n Energy is used to overcome intermolecular attractions.

Summary 10 n Is the example of ice melting an endothermic process or an exothermic process?

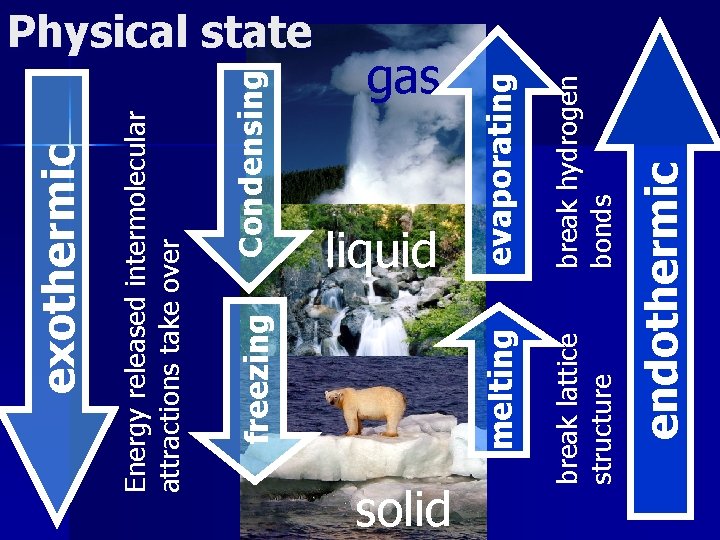
Condensing endothermic break hydrogen bonds solid break lattice structure liquid evaporating gas melting freezing Energy released intermolecular attractions take over exothermic Physical state

Summary 11 1. 2. 3. In which phase do the molecules have the most energy? (solid, liquid, or gas) Is the process of condensing endothermic or exothermic? Is the process of vaporization endothermic or exothermic?

Standard 7 d: Freezing/boiling point graph for water. solving problems Temperature (°C) 110 Energy absorbed = no temp change = physical change boiling ΔHvap 100 steam melting ΔHfus 0 -10 ice energy Water (CH 2 O(l)) Energy absorbed = Change in temperature = Change in K. E.

Summary 12 Which two sections of the graph show no temperature change. n Why is there no temperature change in these sections? n

Standard 7 d: solving problems Latent Heat of fusion. (latent heat = hidden heat) ΔHfus = The energy released when 1 g of a substance is frozen OR the energy needed when 1 g of a substance is melted. ΔHfus = enthalpy of fusion (J/g) • Fusion = freezing (liquid solid) • also used for melting (solid liquid)

Summary 13 n What does ‘fusion’ mean?

Example: freezing water n How much energy is released when 10 g water freezes? (ΔHfus. H 2 O = 334 J/g) 10 g H 2 O(s) 334 J 1 g H 2 O(s) = 3340 J =J = 3. 34 k. J

Summary 14 n How much energy is needed to melt 100 g of water? (show calculation)

Latent Heat of vaporization ΔHvap = The energy needed when 1 g of a substance is evaporated OR the energy released when 1 g of a substance is condensed. ΔHvap = enthalpy of vaporization (J/g) • vaporization • also = evaporating (liquid gas) used for condensing (gas liquid)

Summary 15 What does vaporization mean? n What does condensation mean? n

Example: Boiling water n How much energy is needed to boil 10 g water? (ΔHvap. H 2 O = 2260 J/g) 10 g H 2 O(l) 2260 J 1 g H 2 O(l) = 22600 J =J = 22. 6 k. J

Summary 16 n How much energy is released when 100 g of water vapor is condensed? (show work)

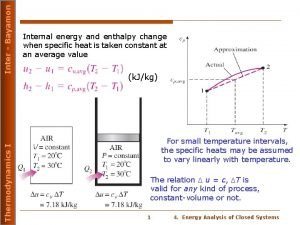
Heat Capacity. n C = specific heat capacity • The amount of heat energy needed to raise the temperature of 1 g of a substance by 1°C n Example: CH 2 O(l) = 4. 18 J/g°C • It takes 4. 18 J of energy to raise the temperature of 1 g of water by 1°C § 1 calorie = 4. 18 J

Summary 17 n How much energy is needed to raise the temperature of 1 g of water by 1°C? (give your answer in joules and calories)

