Summer 2001 Notes June 13 June 15 June
![2+ [Co(H 2 O)6]2+ 2+ [Co(H 2 O)6]2+](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-2.jpg)
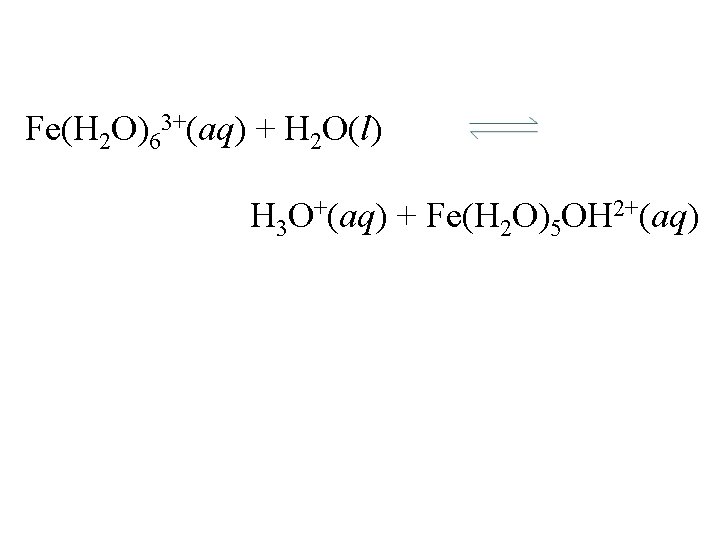
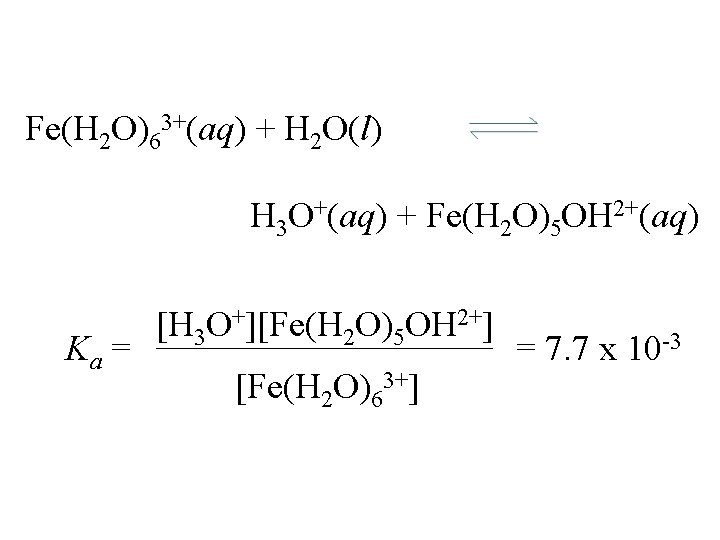
![p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-9.jpg)
![p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-10.jpg)
![p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-11.jpg)
![p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-12.jpg)


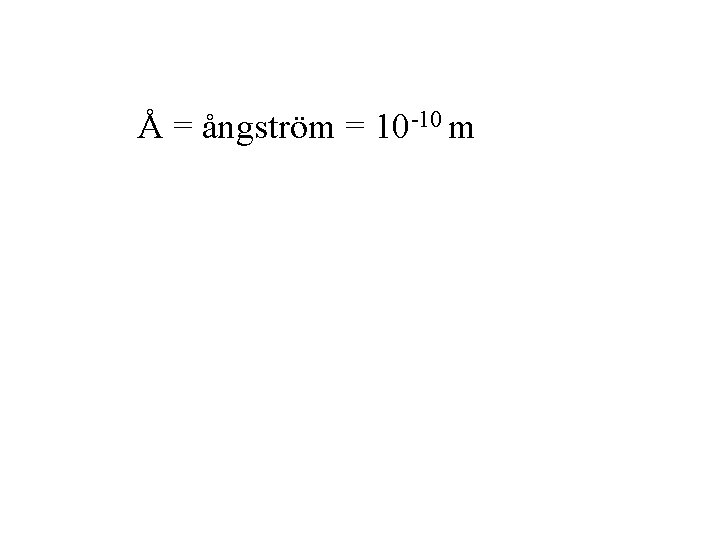









- Slides: 97

Summer 2001 Notes June 13 June 15 June 18 June 20 July 2 Fall 2001 Lectures 9/28 10/1 10/3 10/5 – 10/8
![2 CoH 2 O62 2+ [Co(H 2 O)6]2+](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-2.jpg)
2+ [Co(H 2 O)6]2+

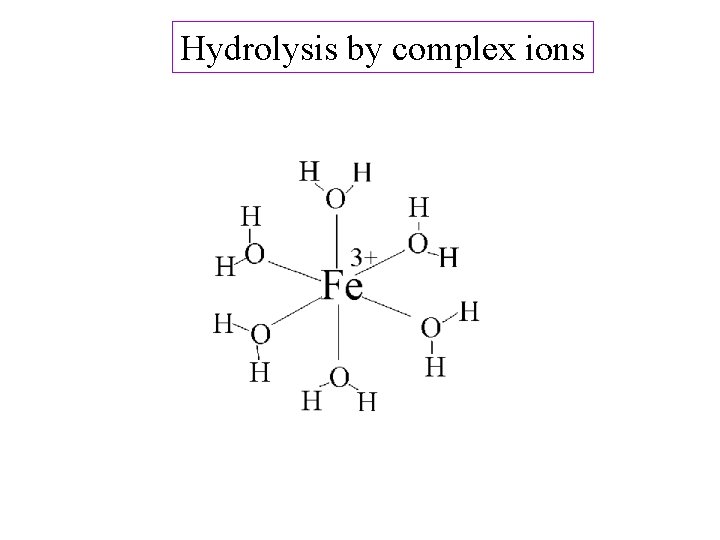
Hydrolysis by complex ions

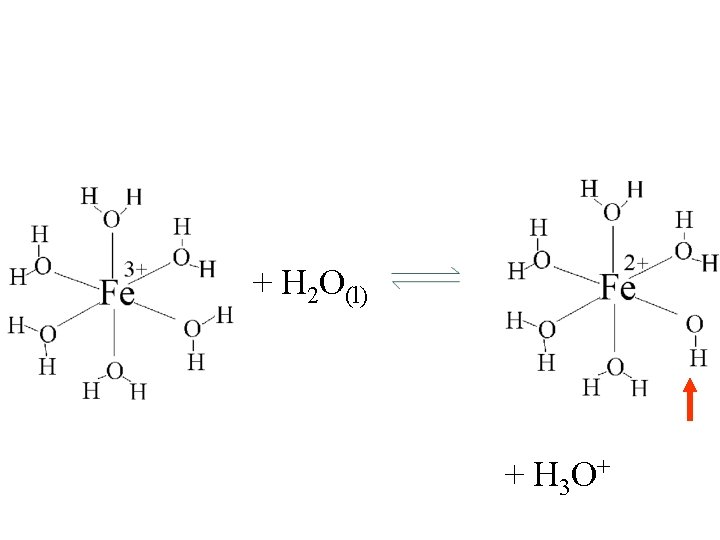
+ H 2 O(l) + H 3 O+

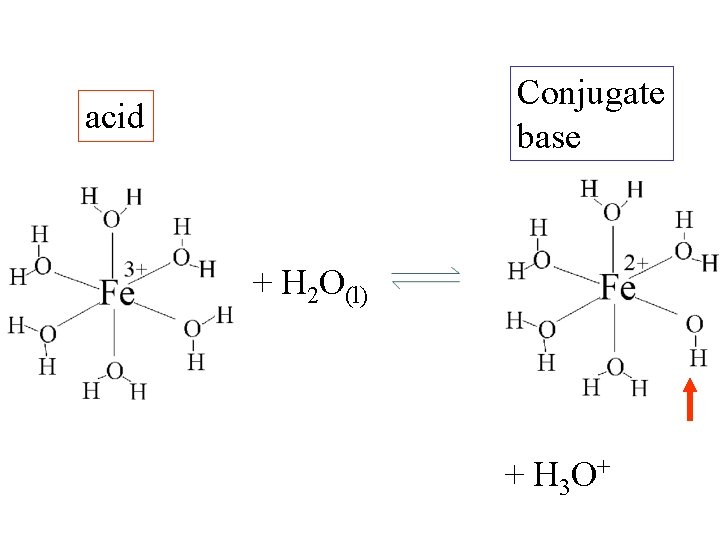
Conjugate base acid + H 2 O(l) + H 3 O+
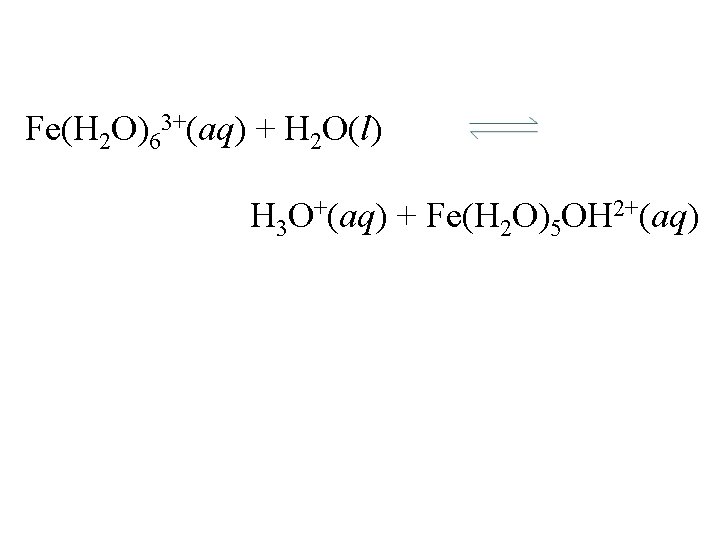
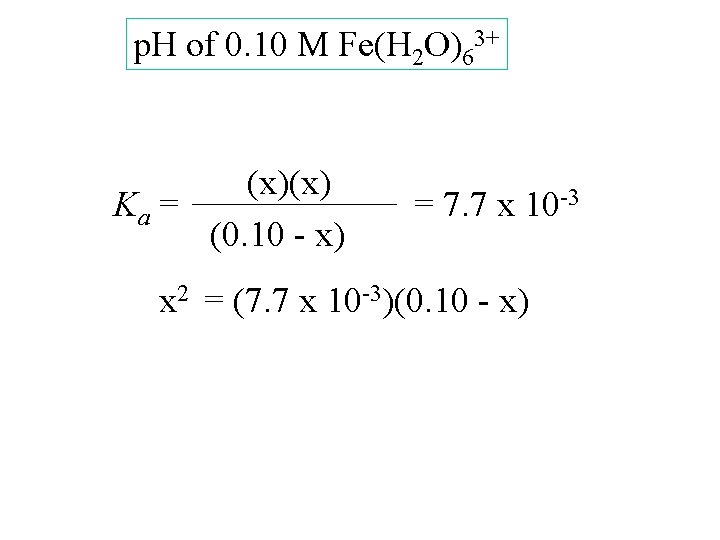
Fe(H 2 O)63+(aq) + H 2 O(l) H 3 O+(aq) + Fe(H 2 O)5 OH 2+(aq)
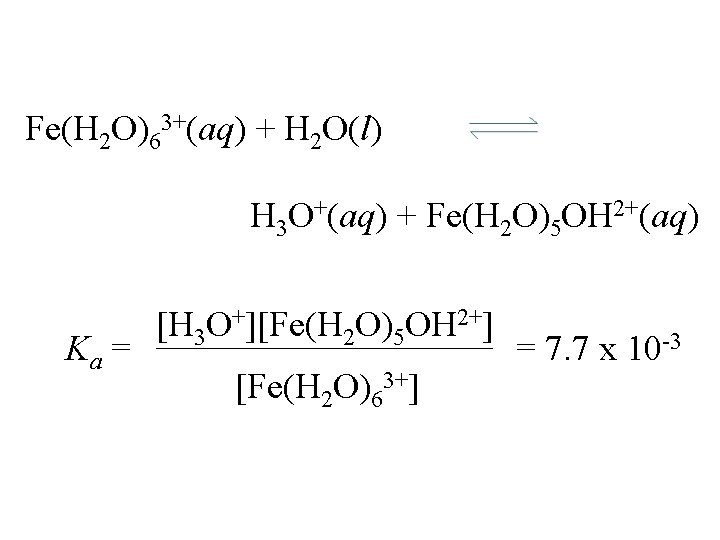
Fe(H 2 O)63+(aq) + H 2 O(l) H 3 O+(aq) + Fe(H 2 O)5 OH 2+(aq) Ka = [H 3 O+][Fe(H 2 O)5 OH 2+] [Fe(H 2 O)63+] = 7. 7 x 10 -3

