Reaction Rate Chemistry Energy and Chemical Reactions Reaction
























- Slides: 45

Reaction Rate Chemistry Energy and Chemical Reactions

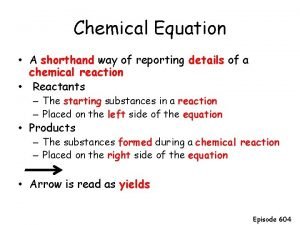
Reaction Rate The reaction rate for a chemical system is defined as the time that it takes for the reactants to be converted into products. The faster this occurs, the faster the chemical reaction, and vice versa. NO CHEMICAL REACTION IS INSTANTANEOUS.

Reaction Rates The reason for this is that in order for a chemical reaction to occur: 1. Compounds must separate-this requires energy, and work is done. Heat =Energy =W=Force X Distance 2. Particles are broken apart and separated by DIPOLAR WATER MOLECULES. 3. See next slide 3. Ions must fight through the water to recombine. 4. All of this takes time.

Dissolving For any chemical reaction to occur, two things must happen. Particles must separate and then they must recombine. The process of dissolving is different for all molecules. The reason being is that not all chemical bonds have the same strength. Some atoms will have a 3+ charge while others have a 1+ charge.

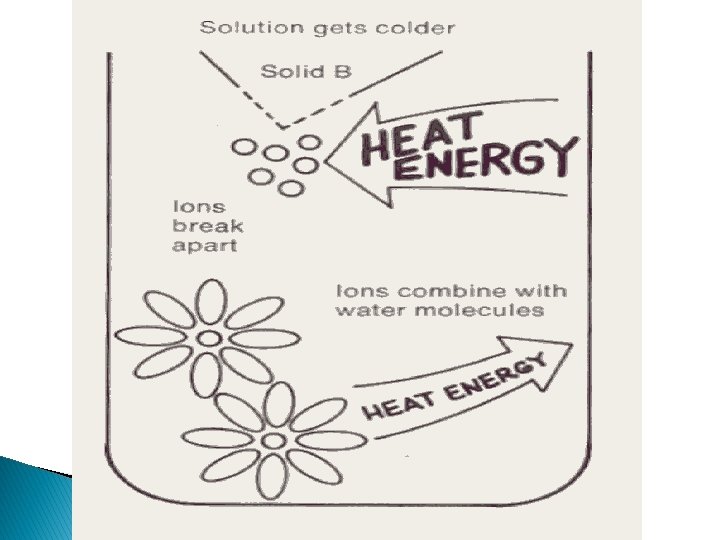
Dissolving For the actual particles to move apart, energy is needed. In this case, the water molecules will use energy to pull the particles apart. When they do this, the temperature of a liquid will go down as heat is removed from the water. When the water molecules surround the ions, they combine with a weak bond, and heat is given back to the water.

Dissolving Depending on the strength of the bond, the dissolving may use a lot or little energy. A strong bond will use a lot of heat energy and the temperature of the beaker will drop. As the ion recombines with water, energy is given back to the solution. If this energy is LESS than what was used to separate the compound, the overall net temperature will go down. The reverse will cause a temperature increase. (You can see these on slides 18 and 24).

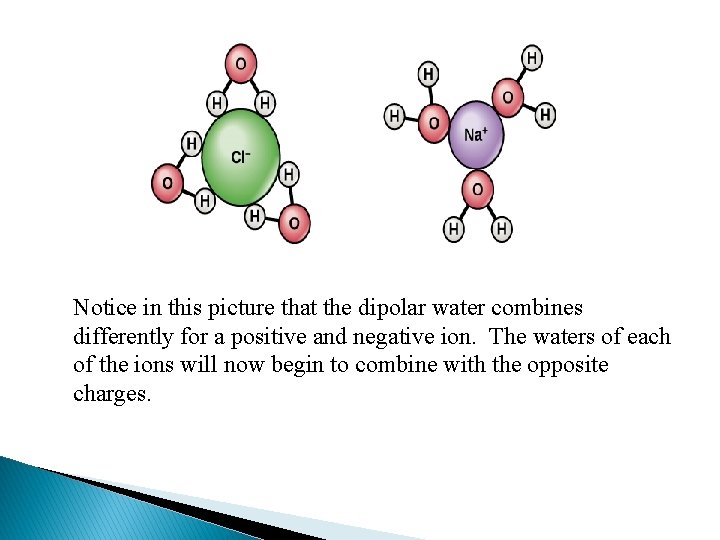
Here you can see an IONIC compound, salt. The dipolar water molecules pull the ions from the crystalline solid. Since the water itself is dipolar, they now combine with the water and keep the particles from recombining.

