Standard 6 Solutions chapter 16 Chemistry Ms Siddall






























- Slides: 39

Standard 6: Solutions chapter 16 Chemistry Ms. Siddall

Standard 6 a: solution definitions • Solution: a homogeneous mixture of 2 or more substances in the same physical state. • Properties: – Particles are small – Particles are evenly mixed – Particles will not separate • Examples: – air (nitrogen & oxygen) – Gatorade (water, sugar, etc) – Na. Cl(aq) (salt & water)

Summary 1 • Is muddy water a solution? Why/why not?

• Solute: substance that is dissolved e. x. sugar • Solvent: does the dissolving e. x. water • Concentration: The amount of solute in a given amount of solvent e. x. [HCl] • (aq) = aqueous = A solution where water is the solvent

Summary 2 Consider lemonade. 1. What is the solvent? 2. What are the solutes?

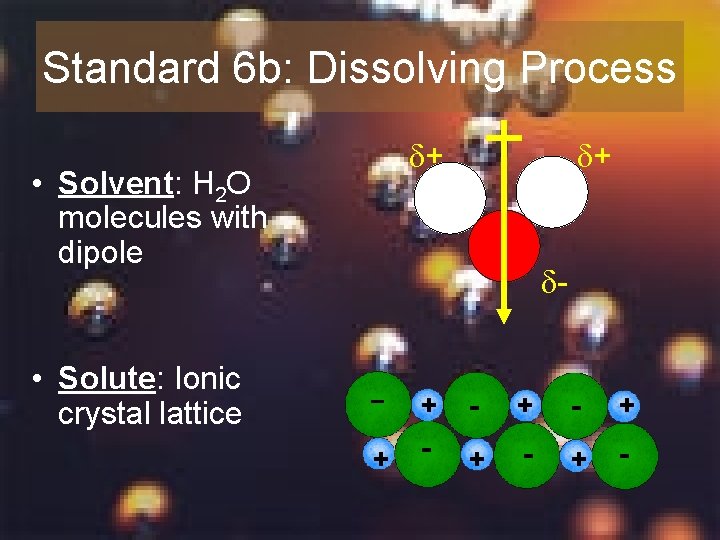
Standard 6 b: Dissolving Process + • Solvent: H 2 O molecules with dipole + - • Solute: Ionic crystal lattice + + - - + + -

• Polar H 2 O molecules surround positive and negative ions and break apart crystal lattice • Water molecules move away (diffusion) so the process is repeated Show animation

Summary 3 Explain how water dissolves ionic compounds

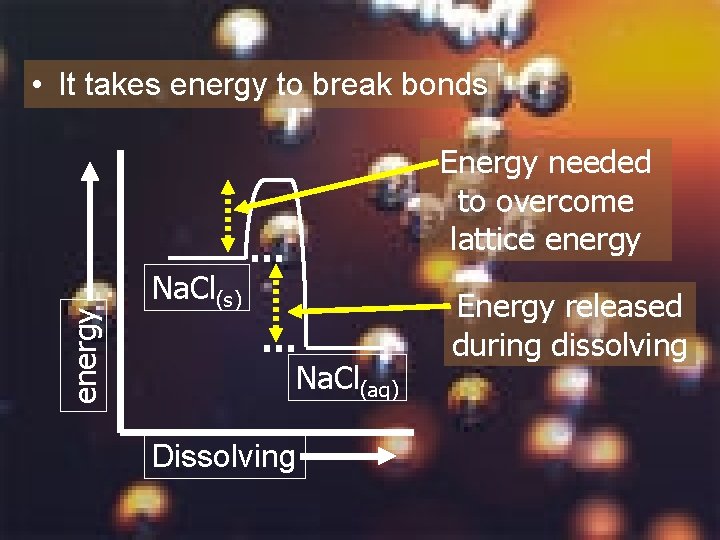
• It takes energy to break bonds energy Energy needed to overcome lattice energy Na. Cl(s) Na. Cl(aq) Dissolving Energy released during dissolving

Summary 4 Does the energy diagram for dissolving Na. Cl represent an endothermic or exothermic process? Explain your answer.

Saturated solution: A solvent can not dissolve any more solute • A saturated solution is at equilibrium. Particles are dissolving and precipitating at the same rate ØNa. Cl(s) Na+(aq) + Cl-(aq)

Summary 5 Does the dissolving process stop at equilibrium? Explain why/why not.

Standard 6 c: Factors that affect the dissolving process • Some factors affect solubility (how much solute is dissolved) • Some factors affect rate (how fast solute is dissolved) • Some factors affect rate and solubility

Standard 6 c: Factors that affect the dissolving process Factors affecting how much solute is dissolved 1. Type of solvent / solute • Polar solvents dissolve polar & ionic solutes • Non-polar solvents dissolve non-polar & covalent solutes e. x oil and water do not mix

Summary 6 Is oil a polar compound or a non polar compound? How do you know?