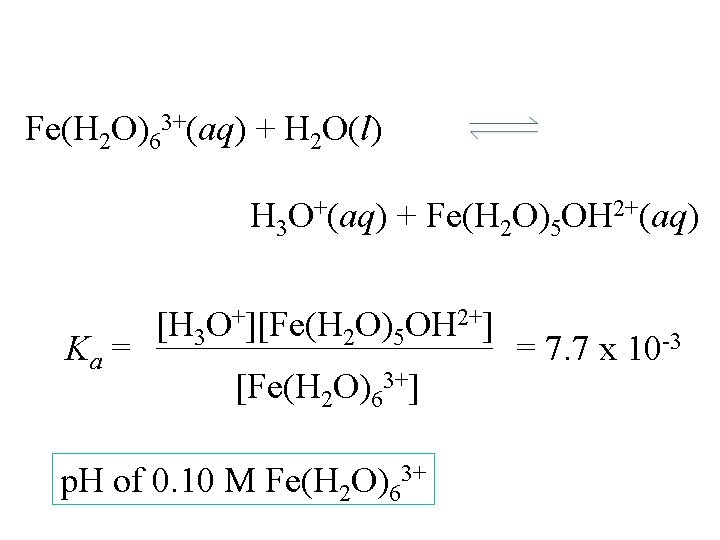
Fe(H 2 O)63+(aq) + H 2 O(l) H 3 O+(aq) + Fe(H 2 O)5 OH 2+(aq) Ka = [H 3 O+][Fe(H 2 O)5 OH 2+] [Fe(H 2 O)63+] p. H of 0. 10 M Fe(H 2 O)63+ = 7. 7 x 10 -3
![p H of 0 10 M FeH 2 O63 FeH 2 O63 H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-9.jpg)
p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 O+] [Fe(H 2 O)5 OH 2+] Start change equil. 0. 10 0 0
![p H of 0 10 M FeH 2 O63 FeH 2 O63 H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-10.jpg)
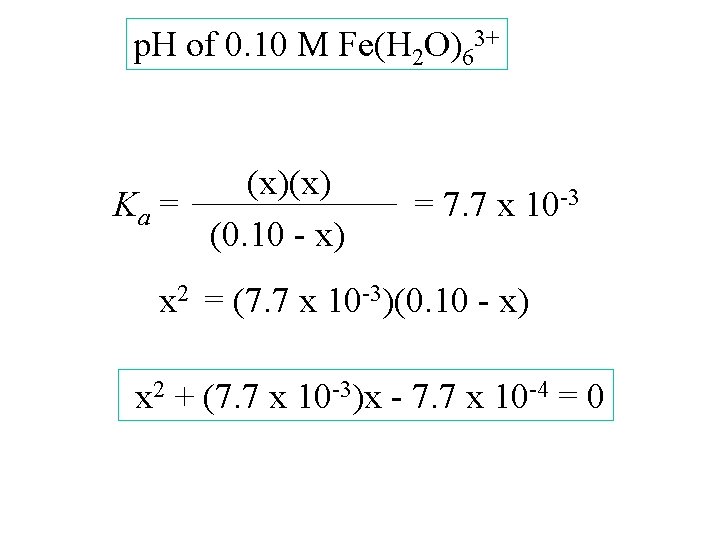
p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 O+] [Fe(H 2 O)5 OH 2+] Start change equil. 0. 10 -x 0 +x
![p H of 0 10 M FeH 2 O63 FeH 2 O63 H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-11.jpg)
p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 O+] [Fe(H 2 O)5 OH 2+] 0. 10 Start change -x equil. 0. 10 - x 0 +x x
![p H of 0 10 M FeH 2 O63 FeH 2 O63 H 3 p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3](https://slidetodoc.com/presentation_image_h2/73cf282b1e01b413c78a51ae070a0257/image-12.jpg)
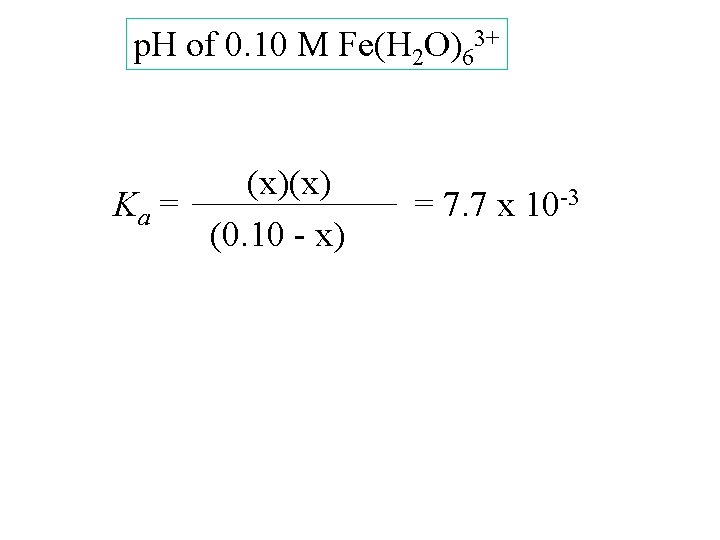
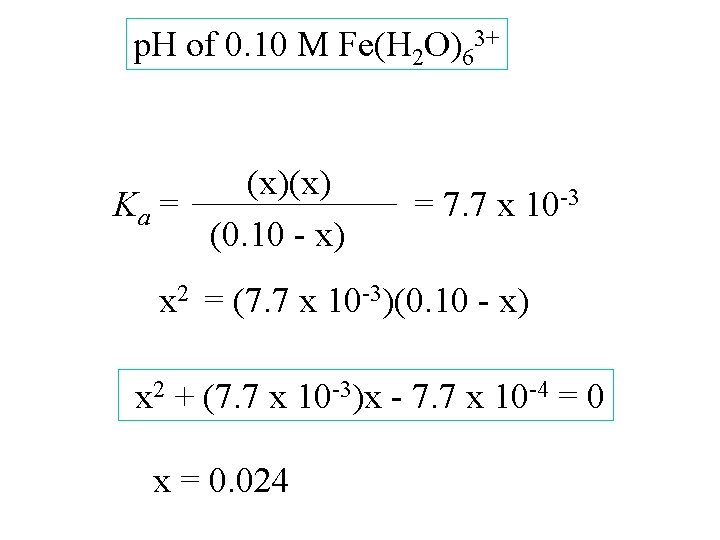
p. H of 0. 10 M Fe(H 2 O)63+ [Fe(H 2 O)63+] [H 3 O+] [Fe(H 2 O)5 OH 2+] 0. 10 Start change -x equil. 0. 10 - x (x)(x) Ka = (0. 10 - x) 0 +x x = 7. 7 x 10 -3

p. H of 0. 10 M Fe(H 2 O)63+ (x)(x) Ka = (0. 10 - x) = 7. 7 x 10 -3

p. H of 0. 10 M Fe(H 2 O)63+ (x)(x) Ka = (0. 10 - x) = 7. 7 x 10 -3 x 2 = (7. 7 x 10 -3)(0. 10 - x)

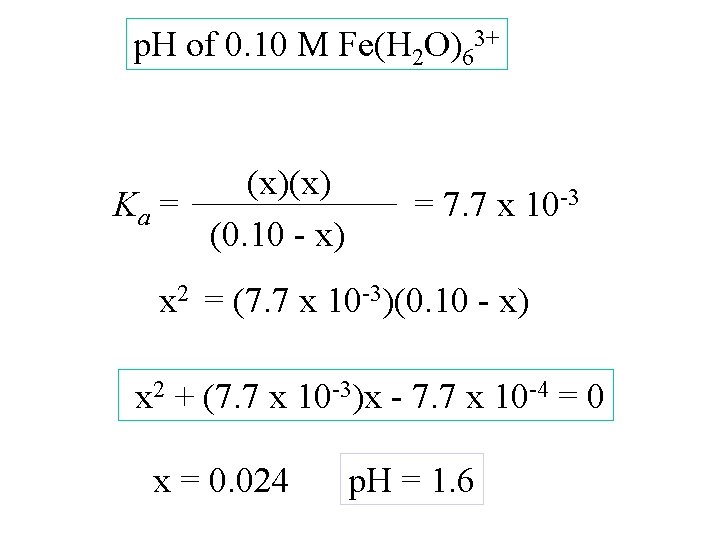
p. H of 0. 10 M Fe(H 2 O)63+ (x)(x) Ka = (0. 10 - x) = 7. 7 x 10 -3 x 2 = (7. 7 x 10 -3)(0. 10 - x) x 2 + (7. 7 x 10 -3)x - 7. 7 x 10 -4 = 0

p. H of 0. 10 M Fe(H 2 O)63+ (x)(x) Ka = (0. 10 - x) = 7. 7 x 10 -3 x 2 = (7. 7 x 10 -3)(0. 10 - x) x 2 + (7. 7 x 10 -3)x - 7. 7 x 10 -4 = 0 x = 0. 024

p. H of 0. 10 M Fe(H 2 O)63+ (x)(x) Ka = (0. 10 - x) = 7. 7 x 10 -3 x 2 = (7. 7 x 10 -3)(0. 10 - x) x 2 + (7. 7 x 10 -3)x - 7. 7 x 10 -4 = 0 x = 0. 024 p. H = 1. 6

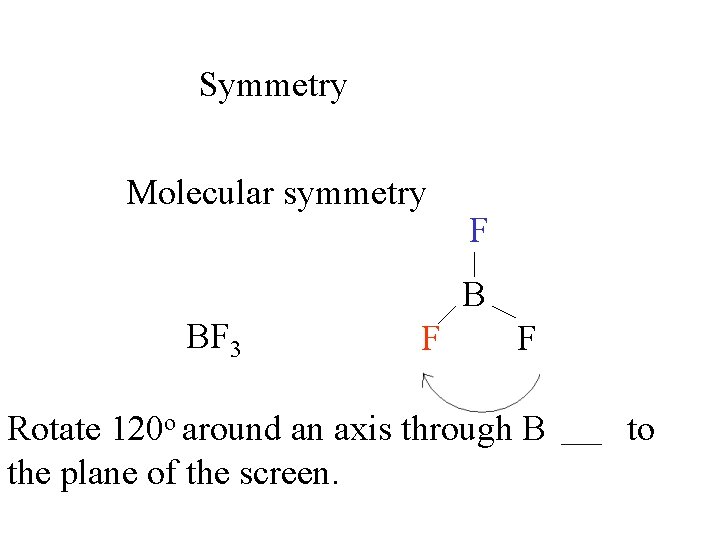
Symmetry

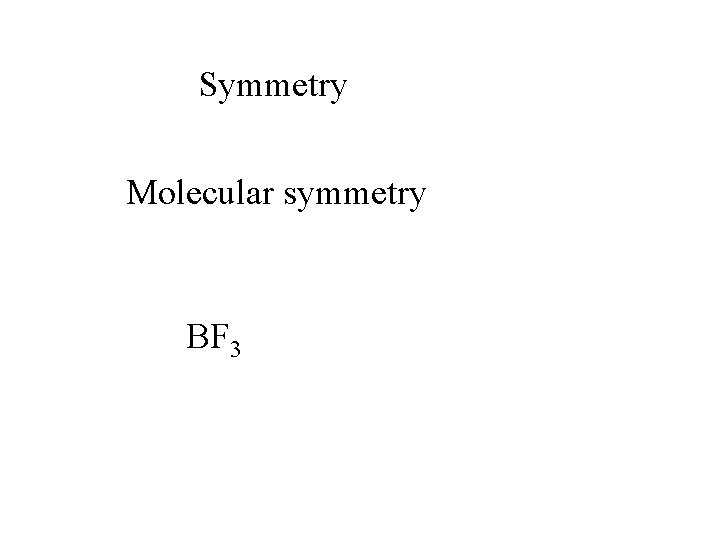
Symmetry Molecular symmetry BF 3

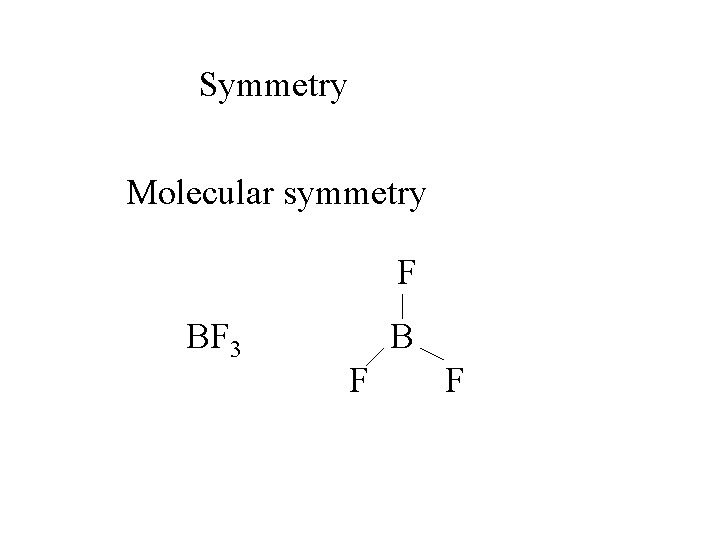
Symmetry Molecular symmetry F BF 3 B F F

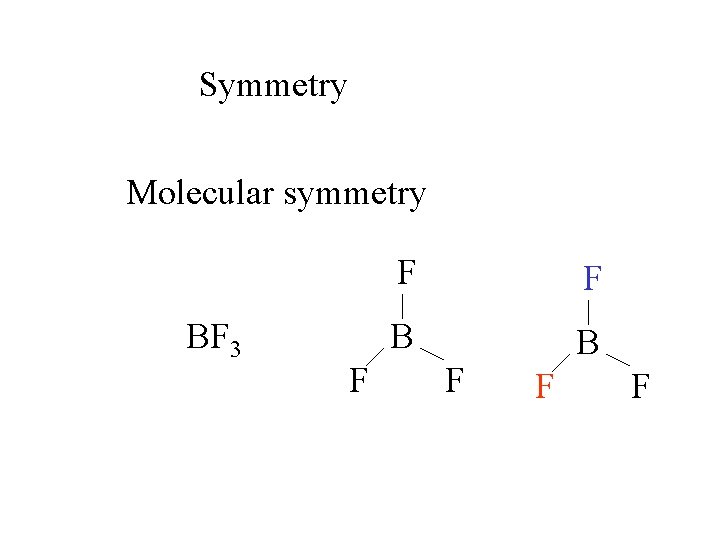
Symmetry Molecular symmetry BF 3 F F F B B F F F