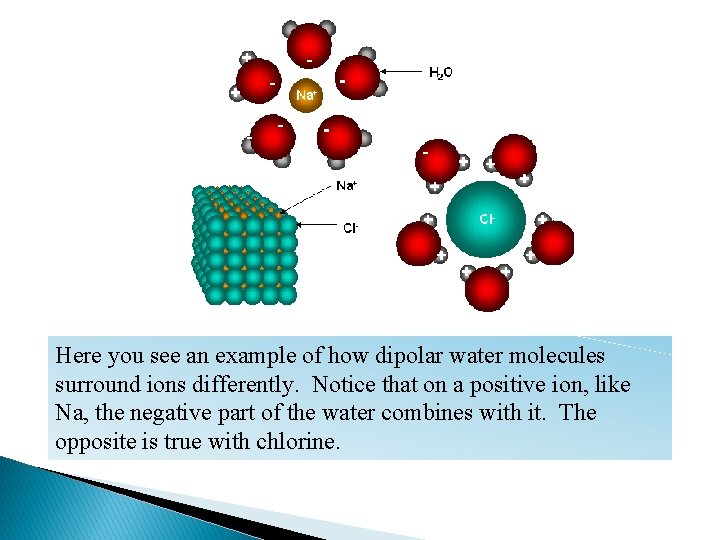
Notice in this picture that the dipolar water combines differently for a positive and negative ion. The waters of each of the ions will now begin to combine with the opposite charges.

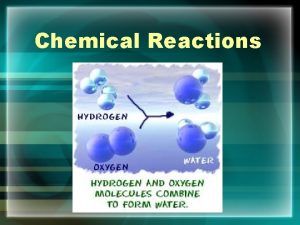
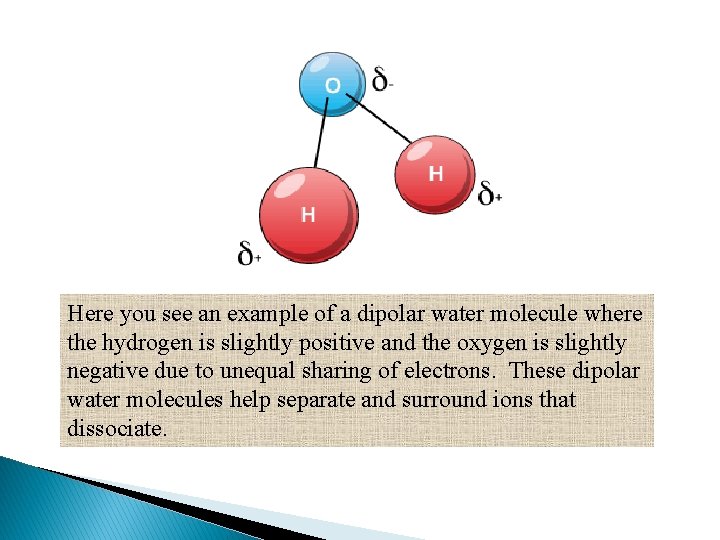
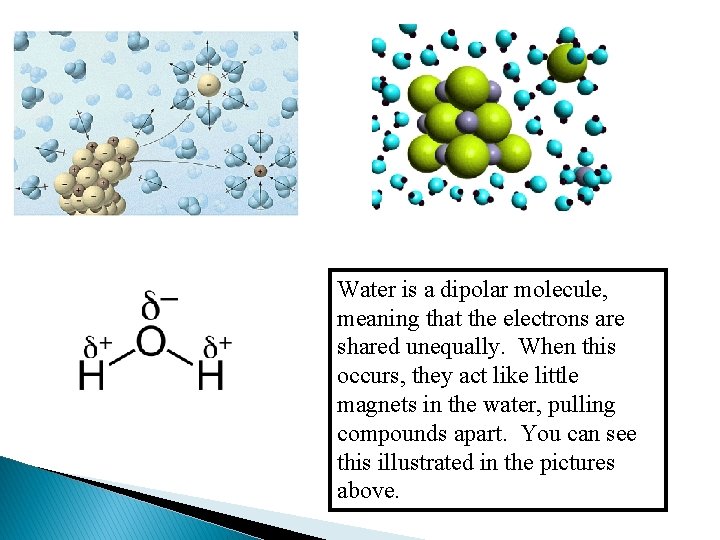
Here you see an example of a dipolar water molecule where the hydrogen is slightly positive and the oxygen is slightly negative due to unequal sharing of electrons. These dipolar water molecules help separate and surround ions that dissociate.