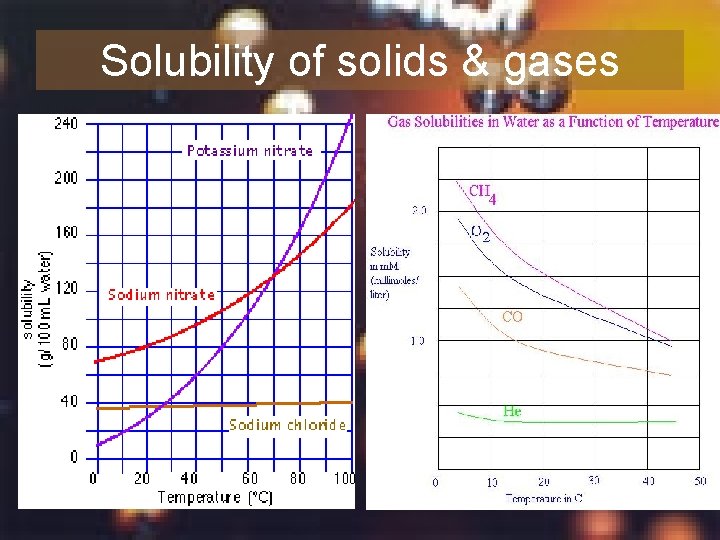
2. Temperature • For solids: Temperature solubility Temperature increases kinetic energy of solvent particles therefore more solute can be dissolved • For gases: Temperature solubility Temperature increases the kinetic energy of solute particles therefore more particles escape from solution

Summary 7 Why does the solubility of gas in solution decrease with increasing temperature?

Solubility of solids & gases

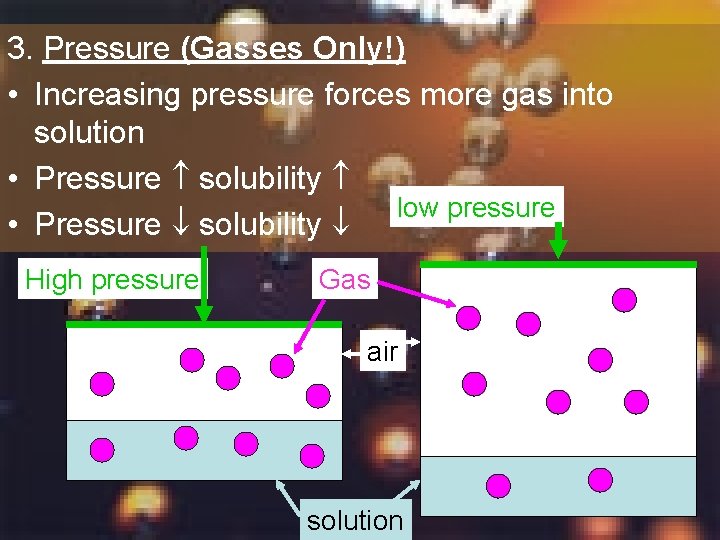
3. Pressure (Gasses Only!) • Increasing pressure forces more gas into solution • Pressure solubility low pressure • Pressure solubility High pressure Gas air solution

Summary 8 Does the concentration of carbon dioxide in your soda increase or decrease when you open the bottle? Why?

Factors that affect the rate of solubility (how quickly something dissolves) 1. Temperature: • T rate • Increasing temperature increases kinetic energy = increased motion = increased diffusion

2. Surface Area (particle size): • S. A. (particle size ) rate • Increasing surface area increases opportunity for interaction between solute and solvent

3. Stirring: • stirring rate • stirring rate • Stirring increases particle motion so more particles can be dissolved at the surface of the solid

Summary 9 1. Name one factor that affects only solubility 2. Name one factor that affects only rate 3. Name one factor that affects rate and solubility

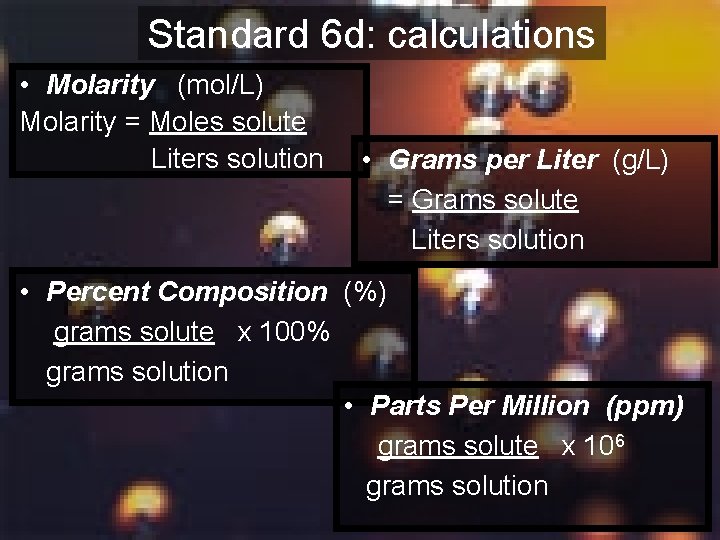
Standard 6 d: calculations • Molarity (mol/L) Molarity = Moles solute Liters solution • Grams per Liter (g/L) = Grams solute Liters solution • Percent Composition (%) grams solute x 100% grams solution • Parts Per Million (ppm) grams solute x 106 grams solution