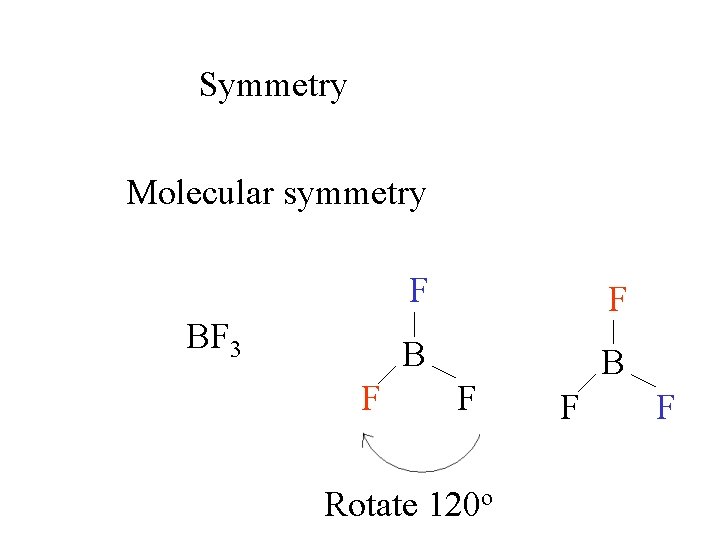
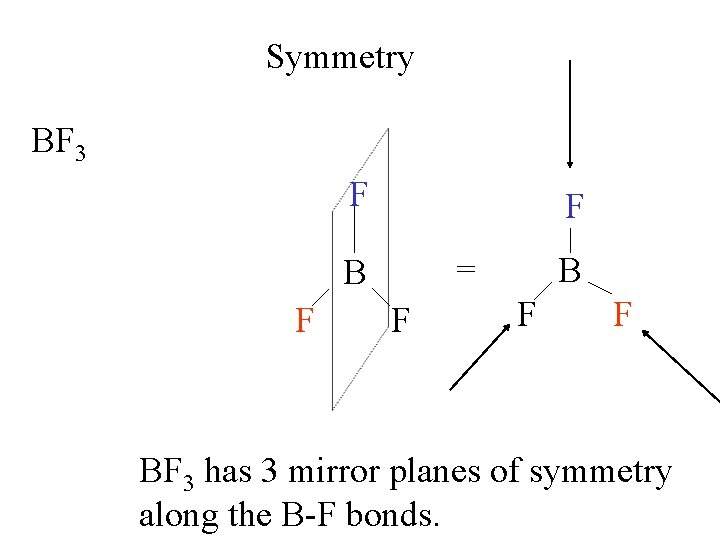
Symmetry Molecular symmetry F B BF 3 F F Rotate 120 o around an axis through B the plane of the screen. to

Symmetry Molecular symmetry BF 3 F F F B B F Rotate 120 o F F

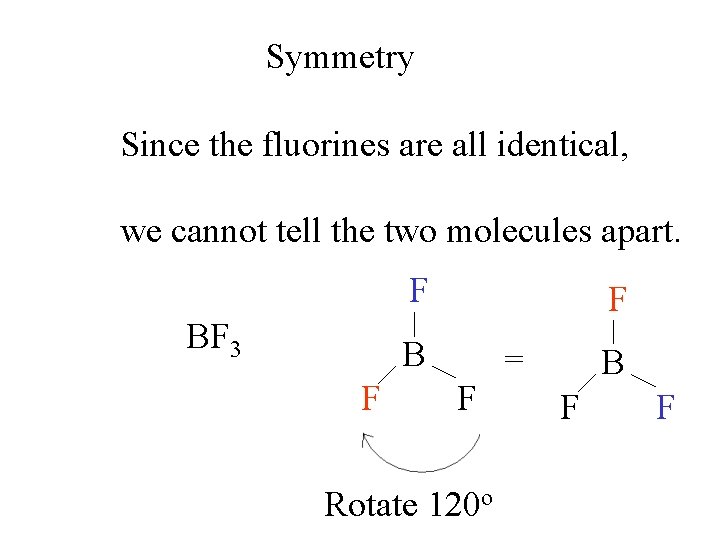
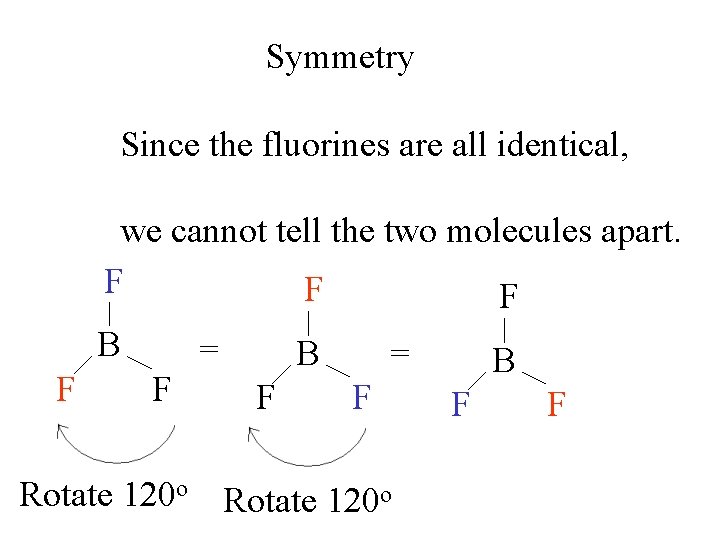
Symmetry Since the fluorines are all identical, we cannot tell the two molecules apart. F BF 3 F B F F Rotate 120 o = B F F

Symmetry Since the fluorines are all identical, we cannot tell the two molecules apart. F F B F F = B F F Rotate 120 o

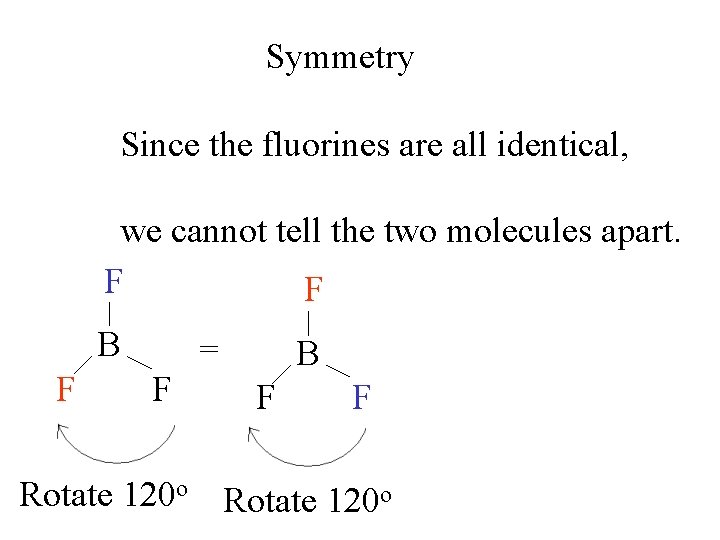
Symmetry Since the fluorines are all identical, we cannot tell the two molecules apart. F F F B F F = B F = F Rotate 120 o B F F

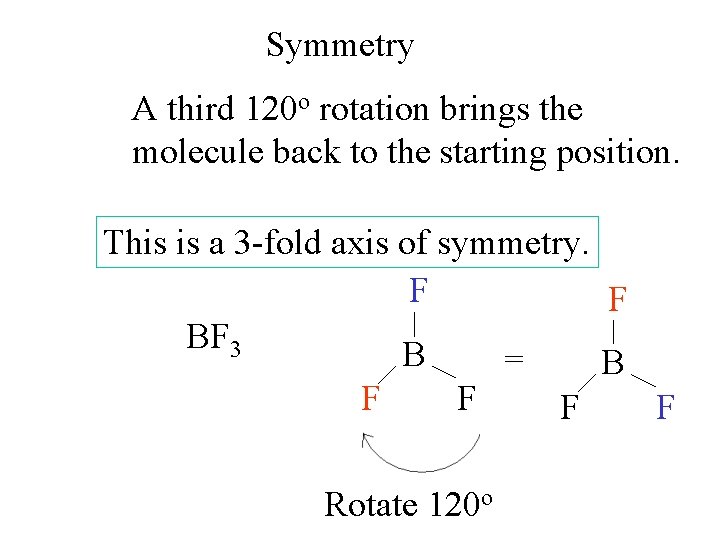
Symmetry A third 120 o rotation brings the molecule back to the starting position. This is a 3 -fold axis of symmetry. F F BF 3 B = B F F Rotate 120 o

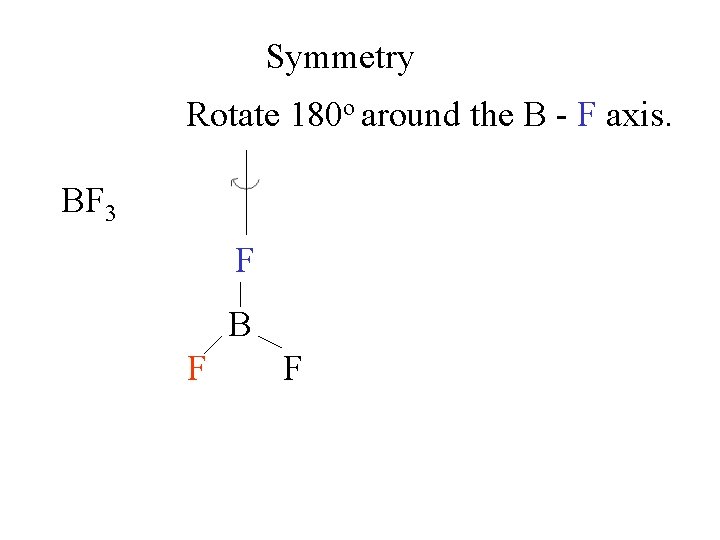
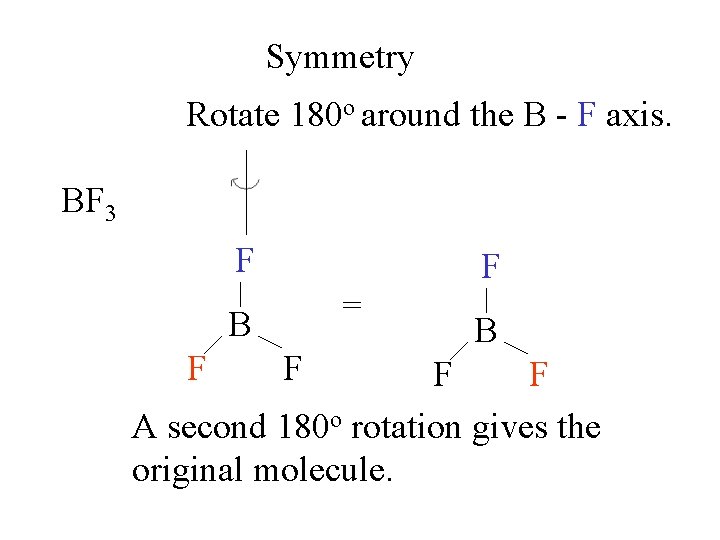
Symmetry Rotate 180 o around the B - F axis. BF 3 F B F F

Symmetry Rotate 180 o around the B - F axis. BF 3 F F F B B F F F

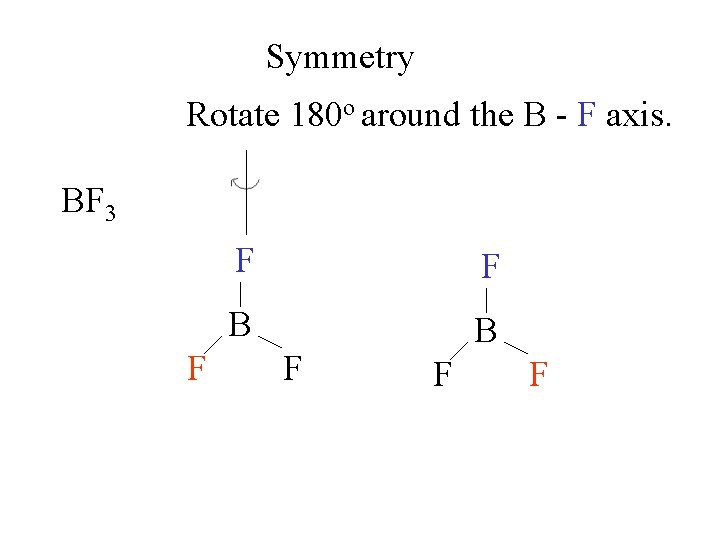
Symmetry Rotate 180 o around the B - F axis. BF 3 F = B F F F B F F A second 180 o rotation gives the original molecule.

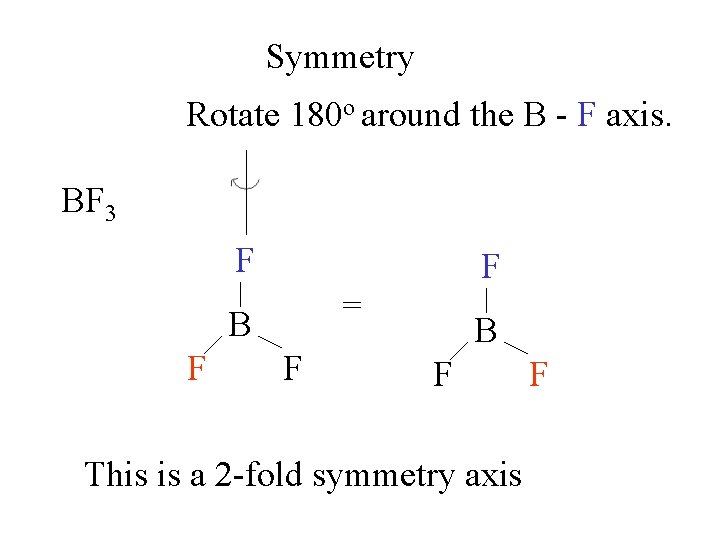
Symmetry Rotate 180 o around the B - F axis. BF 3 F = B F F F B F This is a 2 -fold symmetry axis F

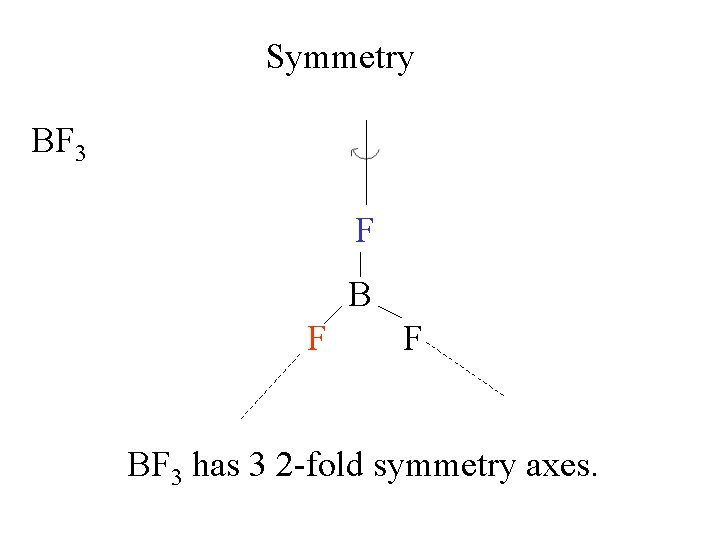
Symmetry BF 3 F B F F BF 3 has 3 2 -fold symmetry axes.