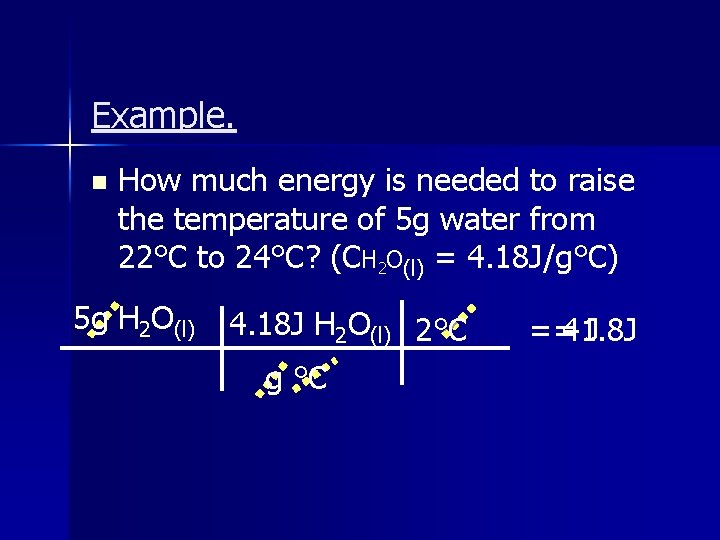
Example. n How much energy is needed to raise the temperature of 5 g water from 22°C to 24°C? (CH 2 O(l) = 4. 18 J/g°C) 5 g H 2 O(l) 4. 18 J H 2 O(l) 2°C g °C ==41. 8 J J

Summary 18 n How much energy is released when 10 g water cools from 40°C to 30°C? 10 g H 2 O(l) 4. 18 J H 2 O(l) 10°C g °C ==418. J J

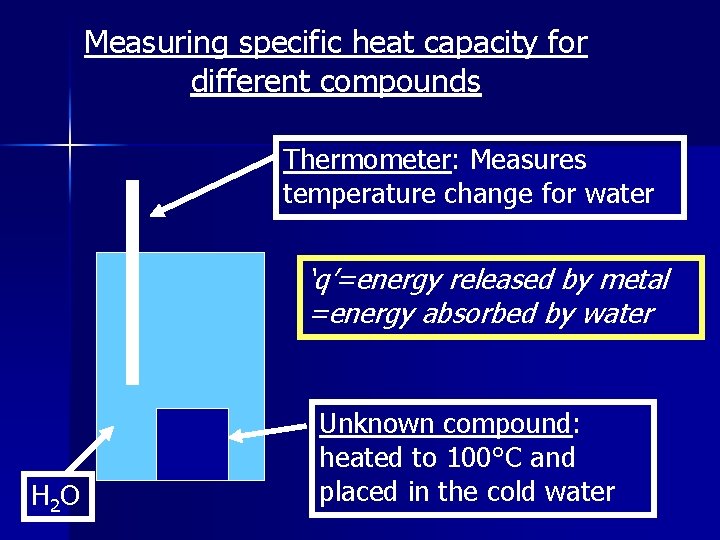
Measuring specific heat capacity for different compounds Thermometer: Measures temperature change for water ‘q’=energy released by metal =energy absorbed by water H 2 O Unknown compound: heated to 100°C and placed in the cold water

Summary 19 n How much energy (q) is released by a metal if the temperature of 100 g of water in the calorimeter rises from 20°C to 30°C?

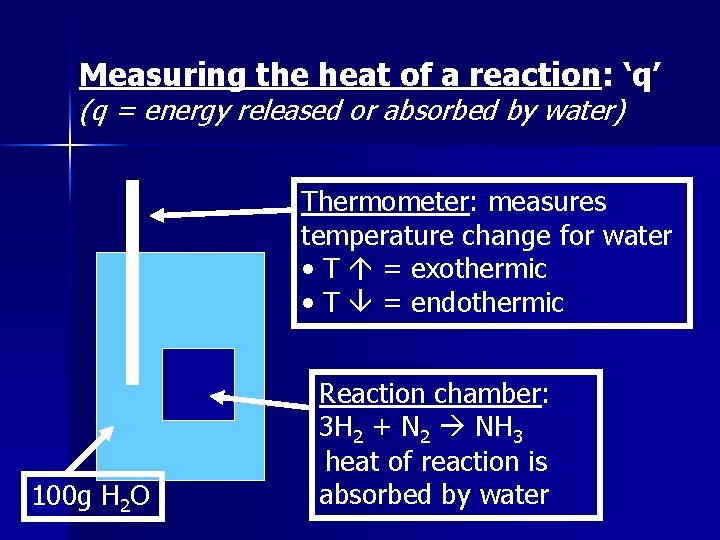
Measuring the heat of a reaction: ‘q’ (q = energy released or absorbed by water) Thermometer: measures temperature change for water • T = exothermic • T = endothermic 100 g H 2 O Reaction chamber: 3 H 2 + N 2 NH 3 heat of reaction is absorbed by water

Example: 10 g NH 3 are produced in the above 1. 2. 3. 4. reaction. The temperature rises from 20. 0°C to 30. 0°C. Calculate ‘q’ (energy) for the reaction. Is the reaction endothermic or exothermic? Calculate ΔH (J/g) for this reaction Calculate ΔH (mol/g) for this reaction

Kinetic energy distribution diagram

Kinetic energy distribution diagram n T 1 = low temperature = low energy n T 2 = higher temperature = higher energy n Emin = minimum energy needed to escape. – More T 2 particles have Emin – Less T 1 particles have Emin

Summary 20 n Explain why more particles evaporate from a cup of hot water compared to a cup of cold water.