Summary 10 A solution contains 80 g of Na. OH in 500 ml of solution. Calculate: 1. ‘grams per liter’ 2. Molarity 3. percent composition for the solution

• Using ‘Parts per Million’ (ppm) • Usually used to measure solutions containing a small amount of solute • e. x air quality or drinking water quality – Air contains about 1 ppm CO 2 (Every 1 million grams of air contains 1 g CO 2) • smaller concentrations are measured in ppb (parts per billion) – Drinking water usually contains ≤ 0. 5 ppb lead

Summary 11 Which solution has the highest concentration of fluoride ions? a) 10 ppm Fb) 100 ppm Fc) 10 ppb Fd) 100 ppb F-

• Example: What is the concentration (ppm) of a 1 L solution containing 5 mg arsenic? (The density of aqueous solutions = 1 g/ml)

Standard 6 e: effect of solute on freezing and boiling point. Molality (m) = moles solute Kg solvent e. x. what is the molality of a solution made by dissolving 180 g sugar (C 6 H 12 O 6) in 500 g water? 180 g sugar 1 mol sugar 1000 g 500 g water 180 g sugar 1 kg = 2 m

Summary 18 what is the molality of a solution made by dissolving 92 g ethanol (C 2 H 6 O) in 200 g water? 92 g ethanol 1 mol ethanol 200 g water 46 g ethanol 1000 g 1 kg = 20 m

Freezing point depression and boiling point elevation: adding solute to a solvent lowers the freezing point and raises the boiling point of the solvent. • Solute particles disrupt crystallization and evaporation. • The change in freezing point or boiling point is directly proportional to the molality of solute particles. • A solute that produces more ions in solution has the greatest effect.


Summary 19 Explain why it takes more energy to boil water when it has more solute particles dissolved in solution.

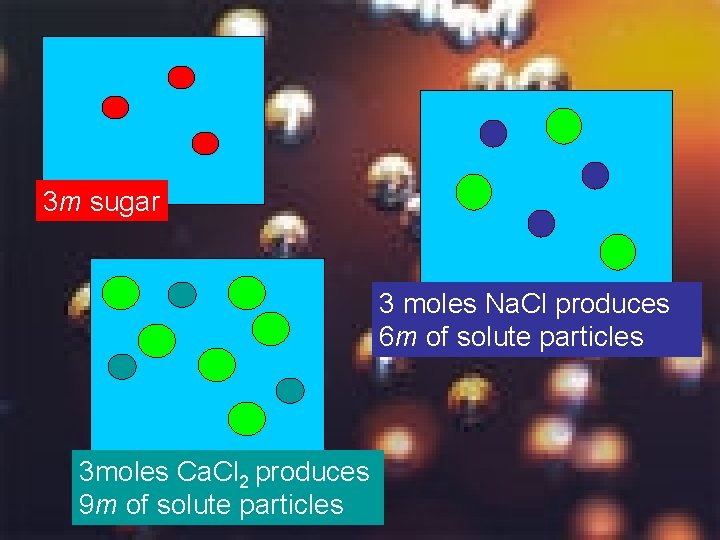
3 m sugar 3 moles Na. Cl produces 6 m of solute particles 3 moles Ca. Cl 2 produces 9 m of solute particles

Summary 19 Which will affect the boiling point of solution more? WHY? a) 1 m Na. Cl b) 1 m Ca. Cl 2 c) 1 m Al. Cl 3

6 f: Chromatography and distillation. Chromatography: the separation of solution into individual substances using differences in polarity. e. x. DNA analysis

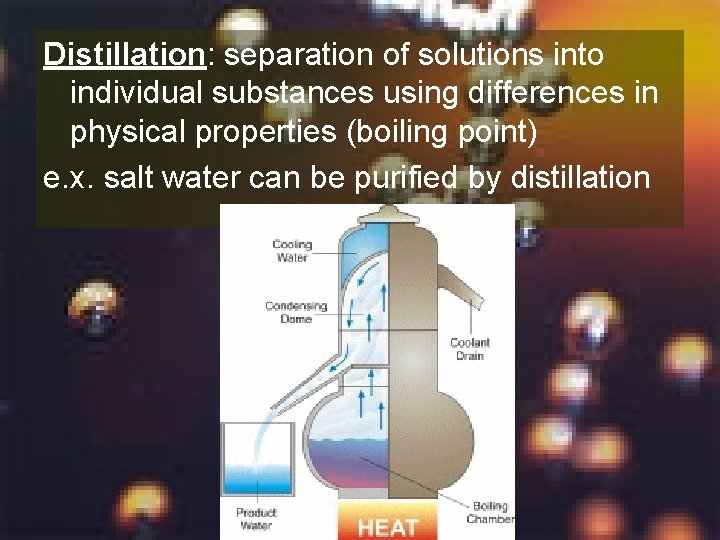
Distillation: separation of solutions into individual substances using differences in physical properties (boiling point) e. x. salt water can be purified by distillation

Summary 20 Why is it possible to separate alcohols by distillation?