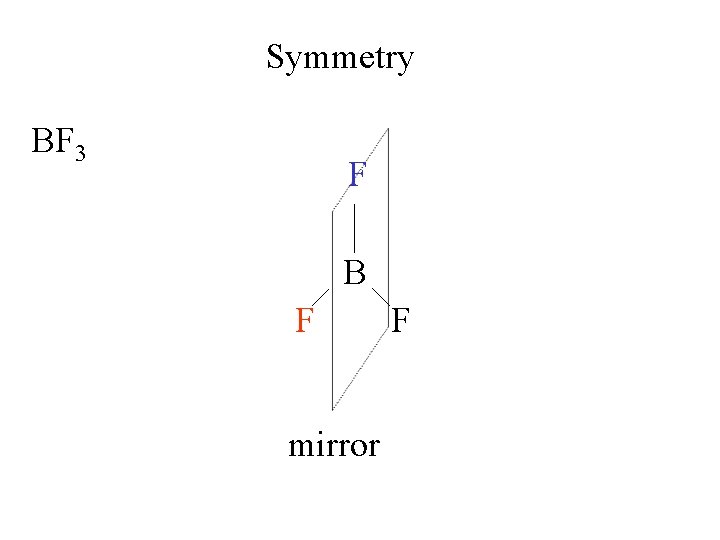
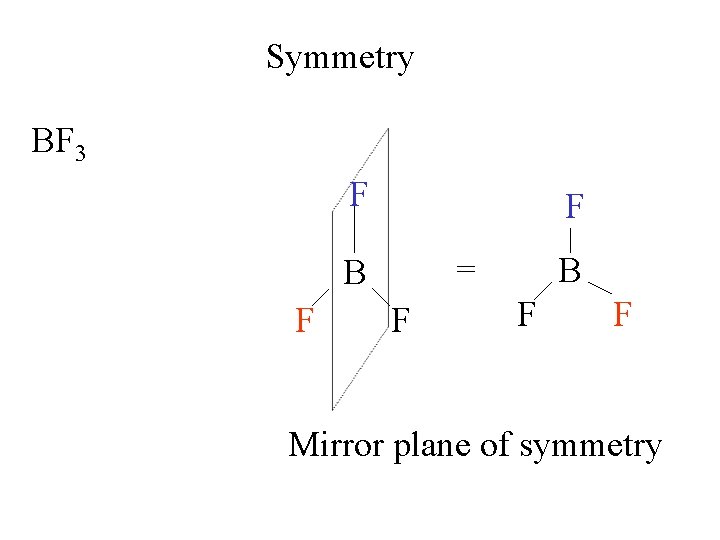
Symmetry BF 3 F F BB F mirror F

Symmetry BF 3 F F F = BB F F Mirror plane of symmetry

Symmetry BF 3 F F F = BB F F BF 3 has 3 mirror planes of symmetry along the B-F bonds.


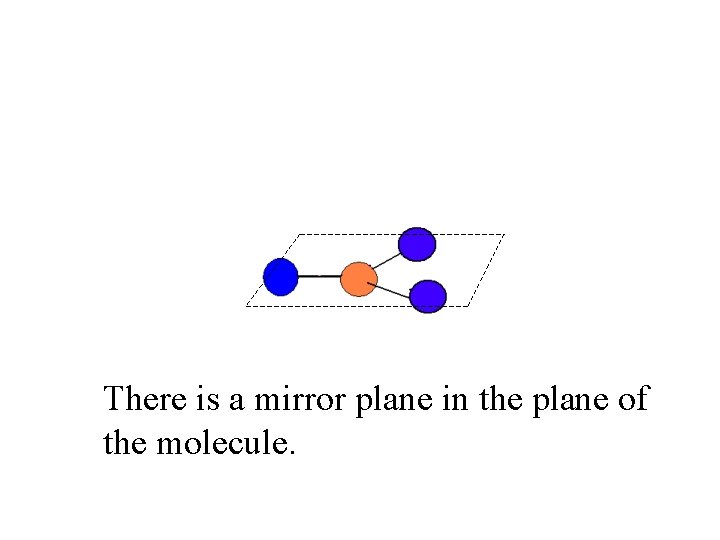
There is a mirror plane in the plane of the molecule.

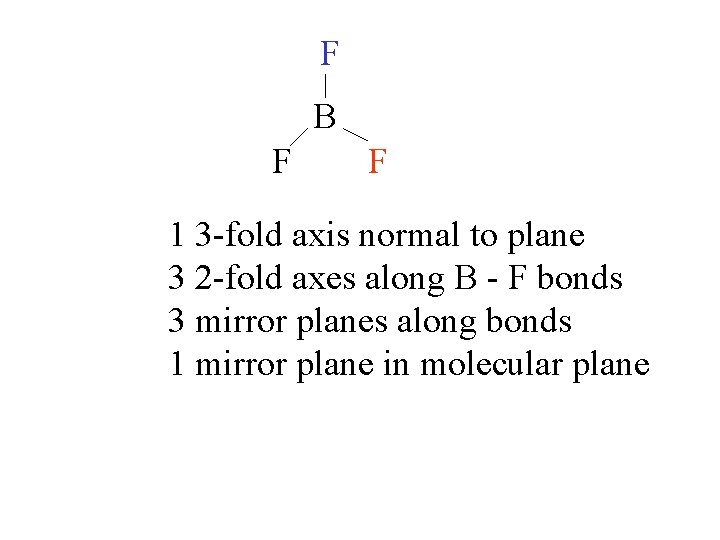
F B F F 1 3 -fold axis normal to plane 3 2 -fold axes along B - F bonds 3 mirror planes along bonds 1 mirror plane in molecular plane

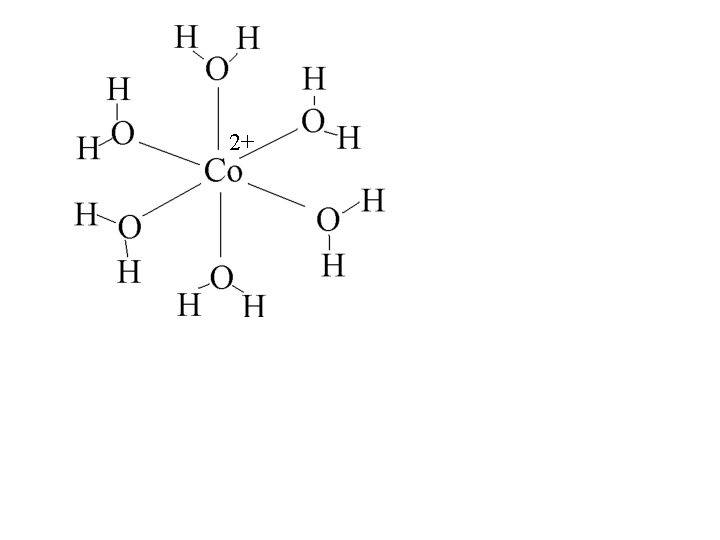
2+

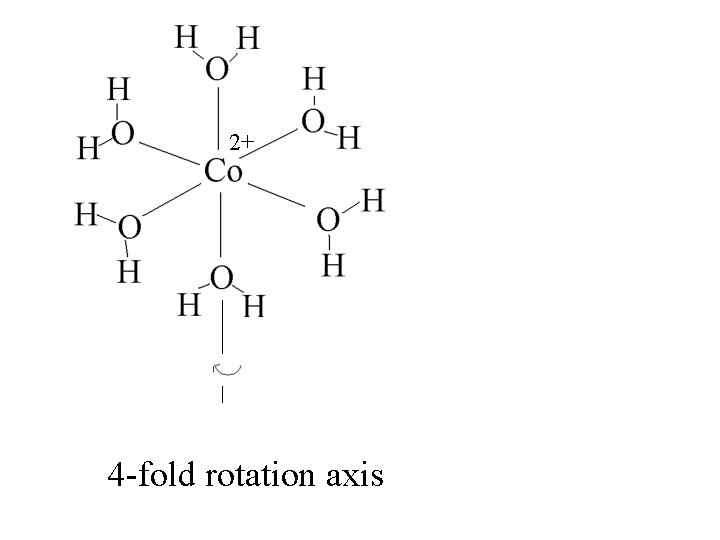
2+ 4 -fold rotation axis

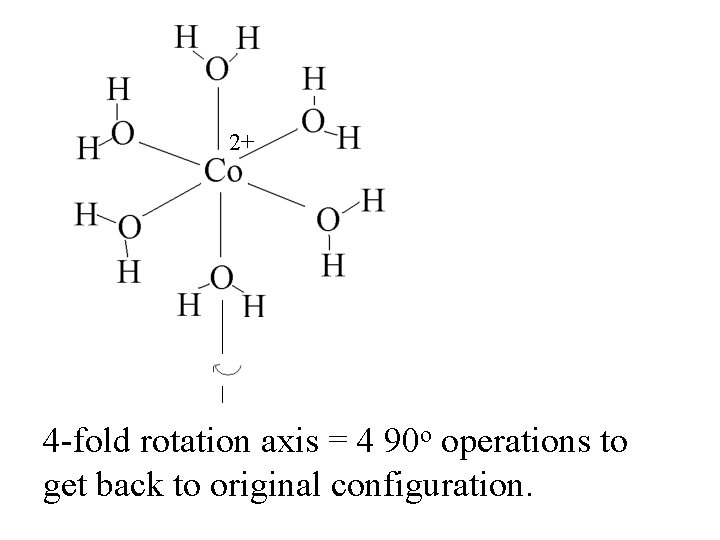
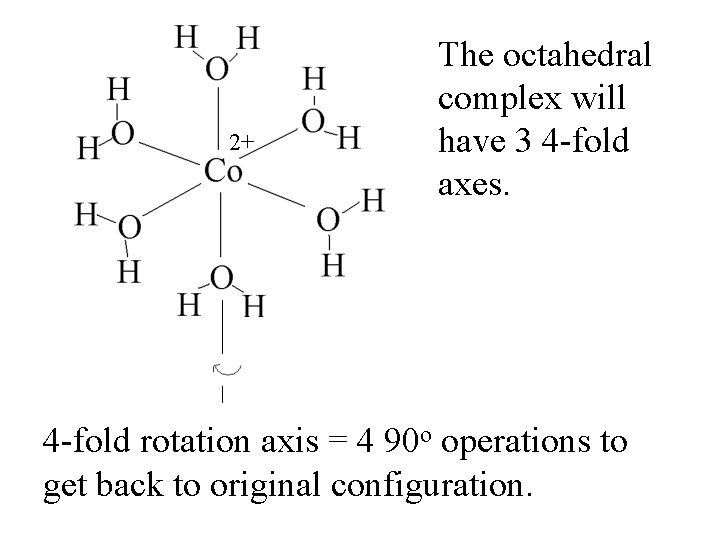
2+ 4 -fold rotation axis = 4 90 o operations to get back to original configuration.

2+ The octahedral complex will have 3 4 -fold axes. 4 -fold rotation axis = 4 90 o operations to get back to original configuration.