Standard 7 e: Apply Hess’s Law to calculate enthalpy change in a reaction Hess’s Law: If a series of reactions are added together the enthalpy change for the net reaction will be the sum of the enthalpy changes for the individual steps. n E. x. N 2(g) + 2 O 2(g) 2 NO 2(g) n • N 2(g) + O 2(g) 2 NO(g) ΔH = +181 k. J • 2 NO(g) + O 2(g) 2 NO 2(g) ΔH = -113 k. J n Find the sum of the 2 equations…

n N 2(g) + O 2(g) 2 NO(g) ΔH = +181 k. J n 2 NO(g) + O 2(g) 2 NO 2(g) ΔH = -113 k. J N 2(g) + O 2(g) + 2 NO(g) + O 2(g) 2 NO(g) + 2 NO 2(g) N 2(g) + 2 O 2(g) 2 NO 2(g) ΔH = +181 k. J + (-113 k. J) = +68 k. J notes: You can reverse reactions (change sign of ΔH) n You can multiply or divide equations (do same to ΔH) n

Hess summary n Complete questions 66, 74, 81 & 84 on page 536 & 537
 Brian siddall
Brian siddall Newton's third law of thermodynamics
Newton's third law of thermodynamics Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Thermodynamics ap chemistry
Thermodynamics ap chemistry Ap chemistry thermodynamics
Ap chemistry thermodynamics 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Mass gfm triangle
Mass gfm triangle Accuracy standard deviation
Accuracy standard deviation Why do we study thermodynamics
Why do we study thermodynamics Thermodynamics ppt
Thermodynamics ppt Rubbing hands together energy
Rubbing hands together energy Thermodynamics property tables
Thermodynamics property tables Entropy equation temperature
Entropy equation temperature Sssf thermodynamics
Sssf thermodynamics First law of thermodynamics for open system
First law of thermodynamics for open system Thermodynamics deals with
Thermodynamics deals with Third tds equation
Third tds equation Microstates in thermodynamics
Microstates in thermodynamics Oth law of thermodynamics
Oth law of thermodynamics Thermodynamics laws
Thermodynamics laws Engineering thermodynamics
Engineering thermodynamics Isobaric process formula
Isobaric process formula Cyclic process thermodynamics
Cyclic process thermodynamics Residual properties in thermodynamics
Residual properties in thermodynamics Laws of thermodynamics simple
Laws of thermodynamics simple Maxwell relations
Maxwell relations Spontaneous irreversible process
Spontaneous irreversible process Kelvin statement
Kelvin statement Thermodynamics 1 formula sheet
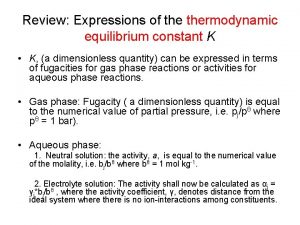
Thermodynamics 1 formula sheet Equilibrium constant in thermodynamics
Equilibrium constant in thermodynamics Cop of refrigerator
Cop of refrigerator Entropy thermodynamics
Entropy thermodynamics Properties of pure substances thermodynamics
Properties of pure substances thermodynamics Thermodynamics study guide
Thermodynamics study guide Deriving maxwell relations
Deriving maxwell relations Zeroth law of thermodynamics
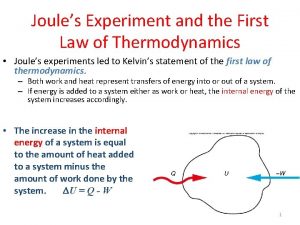
Zeroth law of thermodynamics Joule's experiment first law of thermodynamics
Joule's experiment first law of thermodynamics Specific heat capacity
Specific heat capacity What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Chemical engineering thermodynamics 8th solution chapter 4
Chemical engineering thermodynamics 8th solution chapter 4 Si unit of energy
Si unit of energy Thermodynamic potentials
Thermodynamic potentials Thermodynamics equations
Thermodynamics equations Isothermal process in thermodynamics
Isothermal process in thermodynamics Conservation of energy thermodynamics
Conservation of energy thermodynamics