Terahertz spectroscopy of molecules radiacls and ions using






![Dunham coefficient Ykl Ev. J = ΣYkl(v+1/2 )k[J(J+1)]l ( a set of coefficients. Ykl Dunham coefficient Ykl Ev. J = ΣYkl(v+1/2 )k[J(J+1)]l ( a set of coefficients. Ykl](https://slidetodoc.com/presentation_image_h/a8ae59543984257f8747d2a955de79af/image-30.jpg)





- Slides: 59

Terahertz spectroscopy of molecules, radiacls and ions using Evenson-type tunable FIR spectrometer Fusakazu Matsushima Department of Physics, University of Toyama, Japan 67 th Columbus meeting, June 20, 2012

Scope of this talk “Evenson-type” = difference frequency of CO 2 laser lines = Tu. FIR spectrometer 1. Property of the spectrometer (Review) Tunability, accuracy, sensitivity Trial to extend the properties 2. Examples (mainly of ions) Glow discharge cell Molecular cation (He. H+, H 2 D+) Molecular anion (OH-) Extended negative glow discharge cell Temperature ( N 2 H+) Recent work (H 2 F+)

Ken. M. Evenson NIST (Boulder, Colorado) Time & Frequency Division

late Ken. M. Evenson NIST (Boulder, Colorado) Time & Frequency Division in 1993 at Toyama

His wife called him “a man of curiosity”

“The final measurement of speed of light” Talk in Toyama (1993)

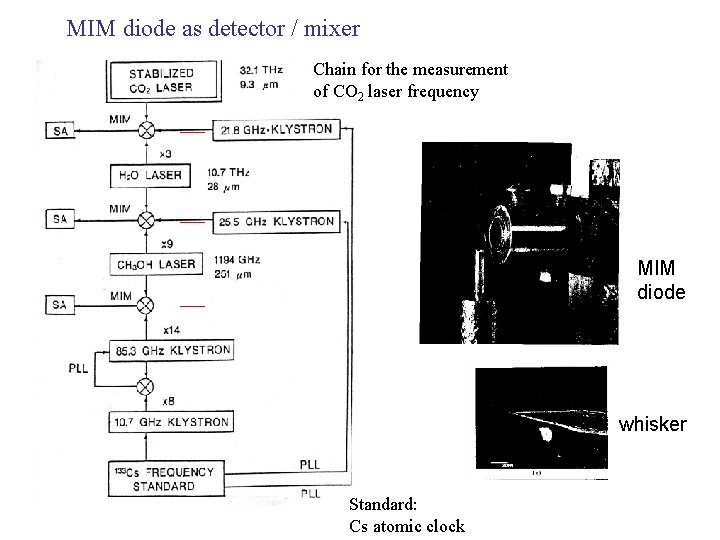
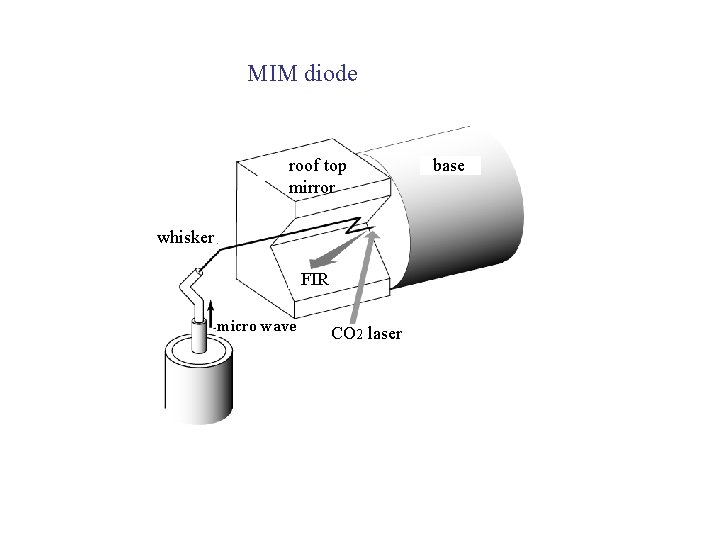
MIM diode as detector / mixer Chain for the measurement of CO 2 laser frequency MIM diode whisker Standard: Cs atomic clock

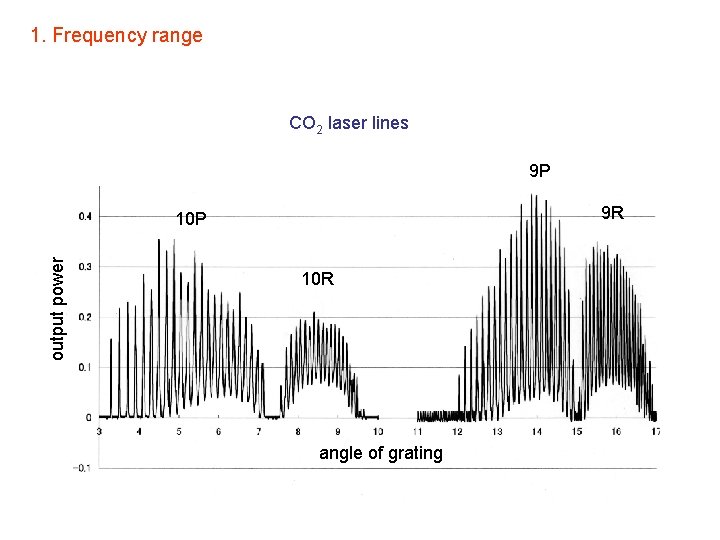
1. Frequency range CO 2 laser lines 9 P 9 R output power 10 P 10 R angle of grating

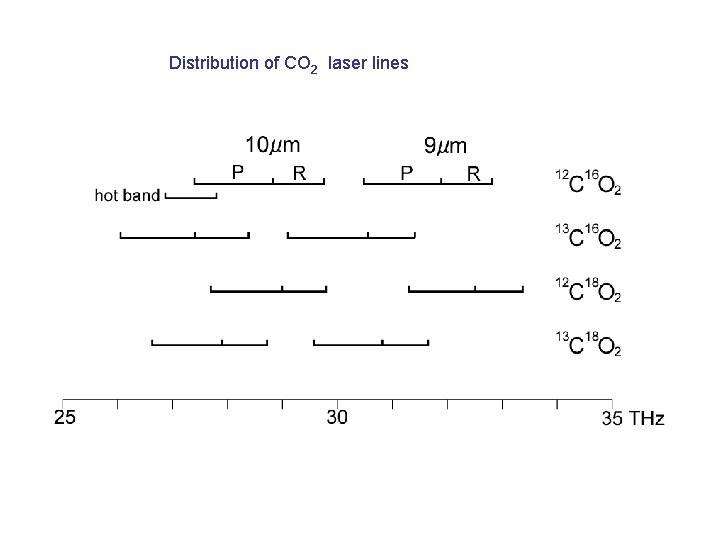
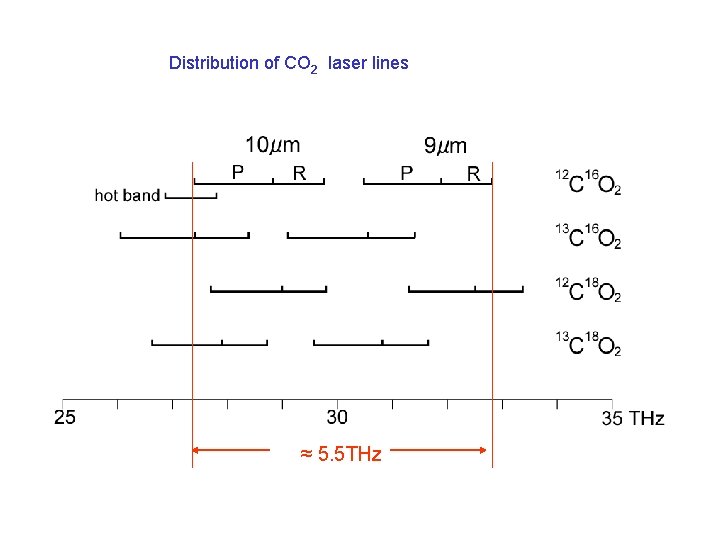
Distribution of CO 2 laser lines

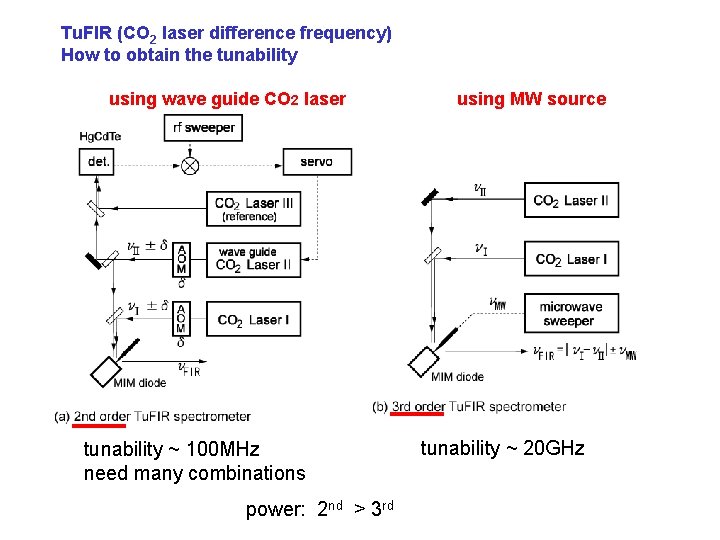
Tu. FIR (CO 2 laser difference frequency) How to obtain the tunability using wave guide CO 2 laser tunability ~ 100 MHz need many combinations power: 2 nd > 3 rd using MW source tunability ~ 20 GHz