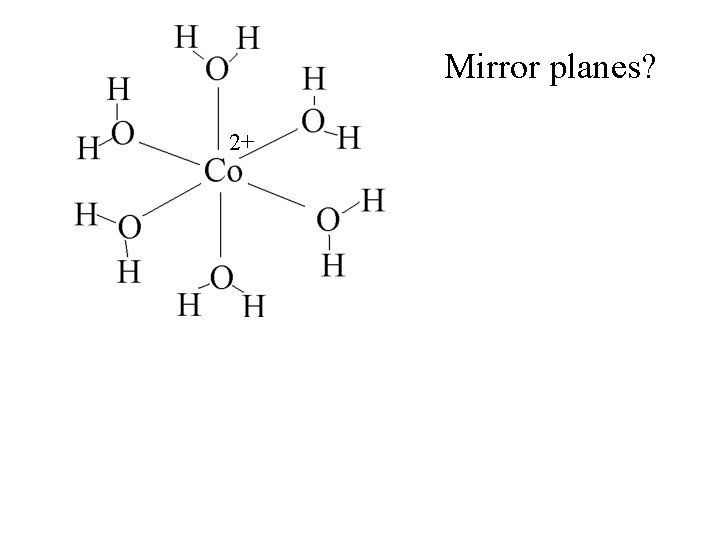
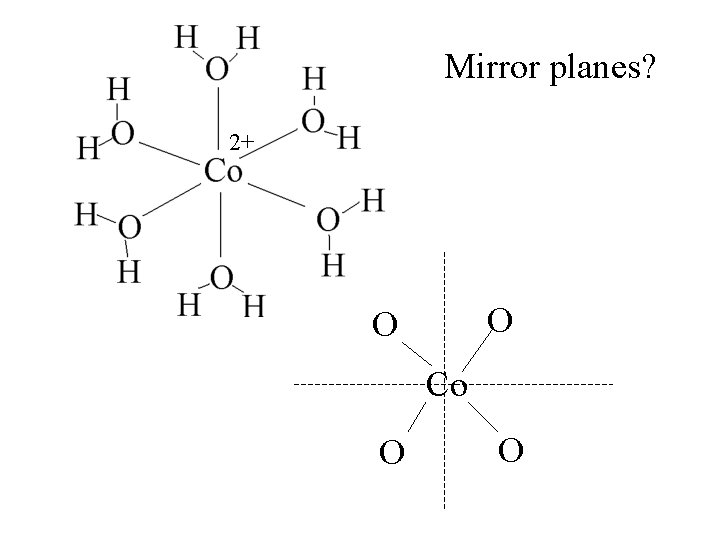
Mirror planes? 2+

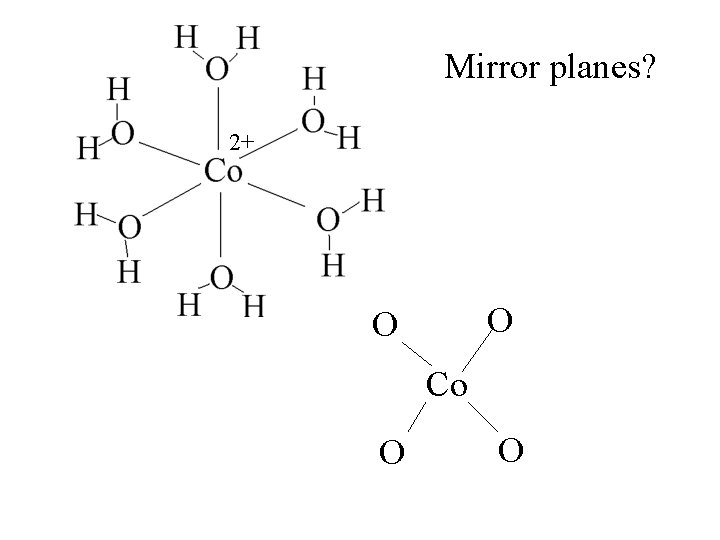
Mirror planes? 2+ O O Co O O

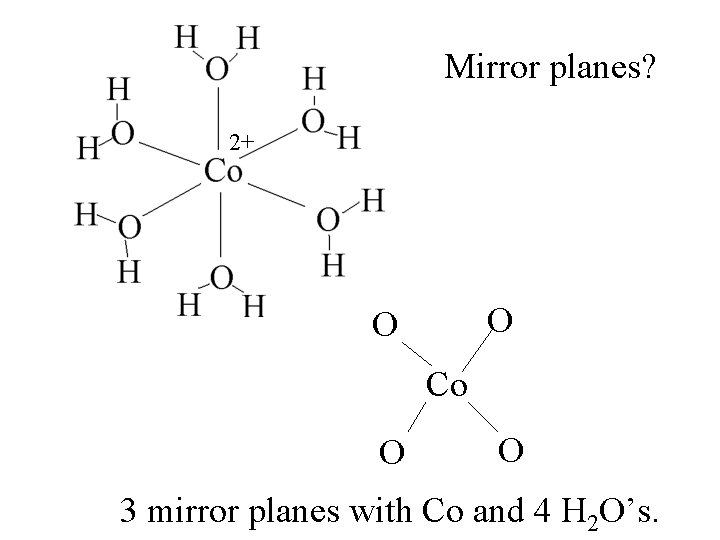
Mirror planes? 2+ O O Co O O 3 mirror planes with Co and 4 H 2 O’s.

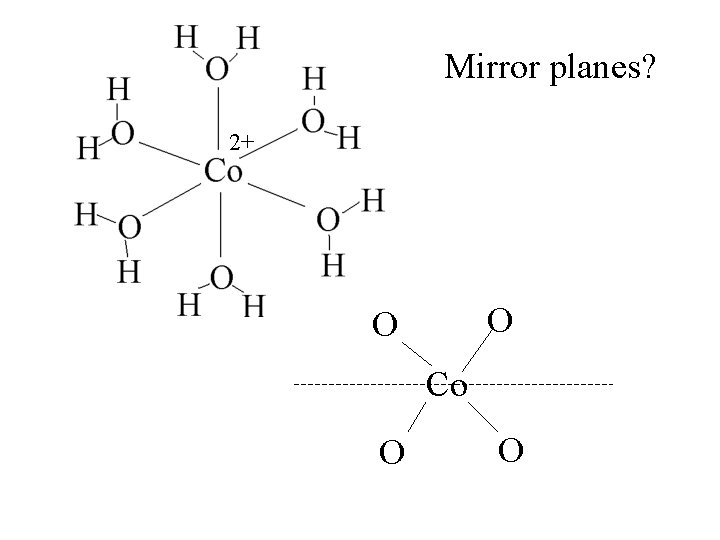
Mirror planes? 2+ O O Co O O

Mirror planes? 2+ O O Co O O

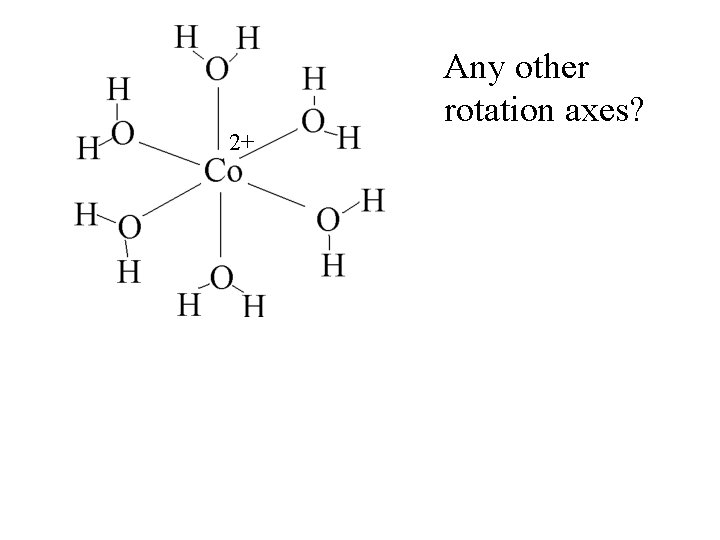
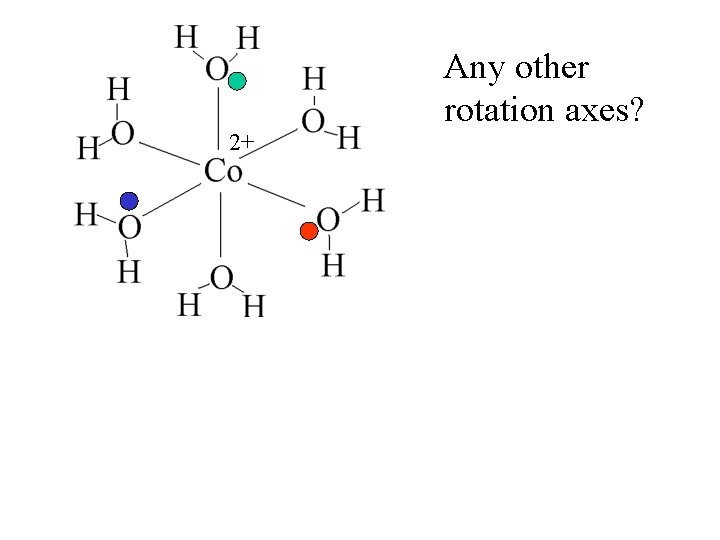
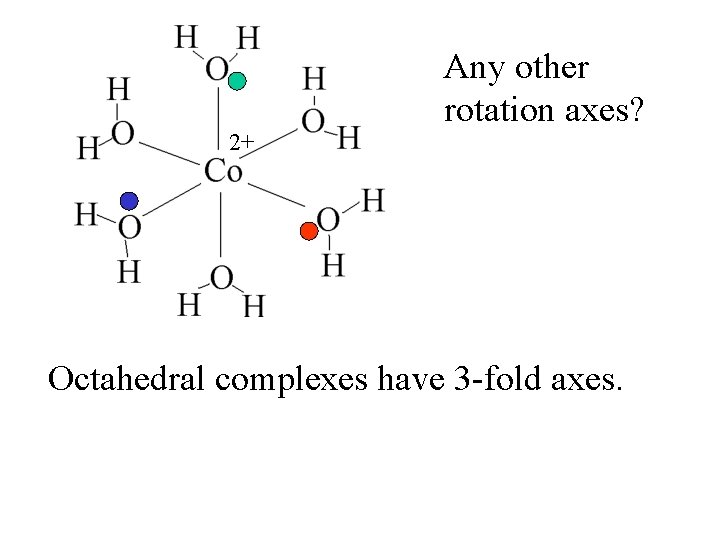
2+ Any other rotation axes?

2+ Any other rotation axes?

2+ Any other rotation axes? Octahedral complexes have 3 -fold axes.

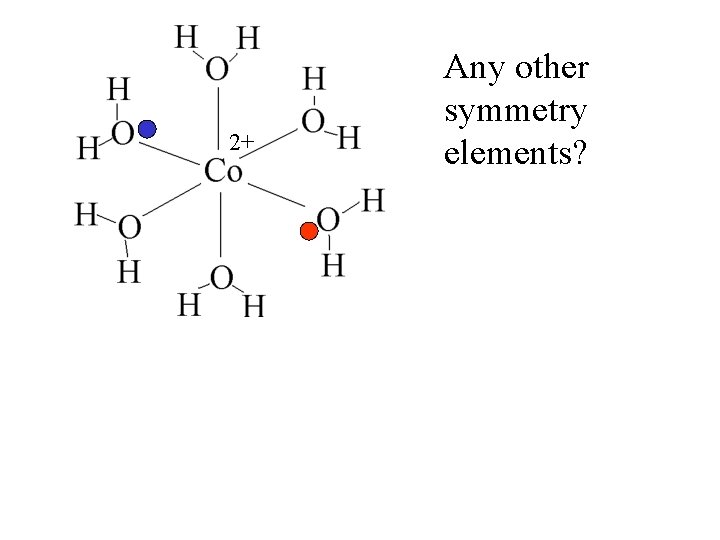
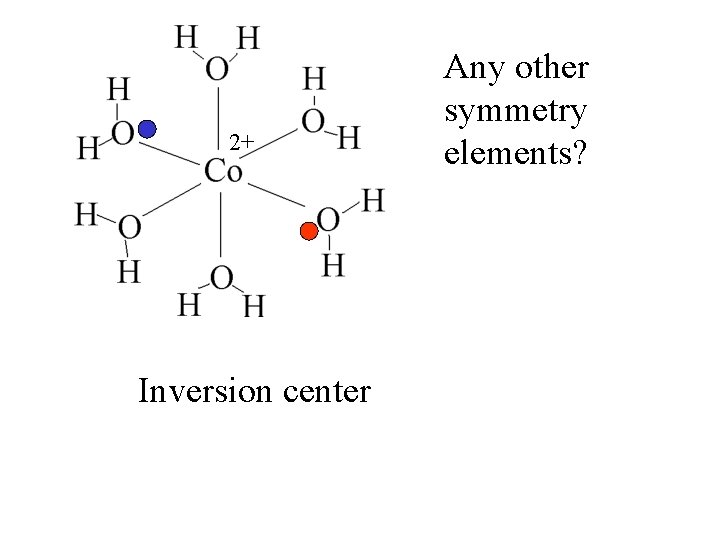
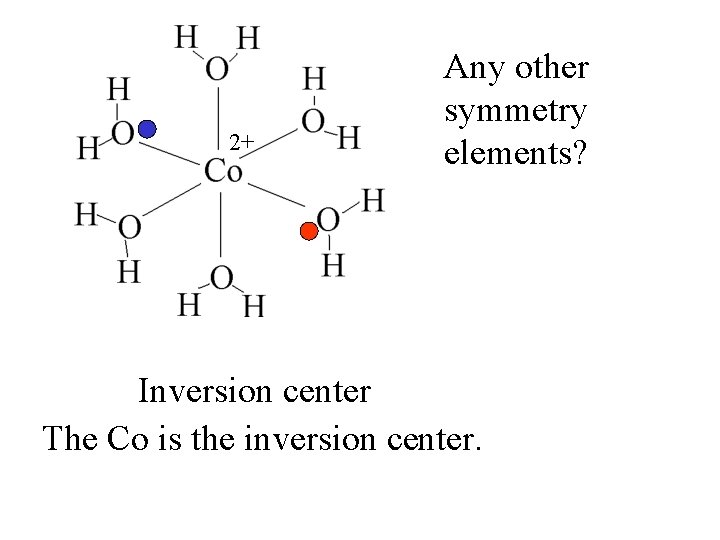
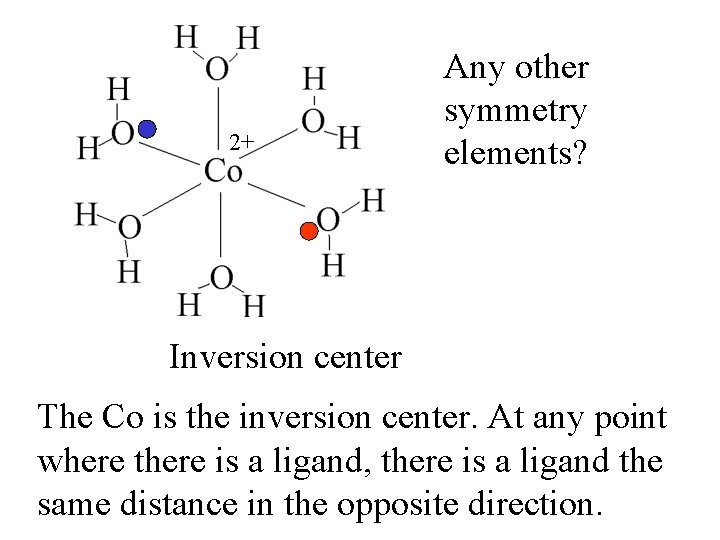
2+ Any other symmetry elements?