Here you see an example of how dipolar water molecules surround ions differently. Notice that on a positive ion, like Na, the negative part of the water combines with it. The opposite is true with chlorine.


Water is a dipolar molecule, meaning that the electrons are shared unequally. When this occurs, they act like little magnets in the water, pulling compounds apart. You can see this illustrated in the pictures above.

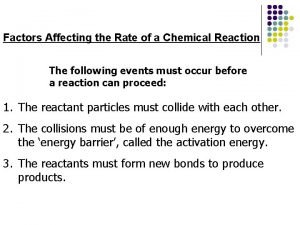
Collision Theory The collision theory relates the rates of particle contact to reaction rate. Anything that increases particle contact will increase the rate of reaction. Anything that decreases particle contact will decrease the rate of reaction.

In the photos above you can see examples of everyday occurrences. On the left, rusting, which has a slow reaction rate, as opposed to burning which is fast.

Collision Theory Stated another way, the Collision Theory states that anything done to alter particle contact will affect the reaction rate. Reaction Rate is the time that it takes for reactants to turn into products.

Activation Energy Activation energy is the minimum energy that is needed for a reaction to occur. In order for a reaction to occur, particles must separate, move, and recombine. This means that particles must hit with sufficient energy as well as with the proper angle.

Energy is always involved in chemical reactions. All reactions are classified depending on whether energy is absorbed or released. Regardless, in any reaction, energy is neither created nor destroyed. It is merely changed from one form to another.

Temperature In the past, temperature has been defined as the “speed or vibration of particles”. While this is somewhat correct, it is not totally correct. Temperature is actually a measure of the total KINETIC ENERGY of a system. To illustrate why this is a better definition, consider two cars of different size and mass. Each car is moving exactly at the same speed. The larger car has more total energy than the smaller one so it would have a higher temperature.

Exothermic Reactions A chemical reaction where more heat energy is released than is absorbed is EXOTHERMIC. When a reaction takes place, the atoms and molecules rearrange. When the energy is released, the molecules in the products have less energy than the molecules in the reactants. See next slide.

Exothermic Dissolving T e m p e r a t u r e Initial Temperature Final Temperature Net Temperature Change Particles Recombine with Particles Water Molecules Separate Time of dissolving

Energy is needed to separate the particles since work is being done. Work = Force x Distance Particles are being separated and recombined. Another way to visualize this is that it takes more energy for the particles to separate than they release when they recombine. Most of the time this energy is heat energy, so the net temperature change in an exothermic reaction will increase. See next slide.


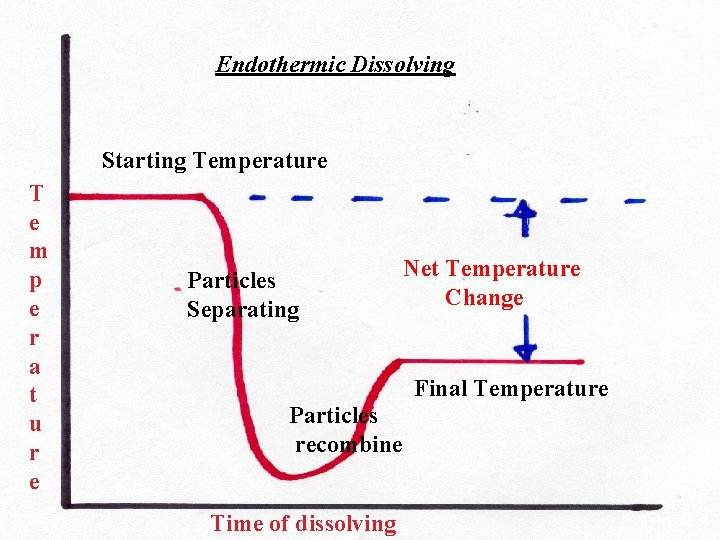
Endothermic Reactions An endothermic reaction is one where energy is absorbed rather than released. It gets COLDER. This means that the energy of the products is now less than the energy of the reactants. If the energy involved is heat energy, the products would appear colder than the reactants. See next slide.

Endothermic Dissolving Starting Temperature T e m p e r a t u r e Particles Separating Net Temperature Change Final Temperature Particles recombine Time of dissolving

With that in mind, the opposite is true for an endothermic reaction. It takes more heat energy to separate the compounds (why? ) than they liberate into the surrounding solution. Since the heat energy is taken from the water, the net temperature difference is a drop in temperature. The solution becomes colder. See next slide.