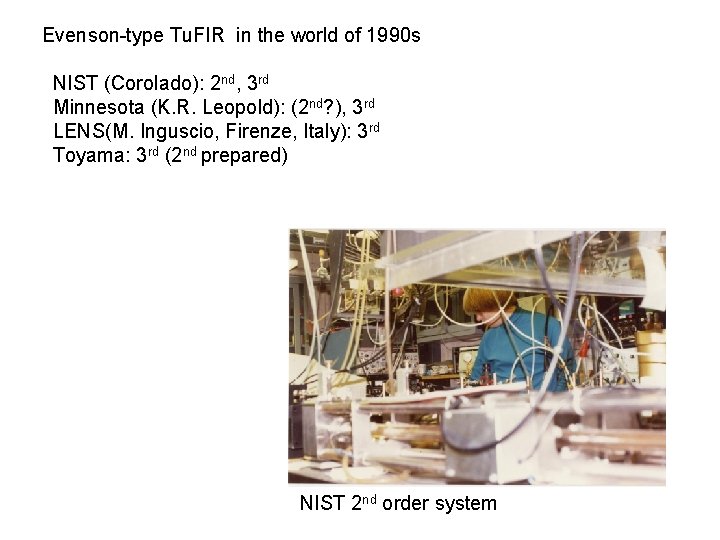
Evenson-type Tu. FIR in the world of 1990 s NIST (Corolado): 2 nd, 3 rd Minnesota (K. R. Leopold): (2 nd? ), 3 rd LENS(M. Inguscio, Firenze, Italy): 3 rd Toyama: 3 rd (2 nd prepared) NIST 2 nd order system

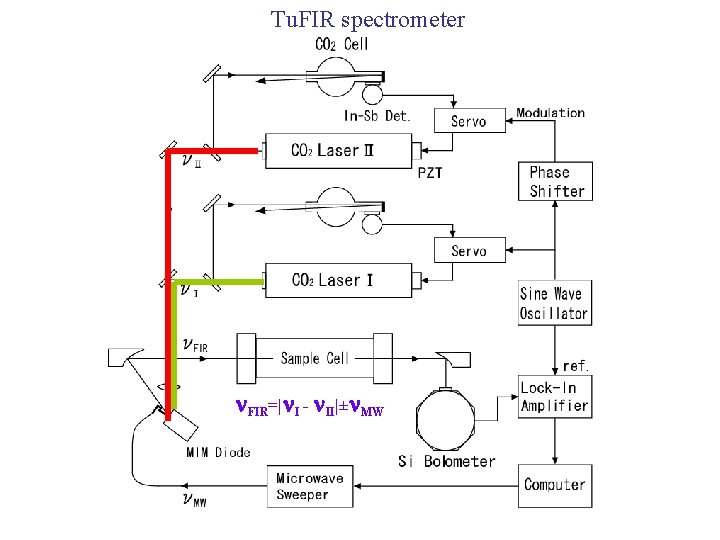
Tu. FIR spectrometer FIR=| I - II|± MW

MIM diode roof top mirror whisker FIR micro wave CO 2 laser base

Properties 1. Frequency Range 2. Precision 3. Sensitivity ( Power of the radiation source)

Distribution of CO 2 laser lines ≈ 5. 5 THz

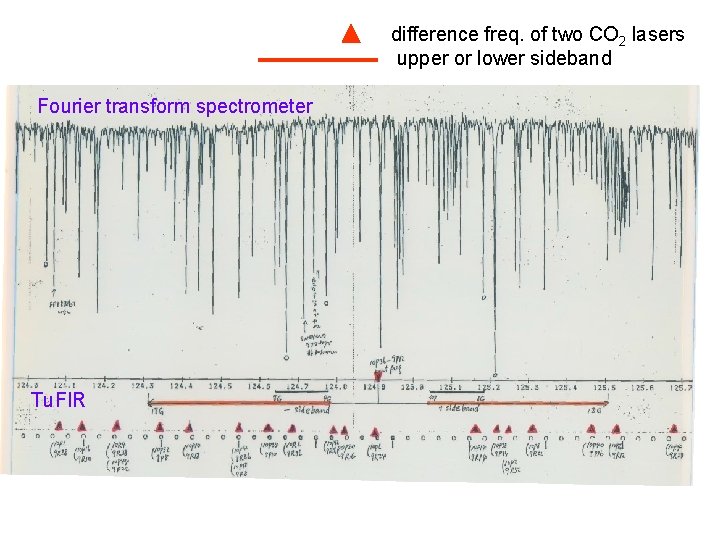
difference freq. of two CO 2 lasers upper or lower sideband Fourier transform spectrometer Tu. FIR

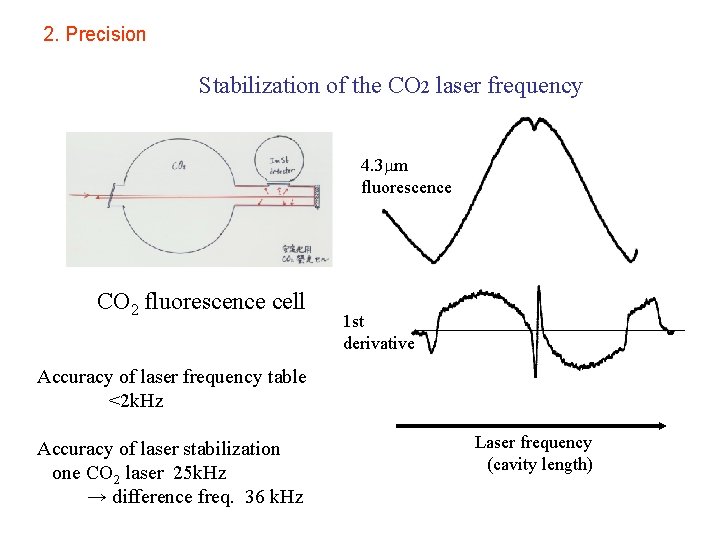
2. Precision Stabilization of the CO 2 laser frequency 4. 3 m fluorescence CO 2 fluorescence cell 1 st derivative Accuracy of laser frequency table <2 k. Hz Accuracy of laser stabilization one CO 2 laser 25 k. Hz → difference freq. 36 k. Hz Laser frequency (cavity length)

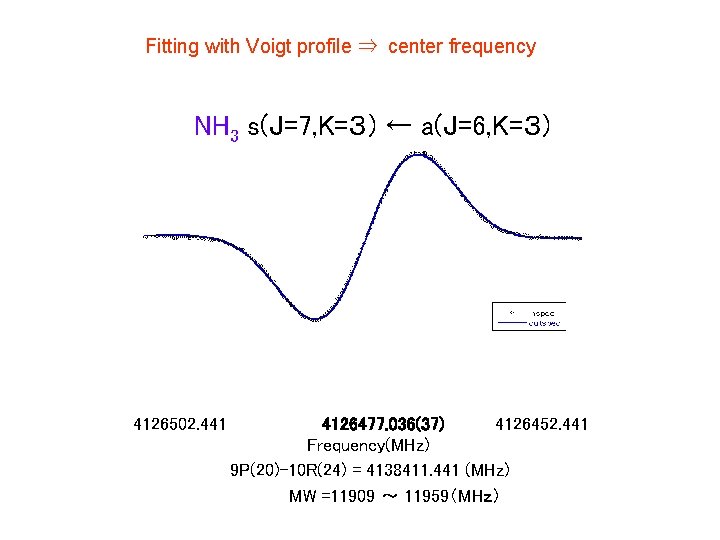
Fitting with Voigt profile ⇒ center frequency NH 3 s(J=7, K=3) ← a(J=6, K=3) 4126502. 441 4126477. 036(37) 4126452. 441 Frequency(MHz) 9 P(20)-10 R(24) = 4138411. 441 (MHz) MW =11909 ~ 11959(MHz)

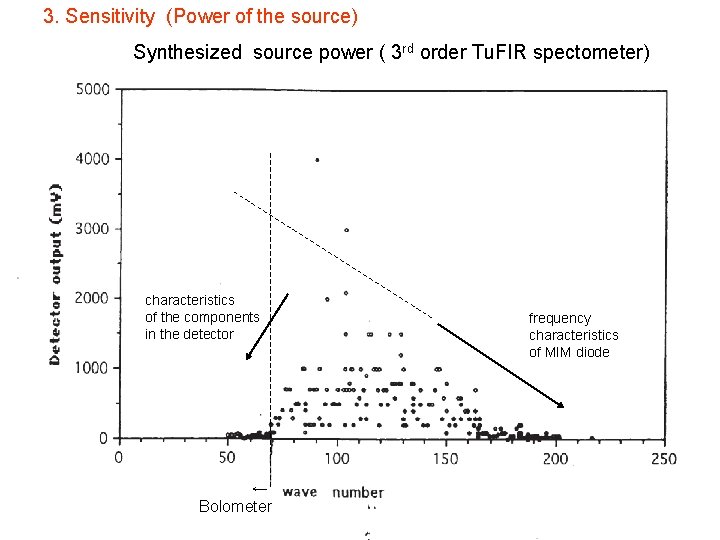
3. Sensitivity (Power of the source) Synthesized source power ( 3 rd order Tu. FIR spectometer) characteristics of the components in the detector ← Bolometer frequency characteristics of MIM diode