2+ Inversion center Any other symmetry elements?

2+ Any other symmetry elements? Inversion center The Co is the inversion center.

2+ Any other symmetry elements? Inversion center The Co is the inversion center. At any point where there is a ligand, there is a ligand the same distance in the opposite direction.

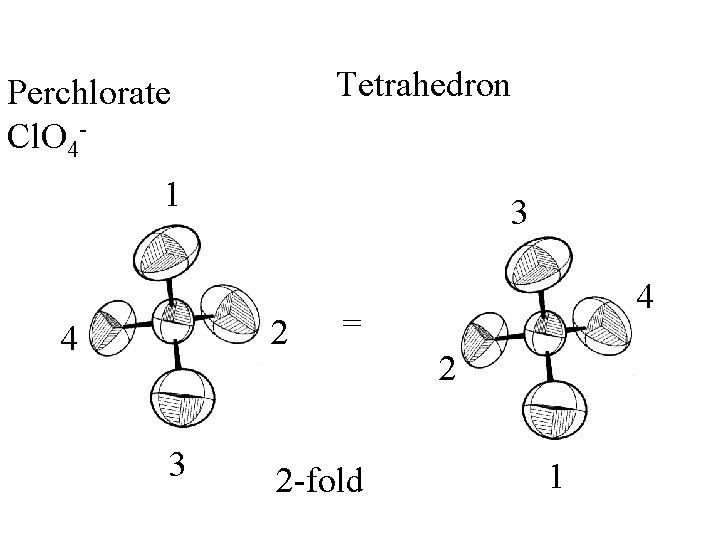
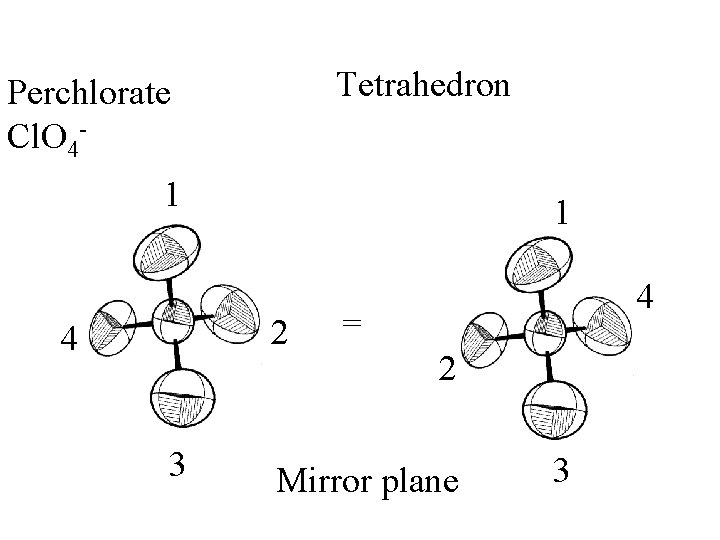
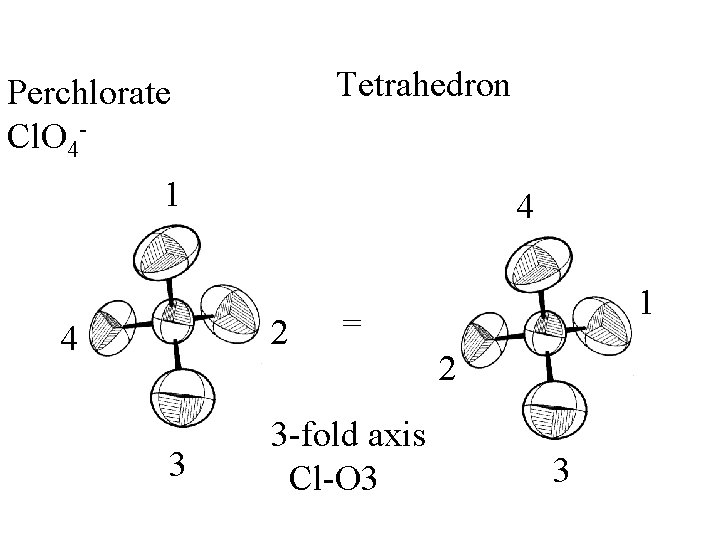
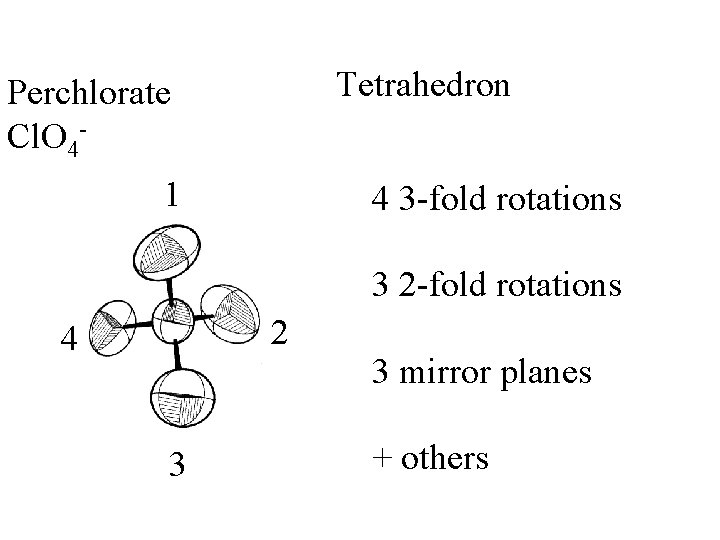
Tetrahedron

Tetrahedron Perchlorate Cl. O 4 -

Tetrahedron Perchlorate Cl. O 41 3 2 4 3 4 = 2 -fold 2 1

Tetrahedron Perchlorate Cl. O 41 1 2 4 3 4 = 2 Mirror plane 3

Tetrahedron Perchlorate Cl. O 41 4 2 4 3 1 = 3 -fold axis Cl-O 3 2 3

Tetrahedron Perchlorate Cl. O 41 4 3 -fold rotations 3 2 -fold rotations 2 4 3 3 mirror planes + others

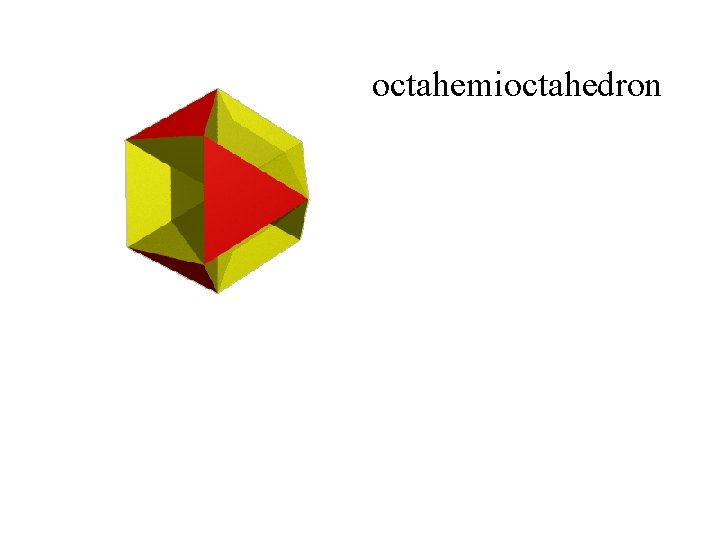
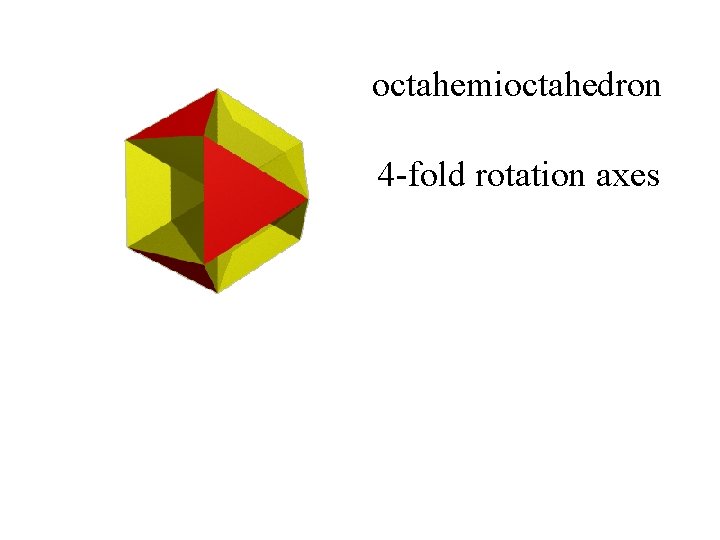
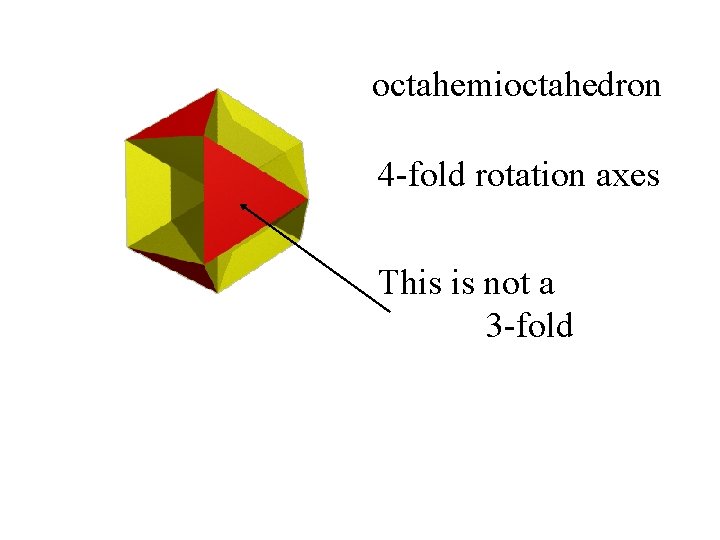
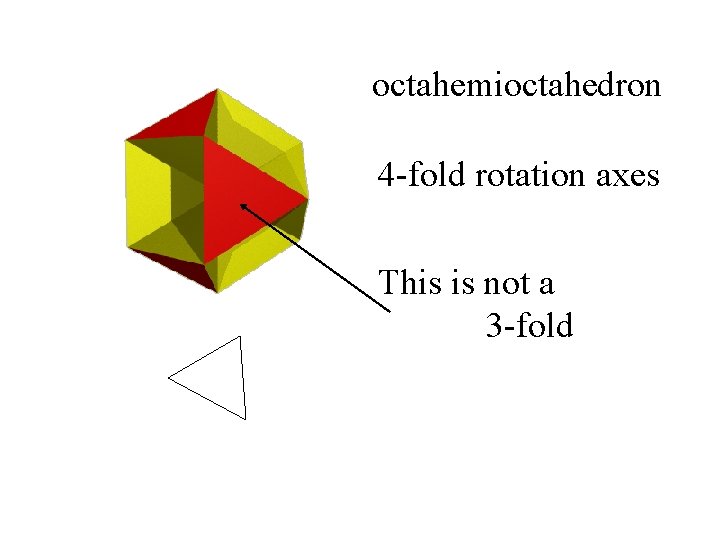
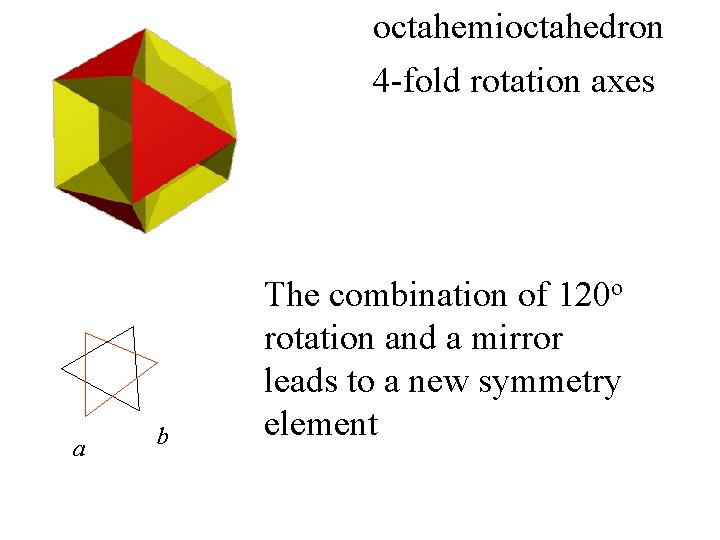
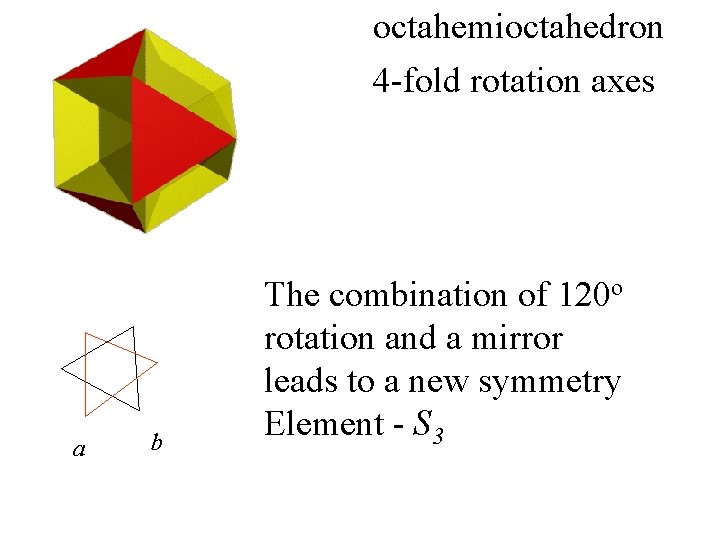
octahemioctahedron

octahemioctahedron 4 -fold rotation axes

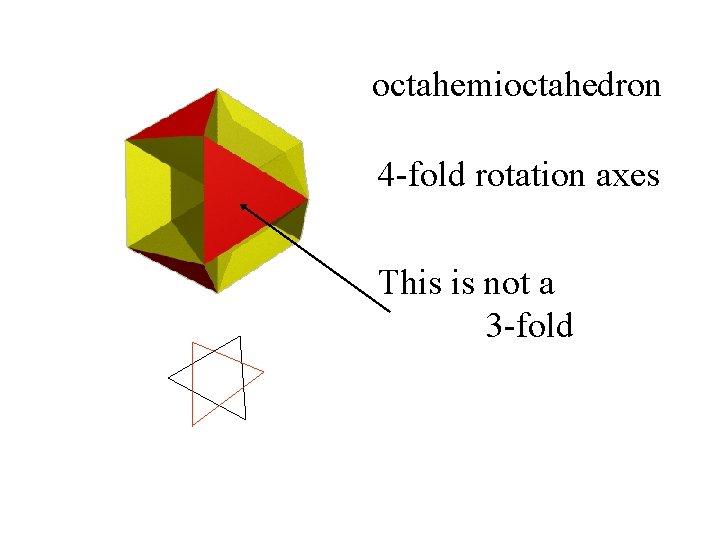
octahemioctahedron 4 -fold rotation axes This is not a 3 -fold

octahemioctahedron 4 -fold rotation axes This is not a 3 -fold

octahemioctahedron 4 -fold rotation axes This is not a 3 -fold

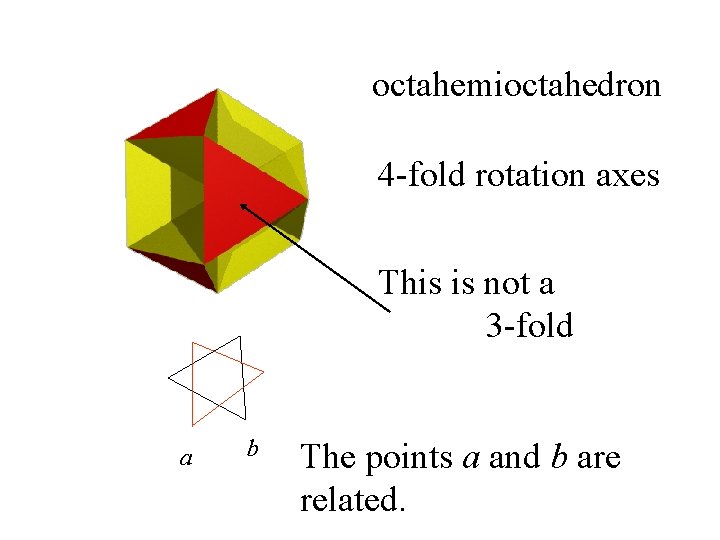
octahemioctahedron 4 -fold rotation axes This is not a 3 -fold a b The points a and b are related.

octahemioctahedron 4 -fold rotation axes a b The combination of 120 o rotation and a mirror leads to a new symmetry element

octahemioctahedron 4 -fold rotation axes a b The combination of 120 o rotation and a mirror leads to a new symmetry Element - S 3

Symmetry elements to look forrotations mirrors inversions


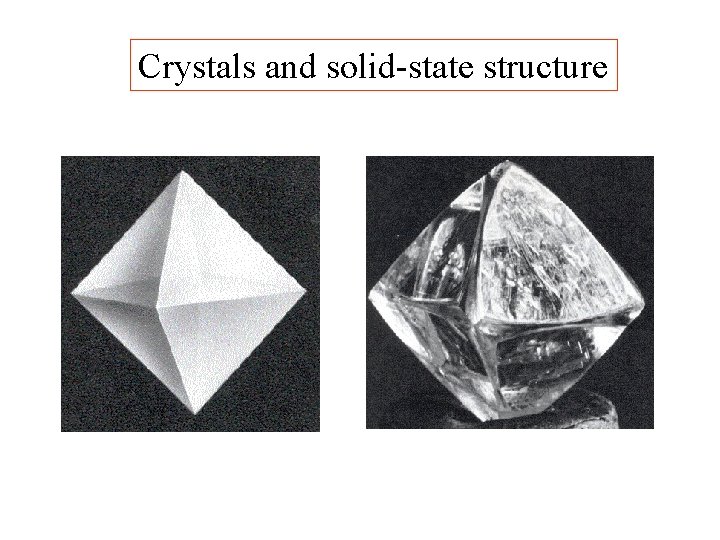
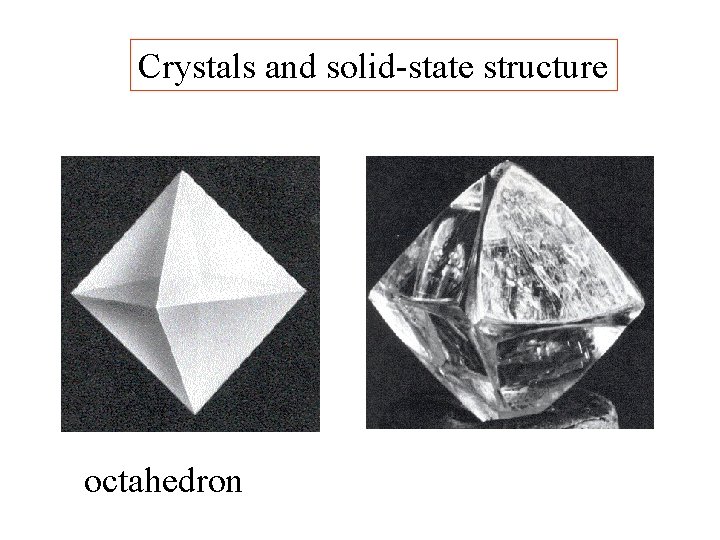
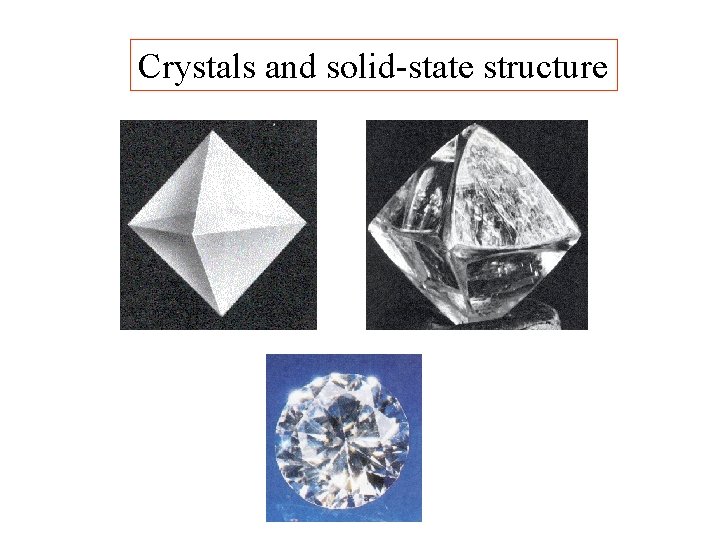
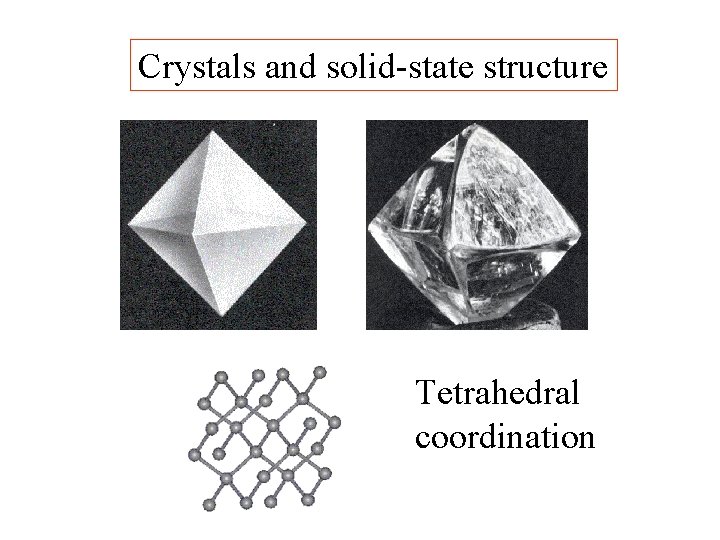
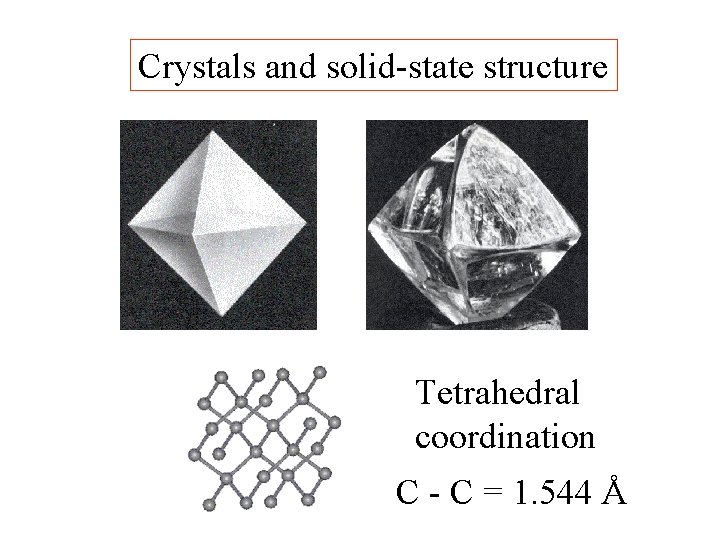
Crystals and solid-state structure

Crystals and solid-state structure octahedron

Crystals and solid-state structure

Crystals and solid-state structure Tetrahedral coordination

Crystals and solid-state structure Tetrahedral coordination C - C = 1. 544 Å
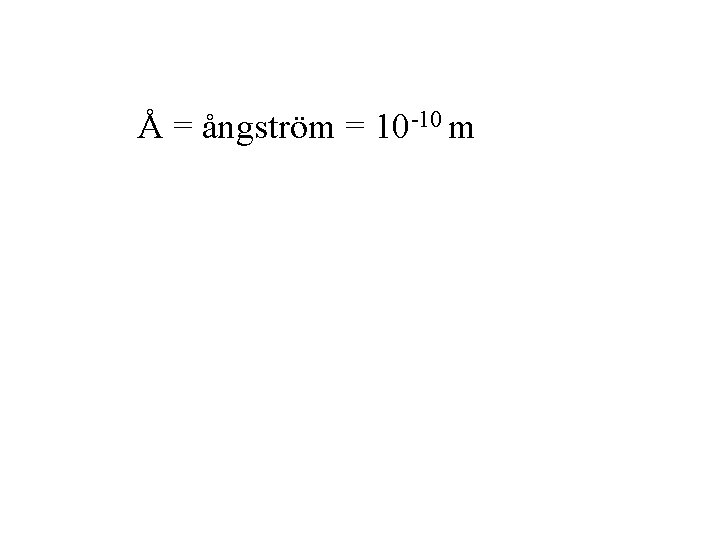
Å = ångström = 10 -10 m

Å = ångström = 10 -10 m The ångström is a useful unit when describing bonding distances.

Symmetry of a tetrahedron

Tetrahedrons and cubes have 3 -fold axes of symmetry

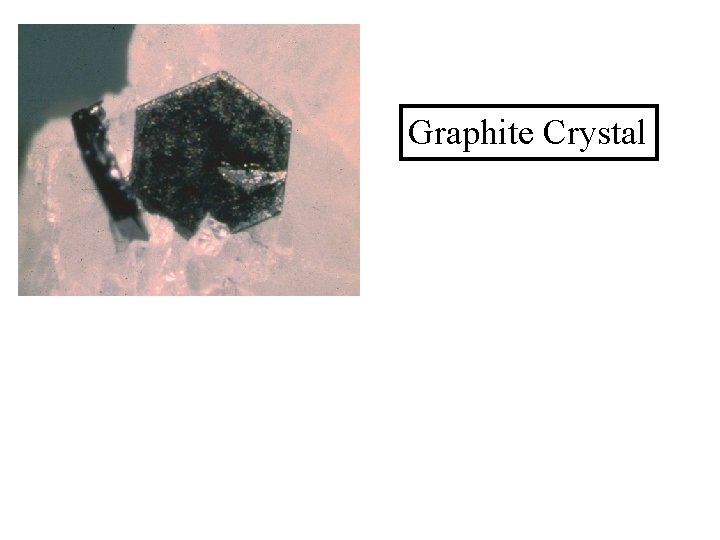
Graphite Crystal

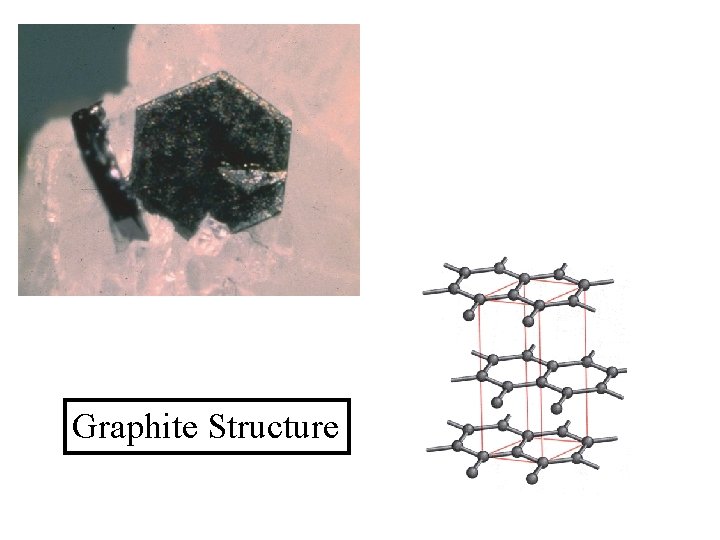
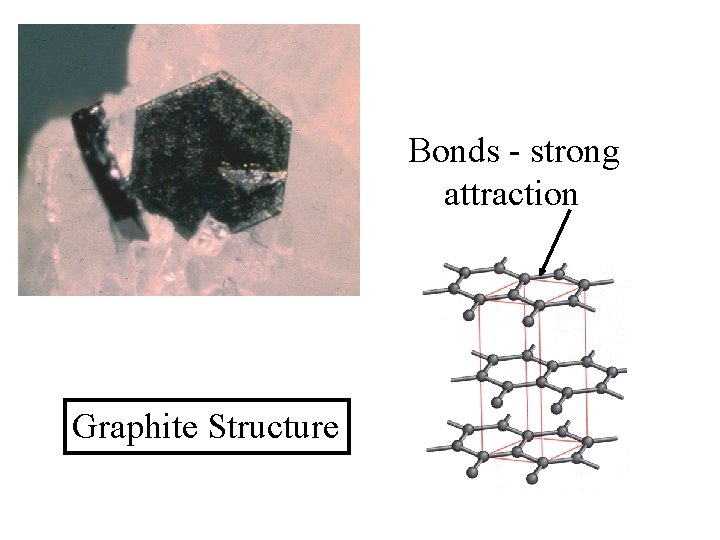
Graphite Structure

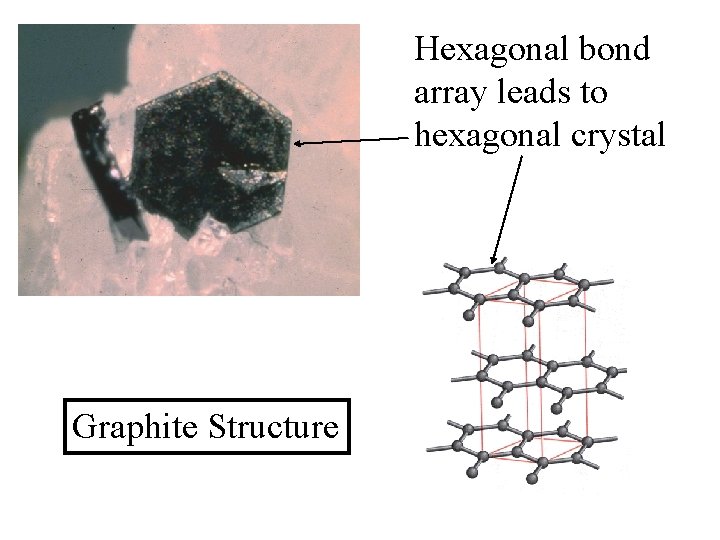
Hexagonal bond array leads to hexagonal crystal Graphite Structure

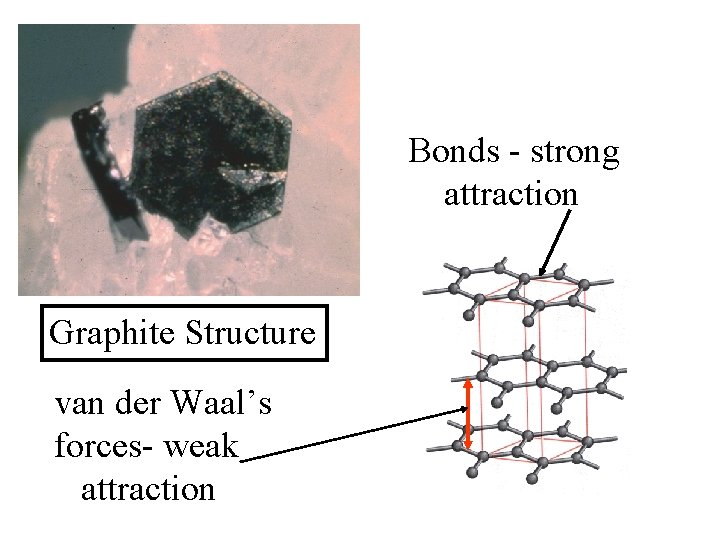
Bonds - strong attraction Graphite Structure

Bonds - strong attraction Graphite Structure van der Waal’s forces- weak attraction

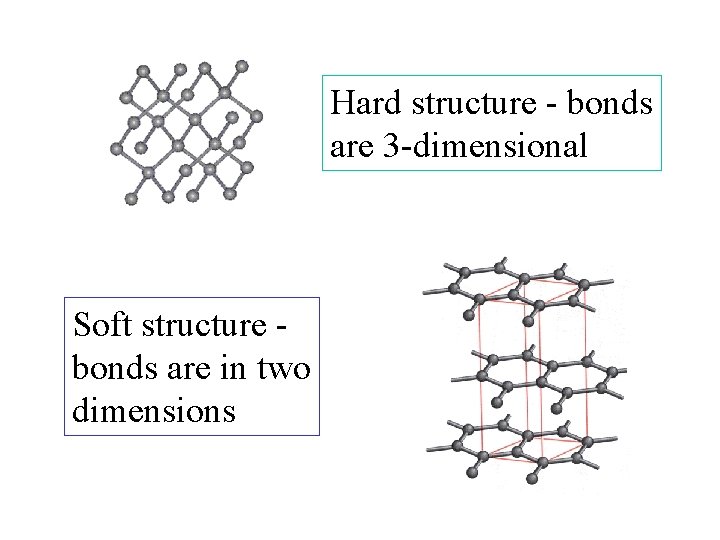
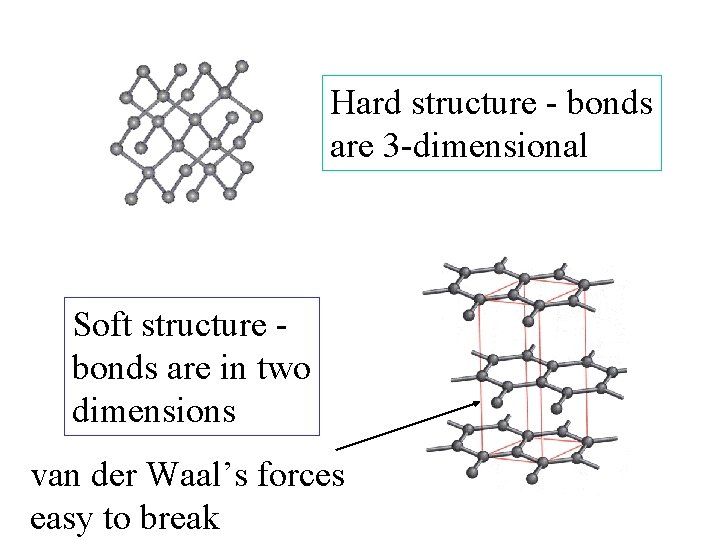
Hard structure - bonds are 3 -dimensional Soft structure bonds are in two dimensions

Hard structure - bonds are 3 -dimensional Soft structure bonds are in two dimensions van der Waal’s forces easy to break

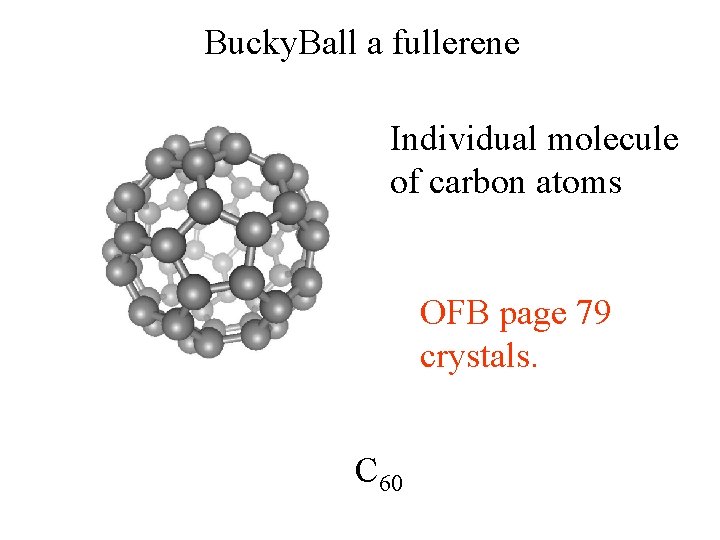
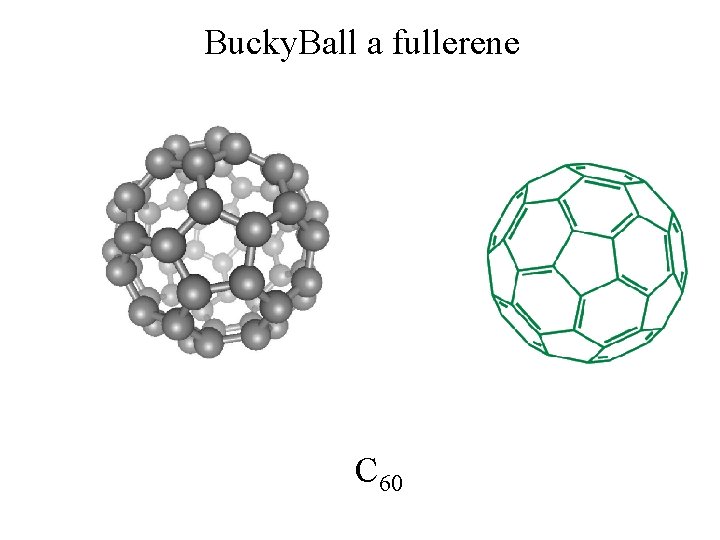
Bucky. Ball a fullerene Individual molecule of carbon atoms OFB page 79 crystals. C 60

Bucky. Ball a fullerene C 60


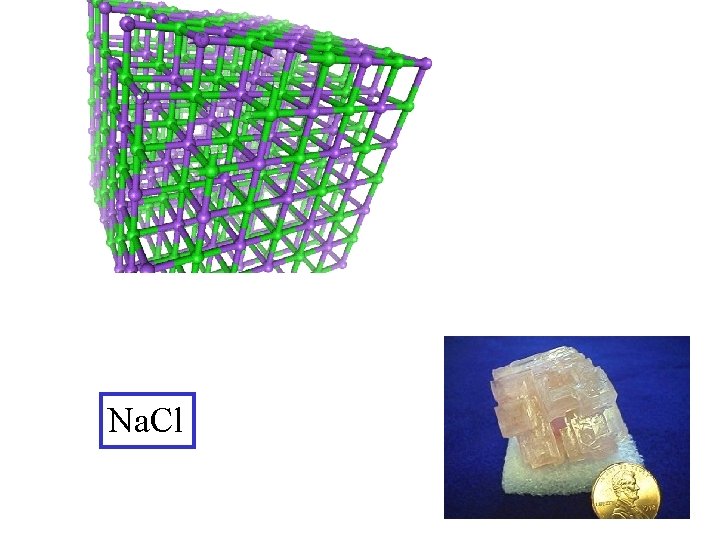
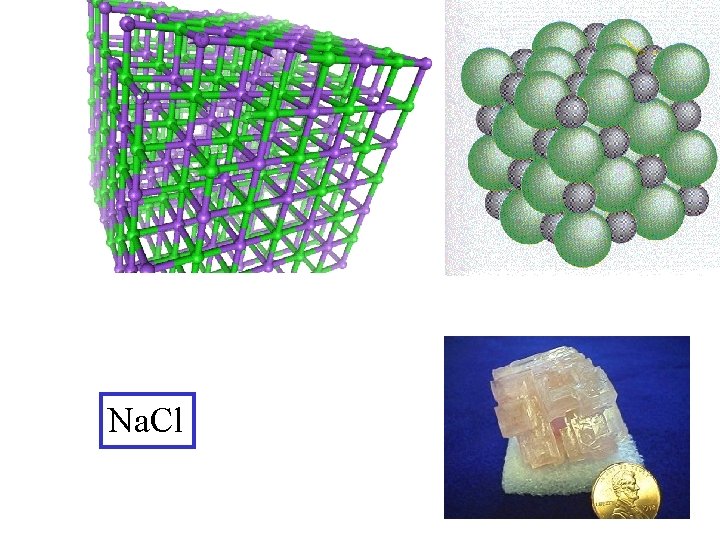
Na. Cl

Na. Cl




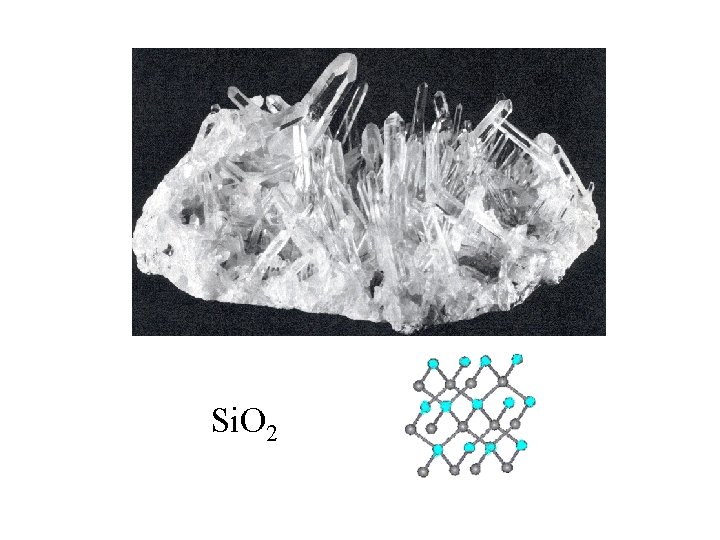
Si. O 2

Si. O 2