How to Remember Them � Think of it this way. When there is a fire and it gets hot, you EXO (exit) the building. Hot = Exothermic � When you are cold outside you go ENDO (into) the house. Cold = Endothermic � If you think these are bad, do better, and let me know.

Altering Particle Contact There are three ways main methods to alter particle contact and therefore alter the reaction rate. They are: Δ Surface Area Δ Temperature Δ Concentration Δ means “change”

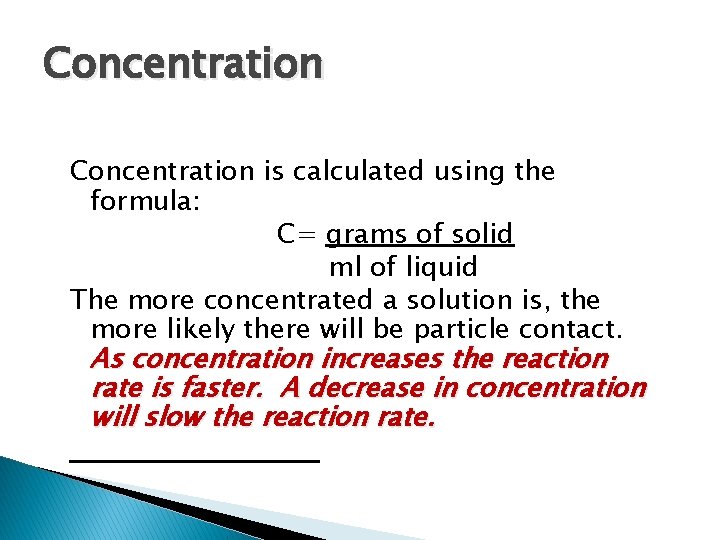
Concentration is calculated using the formula: C= grams of solid ml of liquid The more concentrated a solution is, the more likely there will be particle contact. As concentration increases the reaction rate is faster. A decrease in concentration will slow the reaction rate.

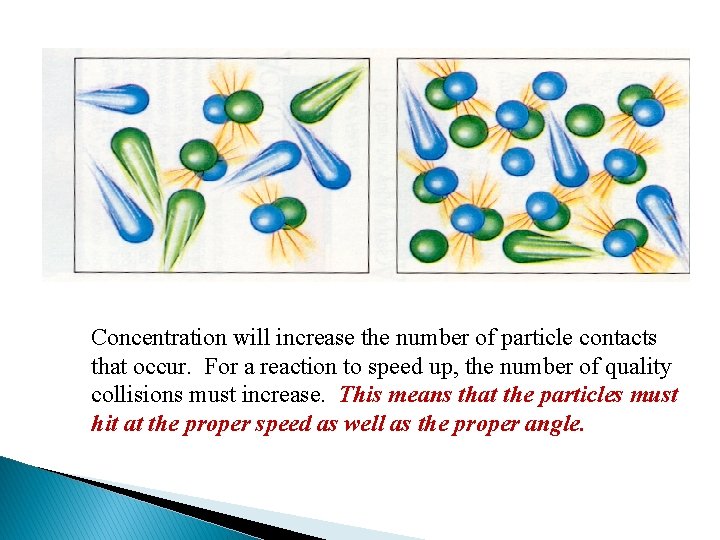
Concentration will increase the number of particle contacts that occur. For a reaction to speed up, the number of quality collisions must increase. This means that the particles must hit at the proper speed as well as the proper angle.

Temperature is defined as a measure of the vibration or motion of atoms. If atoms are moving quickly, they have a lot of energy, and the temperature is high. If the particles have low energy, they are moving slowly, and therefore have a lower temperature. If atoms are moving quickly, more collisions are likely to occur and those collisions will be high energy collisions.

As the temperature increases, particles are moving faster and are more likely to collide with each other. This results in a faster reaction rate. As the temperature decreases, particle motion slows and atoms are less likely to collide. This means that even if a collision occurs, there is not enough energy for the reaction to occur. The result is a slowing of the reaction rate.

Catalysts and Enzymes Catalysts and enzymes are another way to alter reaction rates. Enzymes are custom made catalysts made from proteins by living things. Catalysts are substances that speed reactions but they are not custom made.

Catalysts are substances that, when used in small amounts, speed up reaction rates. It is not fully understood how catalysts work but they do have certain things in common. 1. They are specific. They will not work for all reactions. 2. They are used in small amounts.