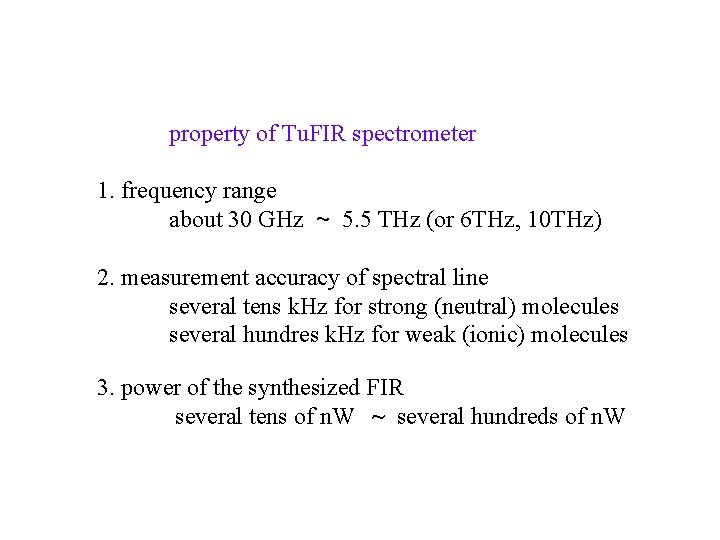
property of Tu. FIR spectrometer 1. frequency range about 30 GHz ~ 5. 5 THz (or 6 THz, 10 THz) 2. measurement accuracy of spectral line several tens k. Hz for strong (neutral) molecules several hundres k. Hz for weak (ionic) molecules 3. power of the synthesized FIR several tens of n. W ~ several hundreds of n. W

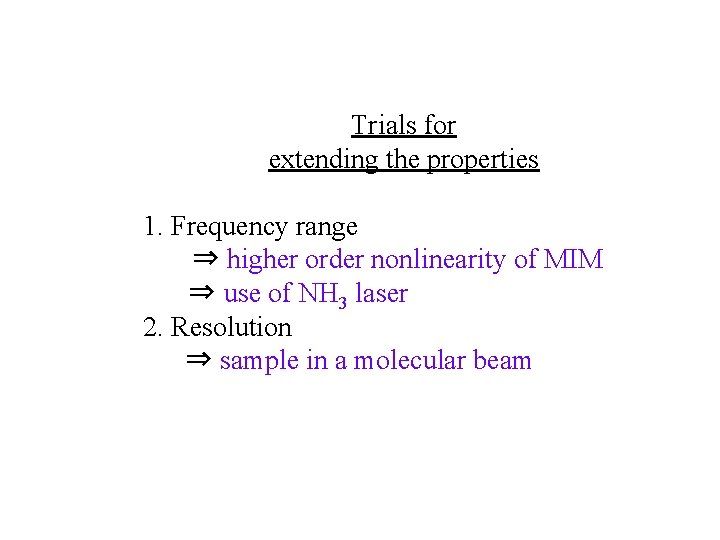
Trials for extending the properties 1. Frequency range ⇒ higher order nonlinearity of MIM ⇒ use of NH 3 laser 2. Resolution ⇒ sample in a molecular beam

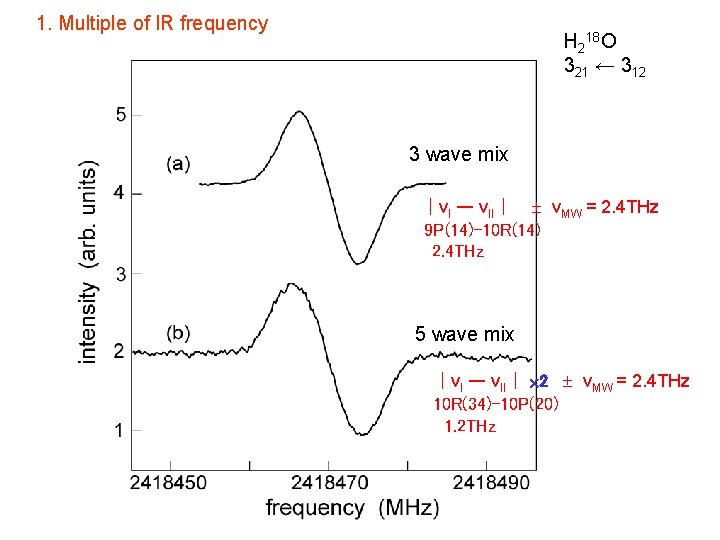
1. Multiple of IR frequency H 218 O 321 ← 312 3 wave mix |νI ー νII| νMW = 2. 4 THz 9 P(14)-10 R(14) 2. 4 THz 5 wave mix |νI ー νII| 2 νMW = 2. 4 THz 10 R(34)-10 P(20) 1. 2 THz

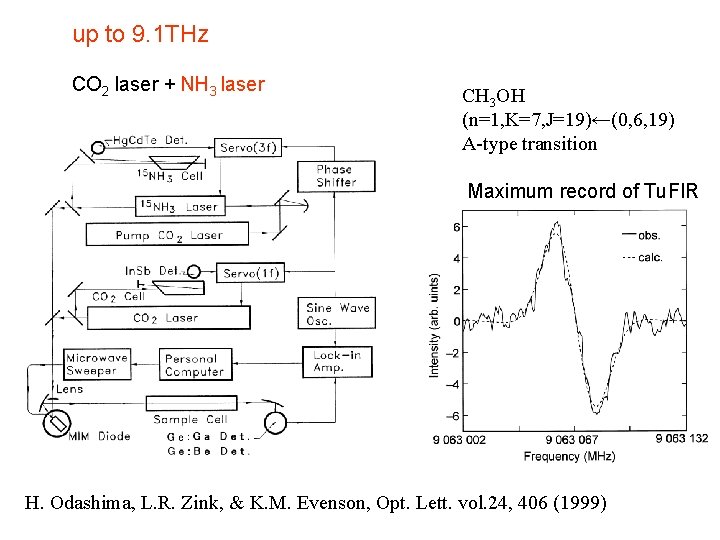
up to 9. 1 THz CO 2 laser + NH 3 laser CH 3 OH (n=1, K=7, J=19)←(0, 6, 19) A-type transition Maximum record of Tu. FIR H. Odashima, L. R. Zink, & K. M. Evenson, Opt. Lett. vol. 24, 406 (1999)

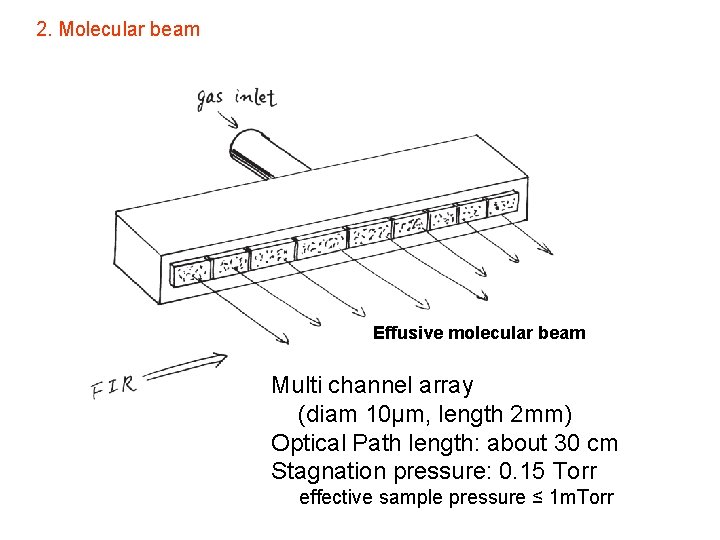
2. Molecular beam Effusive molecular beam Multi channel array (diam 10μm, length 2 mm) Optical Path length: about 30 cm Stagnation pressure: 0. 15 Torr effective sample pressure ≤ 1 m. Torr

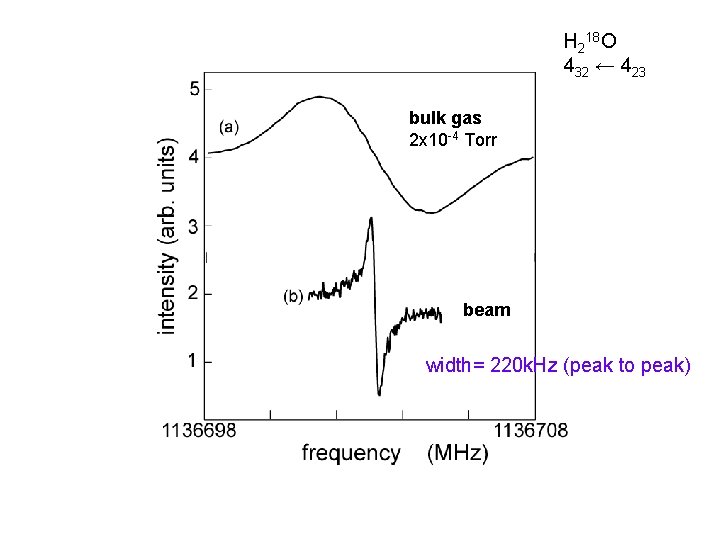
H 218 O 432 ← 423 bulk gas 2 x 10 -4 Torr beam width= 220 k. Hz (peak to peak)

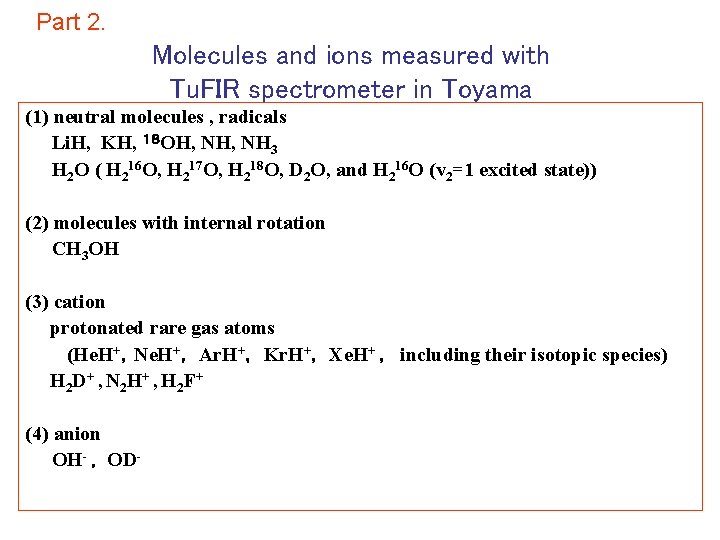
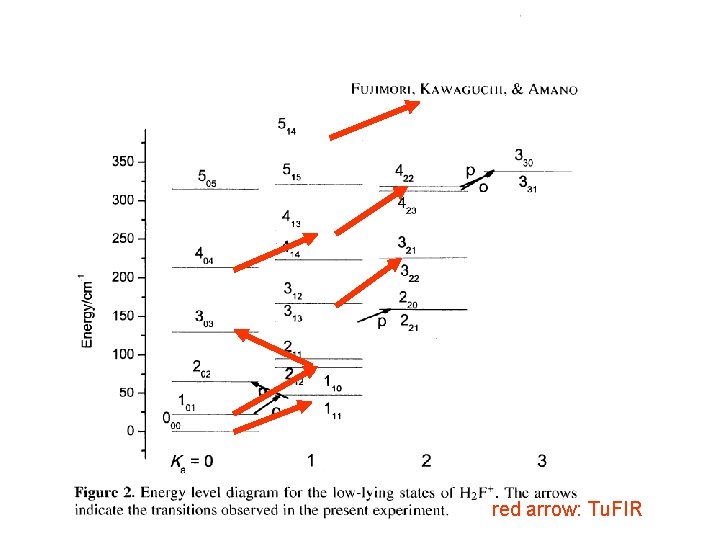
Part 2. Molecules and ions measured with Tu. FIR spectrometer in Toyama (1) neutral molecules , radicals Li. H, KH, 18 OH, NH 3 H 2 O ( H 216 O, H 217 O, H 218 O, D 2 O, and H 216 O (v 2=1 excited state)) (2) molecules with internal rotation CH 3 OH (3) cation protonated rare gas atoms (He. H+,Ne. H+, Ar. H+, Kr. H+, Xe. H+ , including their isotopic species) H 2 D+ , N 2 H + , H 2 F + (4) anion OH- , OD-

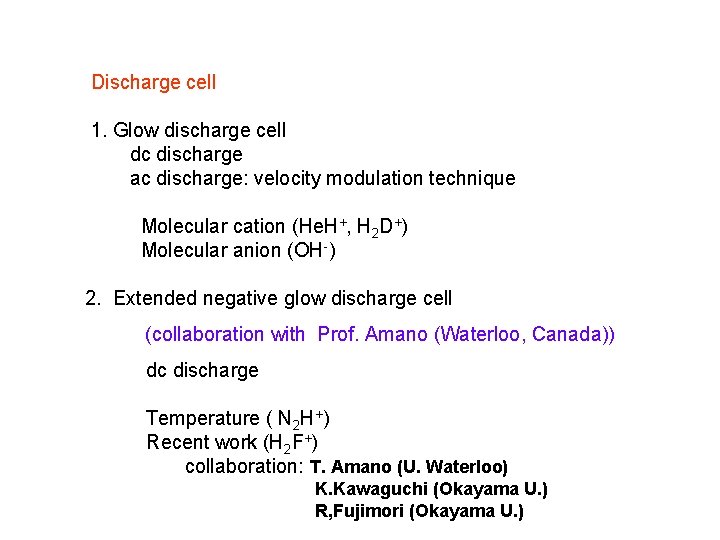
Discharge cell 1. Glow discharge cell dc discharge ac discharge: velocity modulation technique Molecular cation (He. H+, H 2 D+) Molecular anion (OH-) 2. Extended negative glow discharge cell (collaboration with Prof. Amano (Waterloo, Canada)) dc discharge Temperature ( N 2 H+) Recent work (H 2 F+) collaboration: T. Amano (U. Waterloo) K. Kawaguchi (Okayama U. ) R, Fujimori (Okayama U. )

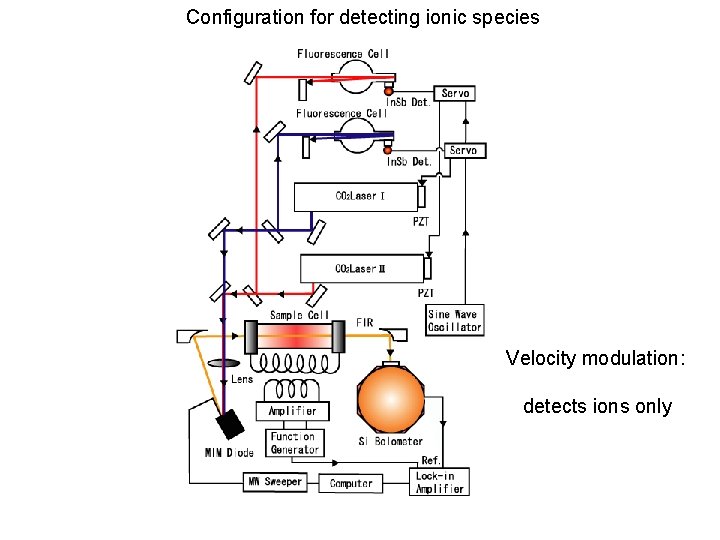
Configuration for detecting ionic species Fig. 1 Tu. FIR分光計 Velocity modulation: detects ions only

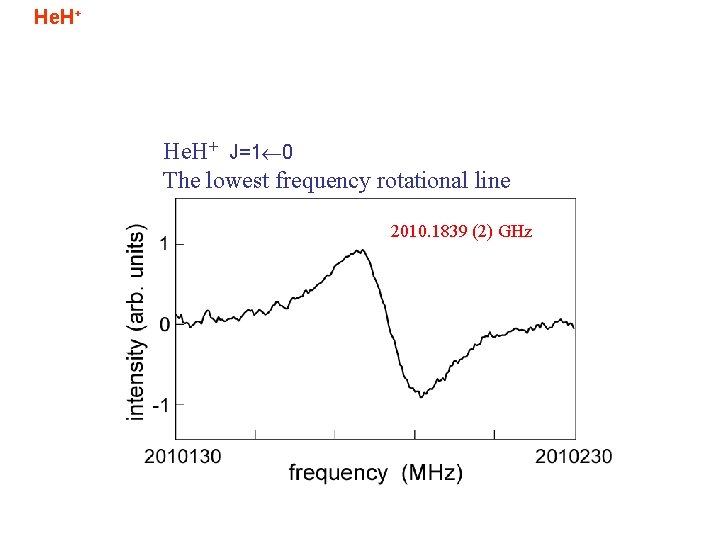
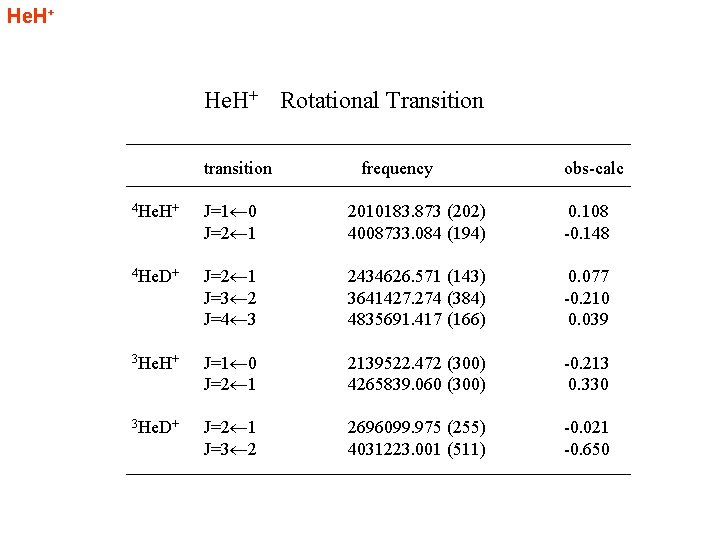
He. H+ J=1 0 The lowest frequency rotational line 2010. 1839 (2) GHz
![Dunham coefficient Ykl Ev J ΣYklv12 kJJ1l a set of coefficients Ykl Dunham coefficient Ykl Ev. J = ΣYkl(v+1/2 )k[J(J+1)]l ( a set of coefficients. Ykl](https://slidetodoc.com/presentation_image_h/a8ae59543984257f8747d2a955de79af/image-30.jpg)
Dunham coefficient Ykl Ev. J = ΣYkl(v+1/2 )k[J(J+1)]l ( a set of coefficients. Ykl for each isotope) To calculate all the isotopes with a set of coefficients Ukl Ykl = μ-(k/2+l)Ukl Reduced mass μis not enough to fit all the isotopes. Ykl = μ-(k/2+l)Ukl [1+meΔHekl/MHe + meΔHkl/MH] Correction terms usingΔvalues are necessary. Breakdown of Born-Oppenheimer approximation.

He. H+ Rotational Transition transition frequency obs-calc 4 He. H+ J=1 0 J=2 1 2010183. 873 (202) 4008733. 084 (194) 0. 108 -0. 148 4 He. D+ J=2 1 J=3 2 J=4 3 2434626. 571 (143) 3641427. 274 (384) 4835691. 417 (166) 0. 077 -0. 210 0. 039 3 He. H+ J=1 0 J=2 1 2139522. 472 (300) 4265839. 060 (300) -0. 213 0. 330 3 He. D+ J=2 1 J=3 2 2696099. 975 (255) 4031223. 001 (511) -0. 021 -0. 650

protonated rare gas atoms He. H+ : 3 He. H+, 4 He. H+, 3 He. D+, 4 He. D+ Ne. H+ : 20 Ne. H+, 22 Ne. D+, 22 Ne. D+ Ar. D+ : Kr. H+ : 82 Kr. D+, 84 Kr. D+, 86 Kr. D+, 82 Kr. H+ Xe. H+ : isotopes of 124 Xe, 126 Xe, 128 Xe, 129 Xe, 130 Xe, 131 Xe, 132 Xe, 134 Xe, 136 Xe, and H, D

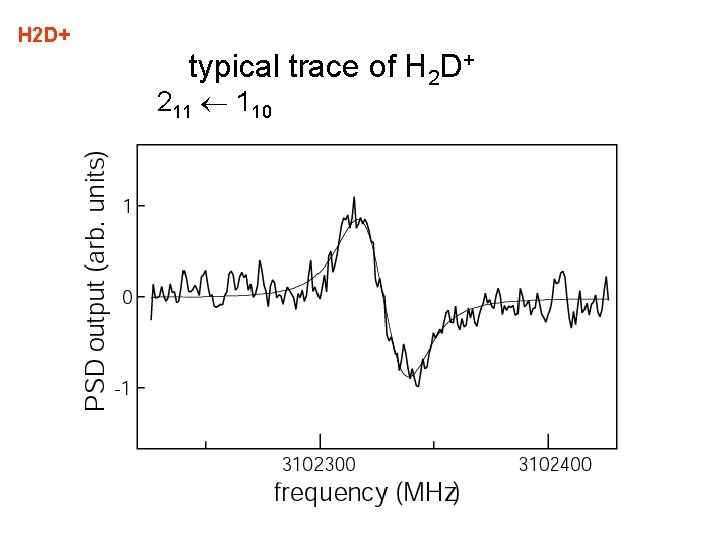
H 2 D+ typical trace of H 2 D+ 211 110

H 2 D+

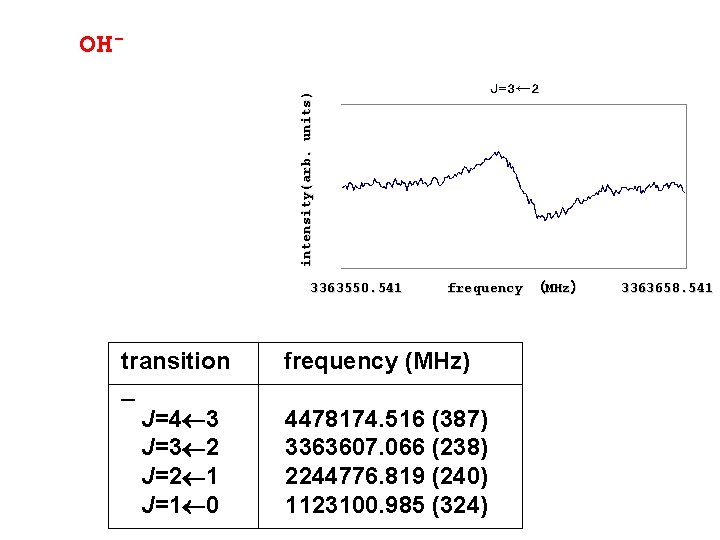
intensity(arb. units) OH- 3363550. 541 transition J=4 3 J=3 2 J=2 1 J=1 0 frequency (MHz) 3363658. 541 frequency (MHz) 4478174. 516 (387) 3363607. 066 (238) 2244776. 819 (240) 1123100. 985 (324)

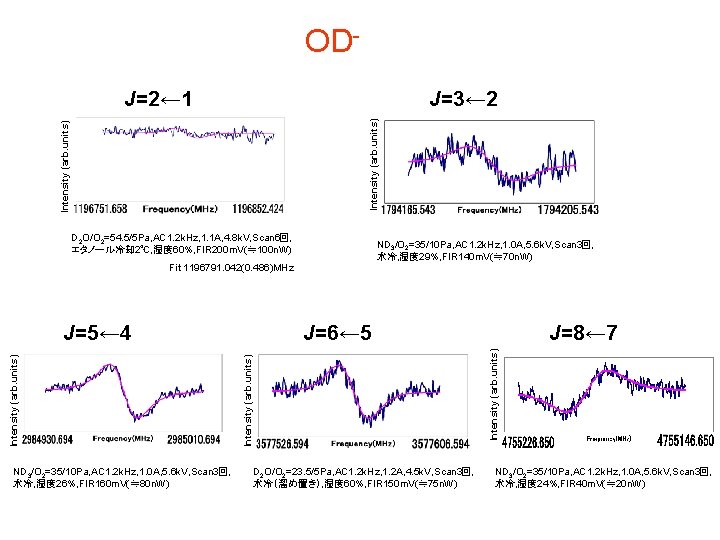
OD J=2← 1 Intensity (arb. units) J=3← 2 D 2 O/O 2=54. 5/5 Pa, AC 1. 2 k. Hz, 1. 1 A, 4. 8 k. V, Scan 6回, エタノール冷却 2℃, 湿度 60%, FIR 200 m. V(≒ 100 n. W) ND 3/O 2=35/10 Pa, AC 1. 2 k. Hz, 1. 0 A, 5. 6 k. V, Scan 3回, 水冷, 湿度 29%, FIR 140 m. V(≒ 70 n. W) Fit 1196791. 042(0. 486)MHz ND 3/O 2=35/10 Pa, AC 1. 2 k. Hz, 1. 0 A, 5. 6 k. V, Scan 3回, 水冷, 湿度 26%, FIR 160 m. V(≒ 80 n. W) J=8← 7 Intensity (arb. units) J=6← 5 Intensity (arb. units) J=5← 4 D 2 O/O 2=23. 5/5 Pa, AC 1. 2 k. Hz, 1. 2 A, 4. 5 k. V, Scan 3回, 水冷(溜め置き), 湿度 60%, FIR 150 m. V(≒ 75 n. W) ND 3/O 2=35/10 Pa, AC 1. 2 k. Hz, 1. 0 A, 5. 6 k. V, Scan 3回, 水冷, 湿度 24%, FIR 40 m. V(≒ 20 n. W)

OH-, OD-

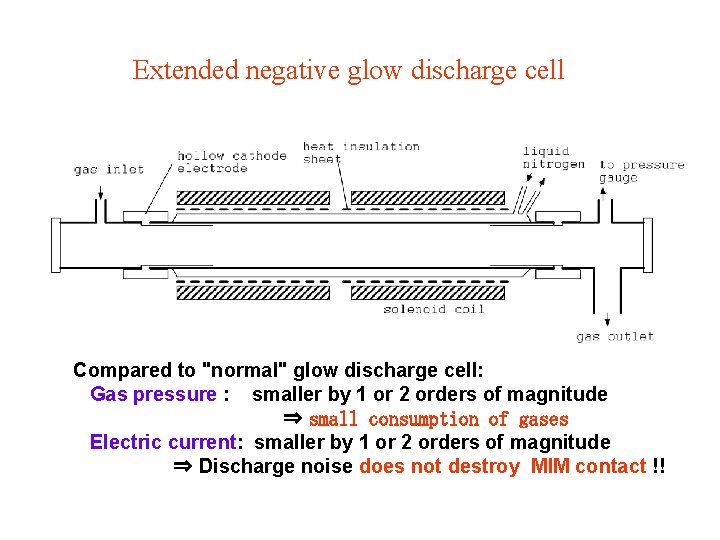
Extended negative glow discharge cell Compared to "normal" glow discharge cell: Gas pressure : smaller by 1 or 2 orders of magnitude ⇒ small consumption of gases Electric current: smaller by 1 or 2 orders of magnitude ⇒ Discharge noise does not destroy MIM contact !!

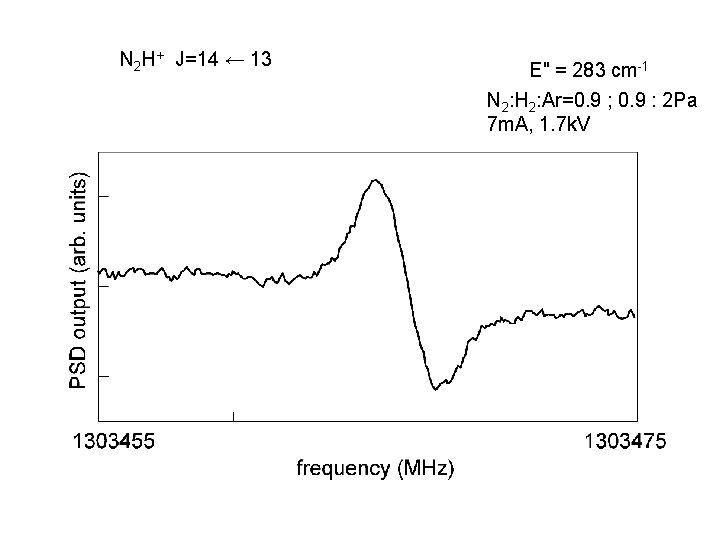
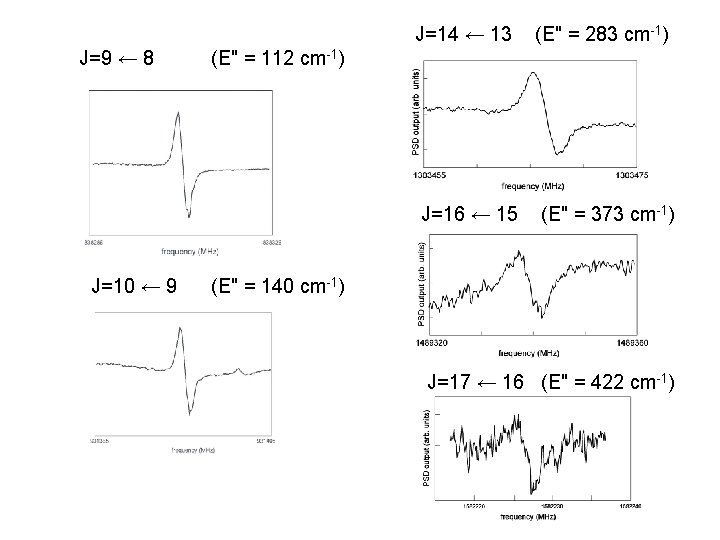
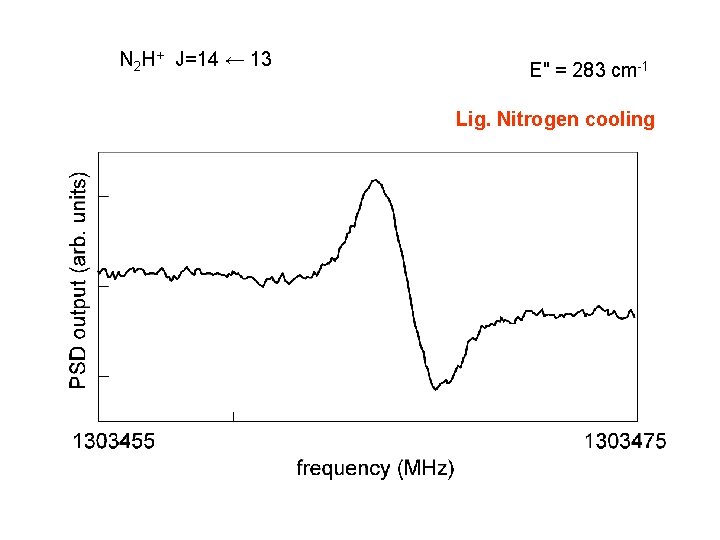
N 2 H+ J=14 ← 13 E" = 283 cm-1 N 2: H 2: Ar=0. 9 ; 0. 9 : 2 Pa 7 m. A, 1. 7 k. V

J=9 ← 8 J=10 ← 9 J=14 ← 13 (E" = 283 cm-1) J=16 ← 15 (E" = 373 cm-1) (E" = 112 cm-1) (E" = 140 cm-1) J=17 ← 16 (E" = 422 cm-1)

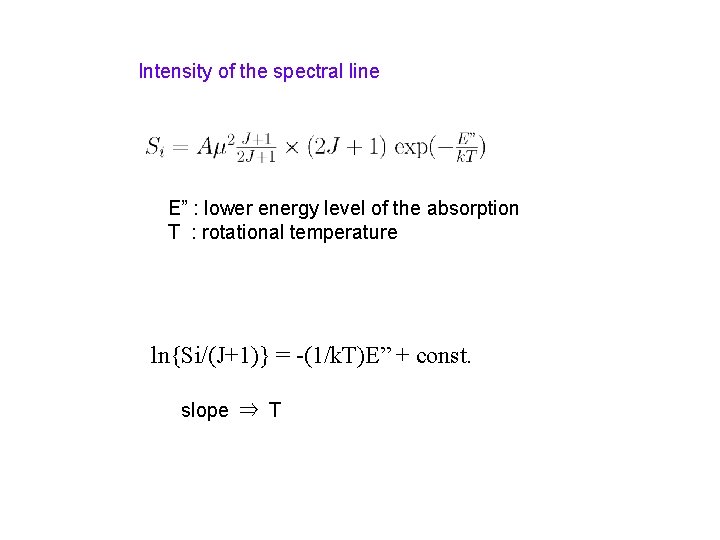
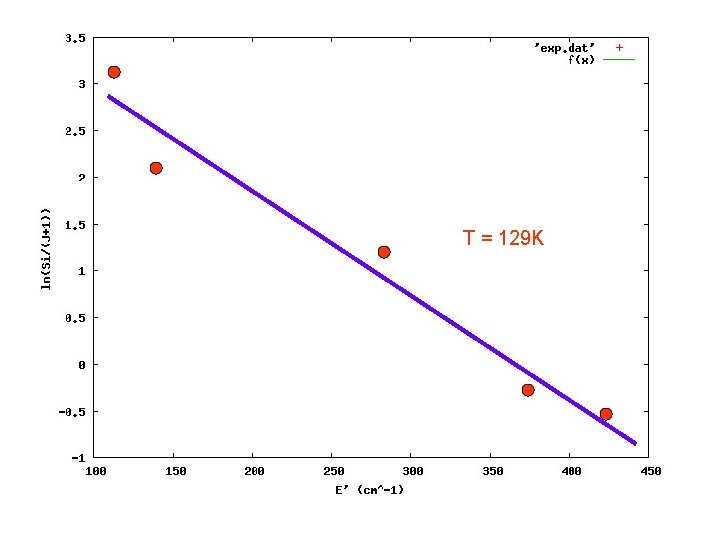
Intensity of the spectral line E” : lower energy level of the absorption T : rotational temperature ln{Si/(J+1)} = -(1/k. T)E” + const. slope ⇒ T

T = 129 K

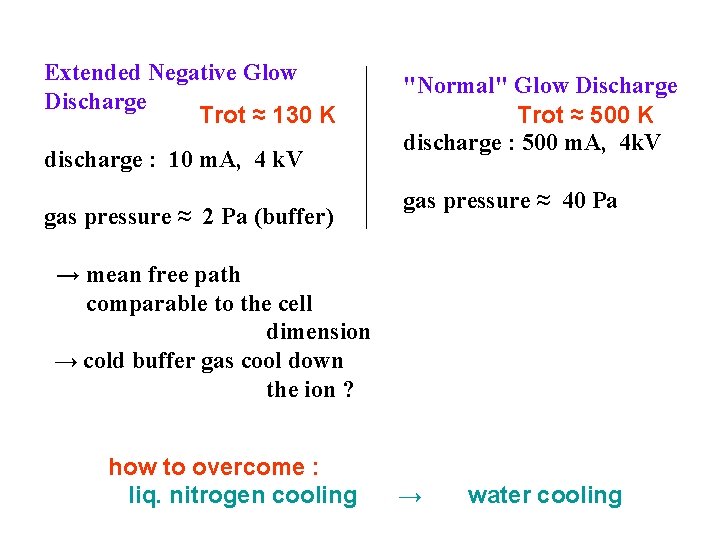
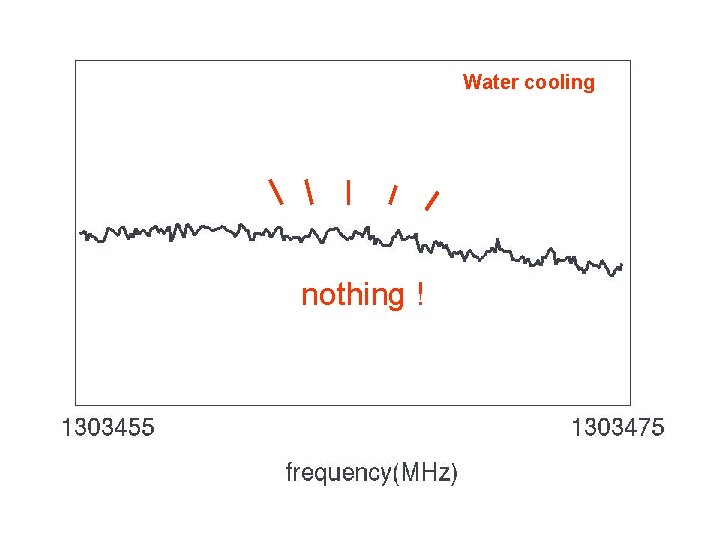
Extended Negative Glow Discharge Trot ≈ 130 K discharge : 10 m. A, 4 k. V gas pressure ≈ 2 Pa (buffer) "Normal" Glow Discharge Trot ≈ 500 K discharge : 500 m. A, 4 k. V gas pressure ≈ 40 Pa → mean free path comparable to the cell dimension → cold buffer gas cool down the ion ? how to overcome : liq. nitrogen cooling → water cooling

N 2 H+ J=14 ← 13 E" = 283 cm-1 Lig. Nitrogen cooling

Water cooling nothing !

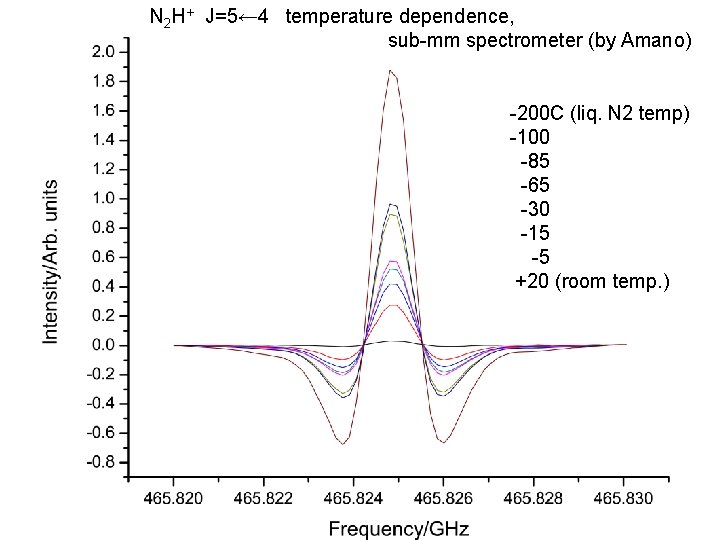
N 2 H+ J=5← 4 temperature dependence, sub-mm spectrometer (by Amano) -200 C (liq. N 2 temp) -100 -85 -65 -30 -15 -5 +20 (room temp. )

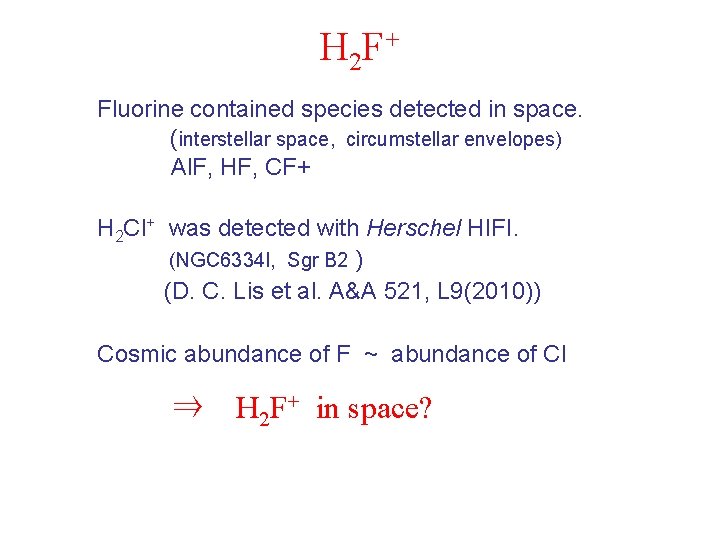
H 2 F+ Fluorine contained species detected in space. (interstellar space, circumstellar envelopes) Al. F, HF, CF+ H 2 Cl+ was detected with Herschel HIFI. (NGC 6334 I, Sgr B 2 ) (D. C. Lis et al. A&A 521, L 9(2010)) Cosmic abundance of F ~ abundance of Cl ⇒ H 2 F+ in space?

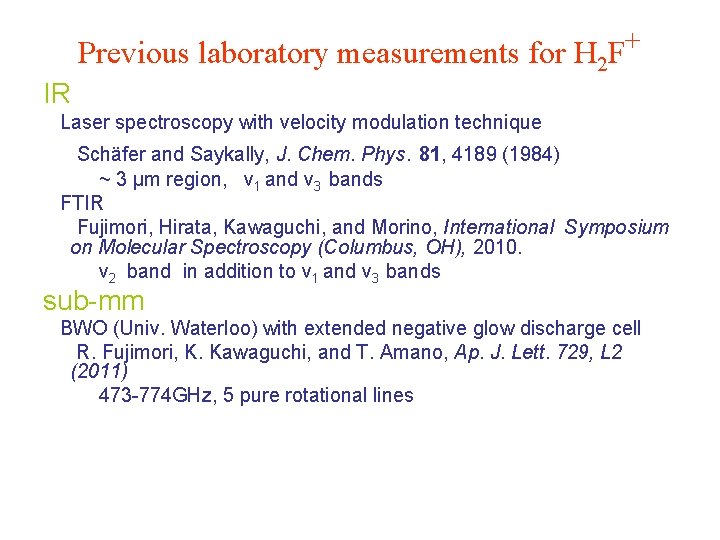
Previous laboratory measurements for H 2 F+ IR Laser spectroscopy with velocity modulation technique Schäfer and Saykally, J. Chem. Phys. 81, 4189 (1984) ~ 3 µm region, ν 1 and ν 3 bands FTIR Fujimori, Hirata, Kawaguchi, and Morino, International Symposium on Molecular Spectroscopy (Columbus, OH), 2010. ν 2 band in addition to ν 1 and ν 3 bands sub-mm BWO (Univ. Waterloo) with extended negative glow discharge cell R. Fujimori, K. Kawaguchi, and T. Amano, Ap. J. Lett. 729, L 2 (2011) 473 -774 GHz, 5 pure rotational lines

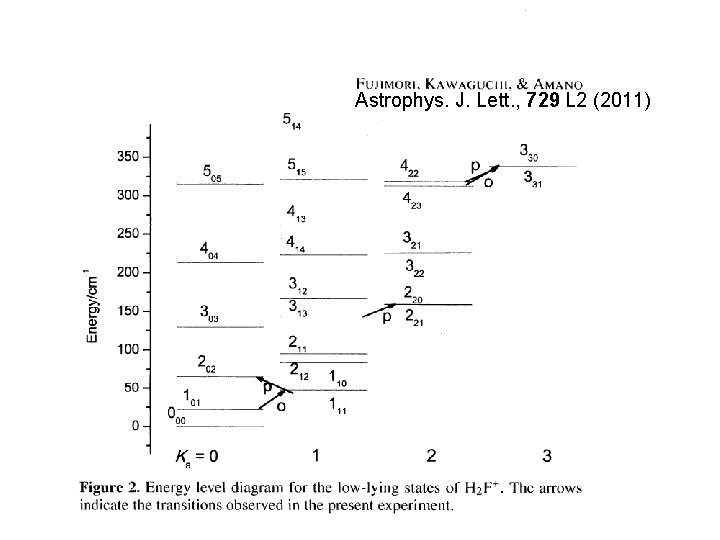
Astrophys. J. Lett. , 729 L 2 (2011)

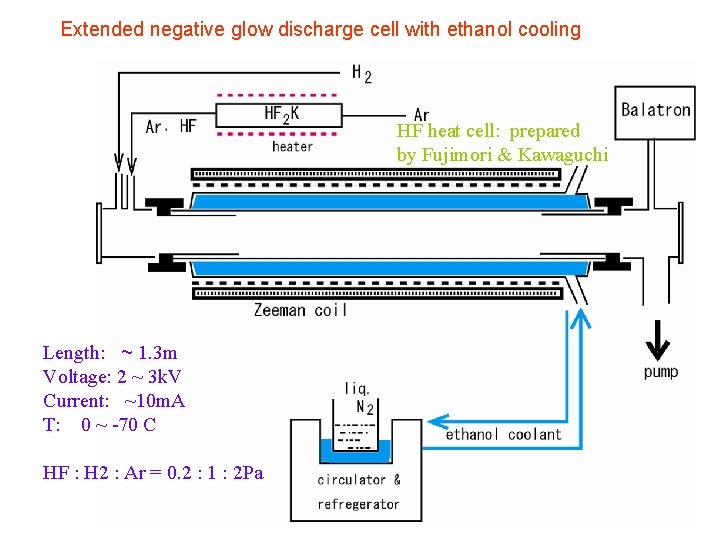
Extended negative glow discharge cell with ethanol cooling HF heat cell: prepared by Fujimori & Kawaguchi Length: ~ 1. 3 m Voltage: 2 ~ 3 k. V Current: ~10 m. A T: 0 ~ -70 C HF : H 2 : Ar = 0. 2 : 1 : 2 Pa


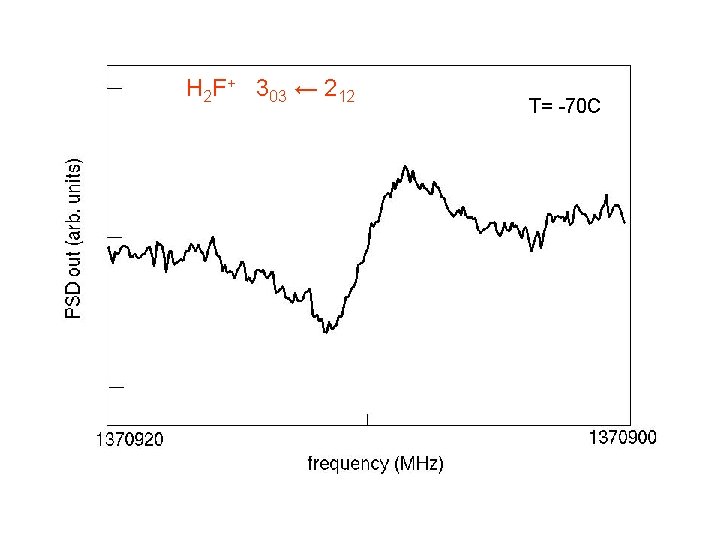
H 2 F+ 303 ← 212 T= -70 C

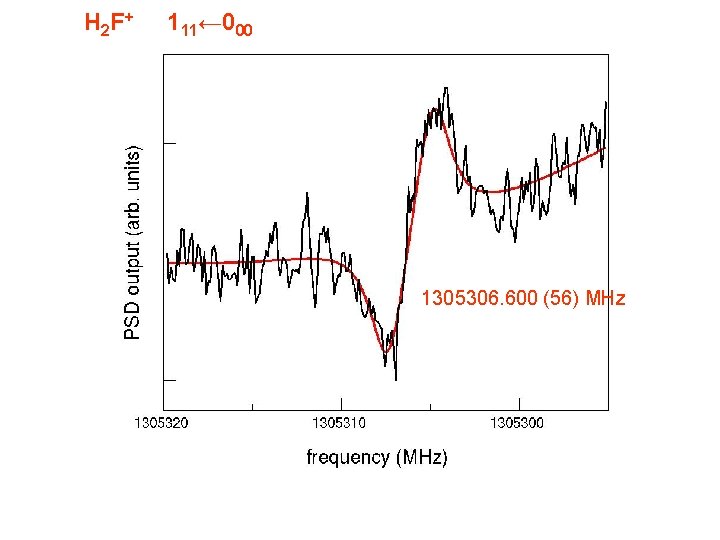
H 2 F + 111← 000 1305306. 600 (56) MHz

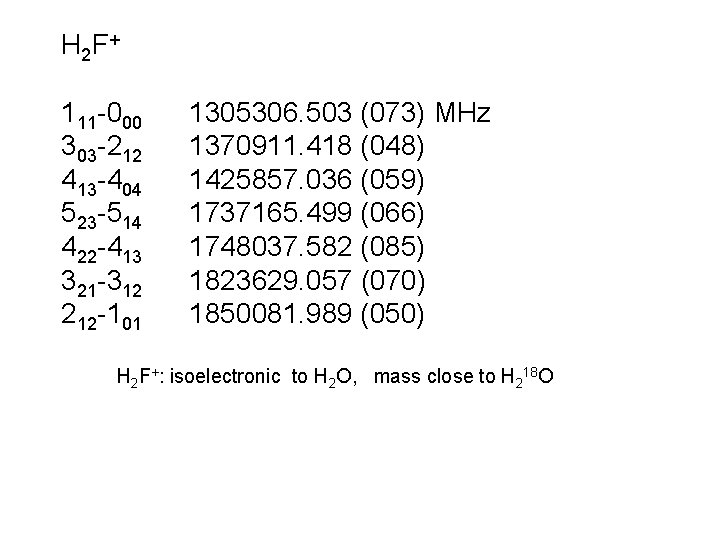
H 2 F + 111 -000 303 -212 413 -404 523 -514 422 -413 321 -312 212 -101 1305306. 503 (073) MHz 1370911. 418 (048) 1425857. 036 (059) 1737165. 499 (066) 1748037. 582 (085) 1823629. 057 (070) 1850081. 989 (050) H 2 F+: isoelectronic to H 2 O, mass close to H 218 O

red arrow: Tu. FIR

Lowest frequency rotational lines para 111 -000 1305306. 503 (73) MHz (Tu. FIR measurement, Toyama) ortho 110 -101 760928. 937 (10) MHz (sub-mm measurement, Waterloo)

liq. N 2 cell without glass-blowing

Collaboration Univ. of Toyama K. Takagi (prof. emeritus) H. Odashima (now in Meiji univ. in Tokyo) Y. Moriwaki K. Kobayashi many students including T. Yonezu (now in Nobeyama Radio Obs. ) T. Oka (Chicago Univ. ) --- start of studies of ionic species T. Amano (Univ. Waterloo, Canada) --- ext. neg. glow discharge cell and works using it K. Kawaguchi (Okayama Univ. ) late J. M. Brown (Oxford Univ. ) and of course late K. M. Evenson (NIST Boulder Colorado)

Thank you !
 Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Radiacls
Radiacls Carbon trichloride
Carbon trichloride Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Interacting molecules or ions
Interacting molecules or ions High-resolution terahertz
High-resolution terahertz Organic molecules vs inorganic molecules
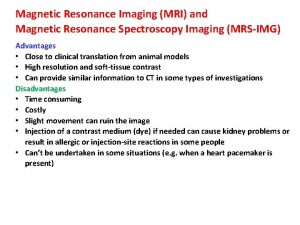
Organic molecules vs inorganic molecules Advantages and disadvantages of spectroscopy
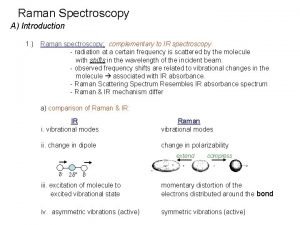
Advantages and disadvantages of spectroscopy Why are raman and ir complementary
Why are raman and ir complementary Difference between ir and raman spectroscopy
Difference between ir and raman spectroscopy Nmr associates
Nmr associates Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Advantages of nmr spectroscopy
Advantages of nmr spectroscopy Introduction to spectrophotometry
Introduction to spectrophotometry Applications of uv and visible spectroscopy
Applications of uv and visible spectroscopy Advantages and disadvantages of spectroscopy
Advantages and disadvantages of spectroscopy Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Advantages and disadvantages of spectroscopy
Advantages and disadvantages of spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Erzeng xue
Erzeng xue What are the different types of spectroscopy
What are the different types of spectroscopy Monatomic ion examples
Monatomic ion examples Plyatomic ions
Plyatomic ions Ionic compounds list
Ionic compounds list Types of polyatomic ions
Types of polyatomic ions Positive negative attract each other
Positive negative attract each other Srim the stopping and range of ions in matter
Srim the stopping and range of ions in matter Multivalent metals and polyatomic ions
Multivalent metals and polyatomic ions Freezing point chapter 13
Freezing point chapter 13 Chemistry formulas list
Chemistry formulas list Ions and ionic bonding cornell doodle notes
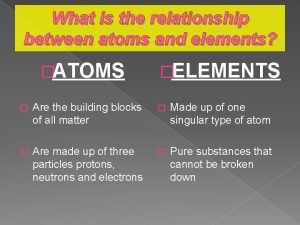
Ions and ionic bonding cornell doodle notes What is the relationship between atoms and elements
What is the relationship between atoms and elements Classify the following unbalanced chemical equations
Classify the following unbalanced chemical equations Solid to gas
Solid to gas Mixture of compounds diagram
Mixture of compounds diagram Absolute vs relative configuration
Absolute vs relative configuration 3bacl2 counting atoms
3bacl2 counting atoms Polar and nonpolar similarities
Polar and nonpolar similarities Fluod
Fluod Molecular shapes polar or nonpolar
Molecular shapes polar or nonpolar Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Bead like structures formed by dna and histone molecules
Bead like structures formed by dna and histone molecules System collections generic
System collections generic Accumulator ac
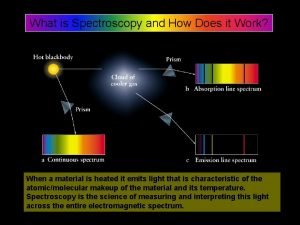
Accumulator ac What is spectroscopy
What is spectroscopy Homocyclic diene component
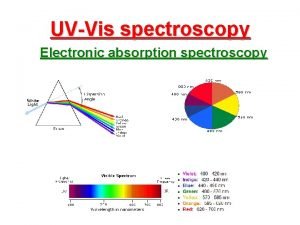
Homocyclic diene component Beer lambert law in uv spectroscopy
Beer lambert law in uv spectroscopy Ir sample preparation
Ir sample preparation Pengertian aas
Pengertian aas Objectives of spectroscopy
Objectives of spectroscopy Pes spectroscopy
Pes spectroscopy Near infrared spectroscopy instrumentation
Near infrared spectroscopy instrumentation Mass spec principle
Mass spec principle