3. Catalysts are not used up in a chemical reaction. They are unchanged. Stated another way, catalysts are not reactants. They exist in the products as they did in the reactants. 4. Catalysts do not cause a reaction to occur. 5. Catalysts are not affected by heat.

A possible theory as to how a catalyst might work is this. Consider this photo to be representative of the surface of a catalyst. As compounds move across its surface, they encounter ridges holes and a very uneven surface. (continued next slide)

How a catalyst might work In moving over this surface, the compounds twist, turn, and eventually separate. Since the surface is uneven, charges are uneven as well. Reactants from other substances adhere to the surface and are more likely to combine. After they combine, they move off of the surface of the catalyst due to a new shape of the new compound that forms.

Enzymes are similar to catalysts that are made by living things. The greatest difference between a catalyst and an enzyme is that enzymes can be regulated whereas catalysts cannot. Enzymes have endings that end with “ase”. ase Example: Salivary amylase. ase Enzymes are also different from catalysts in that they tend to be sensitive to heat.

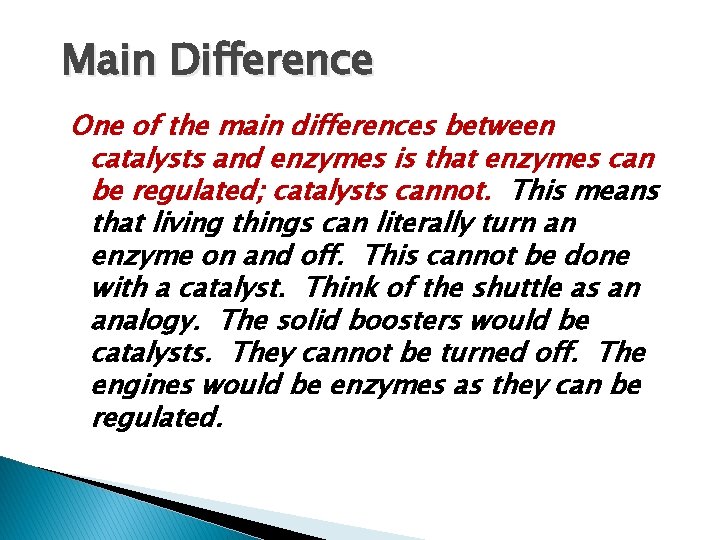
Main Difference One of the main differences between catalysts and enzymes is that enzymes can be regulated; catalysts cannot. This means that living things can literally turn an enzyme on and off. This cannot be done with a catalyst. Think of the shuttle as an analogy. The solid boosters would be catalysts. They cannot be turned off. The engines would be enzymes as they can be regulated.

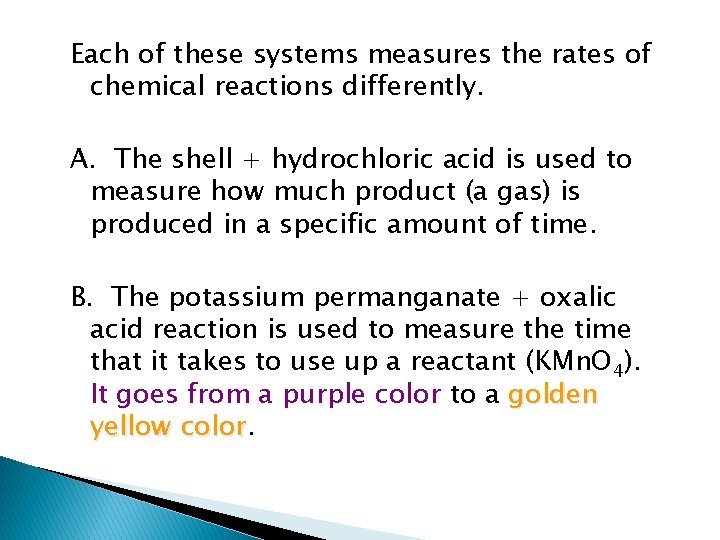
Proof from lab Your lab was intended to verify the collision theory using three different chemical systems. I. HCl + Shell ----> fizz (carbon dioxide) II. KMn. O 4 + H 2 C 2 O 4 ----> golden color III. KI + K 2 S 2 O 8 + starch ----> black

Each of these systems measures the rates of chemical reactions differently. A. The shell + hydrochloric acid is used to measure how much product (a gas) is produced in a specific amount of time. B. The potassium permanganate + oxalic acid reaction is used to measure the time that it takes to use up a reactant (KMn. O 4). It goes from a purple color to a golden yellow color

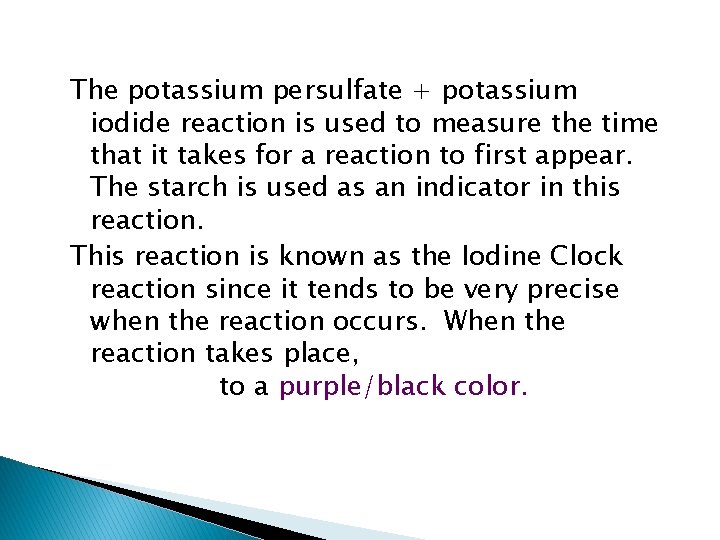
The potassium persulfate + potassium iodide reaction is used to measure the time that it takes for a reaction to first appear. The starch is used as an indicator in this reaction. This reaction is known as the Iodine Clock reaction since it tends to be very precise when the reaction occurs. When the reaction takes place, it goes from colorless reactants to a purple/black color.

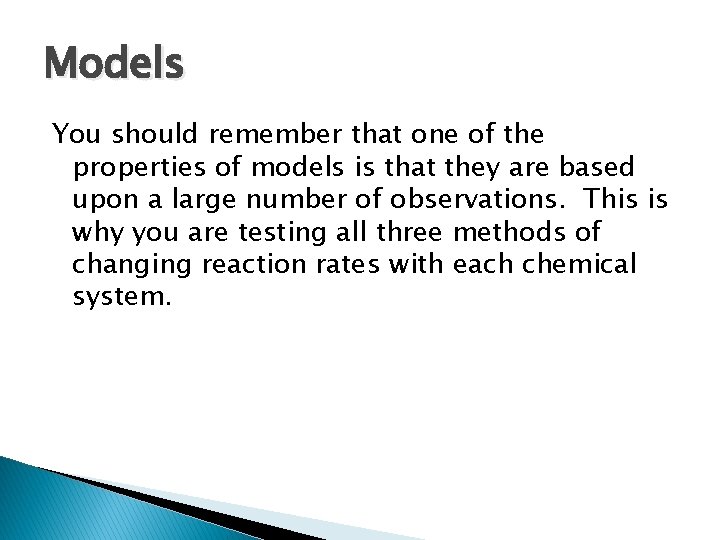
Models You should remember that one of the properties of models is that they are based upon a large number of observations. This is why you are testing all three methods of changing reaction rates with each chemical system.

Conclusions You should have learned that in each of the systems that anything that was done to increase particle contact also increased the reaction rate. The converse is also true. Anything done to decrease particle contact, decreased the reaction rate.

Speed of the Reactions You also should have concluded with several different systems that although you can speed a chemical system, you can only speed it up to a point. NO CHEMICAL REACTION IS INSTANTANEOUS.
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Types of reactions
Types of reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Enzym
Enzym Neutron emission
Neutron emission A chemist shorthand way of
A chemist shorthand way of Rate of reaction graph
Rate of reaction graph Redox reactions examples
Redox reactions examples Reaction order
Reaction order Reactants and products
Reactants and products Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Unit 5 chemical reactions
Unit 5 chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions of copper and percent yield report sheet
Chemical reactions of copper and percent yield report sheet Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Usable chemical energy in food begins as __________ energy.
Usable chemical energy in food begins as __________ energy. ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Potential energy examples
Potential energy examples Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy 4 types of chemical reactions
4 types of chemical reactions 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Types of reactions chemistry
Types of reactions chemistry Chemistry reactions
Chemistry reactions Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Factors that affect the rate of reactions
Factors that affect the rate of reactions Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Types of redox reactions
Types of redox reactions Types of reaction
Types of reaction Types of chemical reactions
Types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions