Rate of Chemical Reactions Rate of Chemical Reactions




- Slides: 15

Rate of Chemical Reactions

Rate of Chemical Reactions �The rate of reaction is a measure of how rapidly a reaction takes place. �Activation energy - is the minimum amount of energy needed for substances to react.

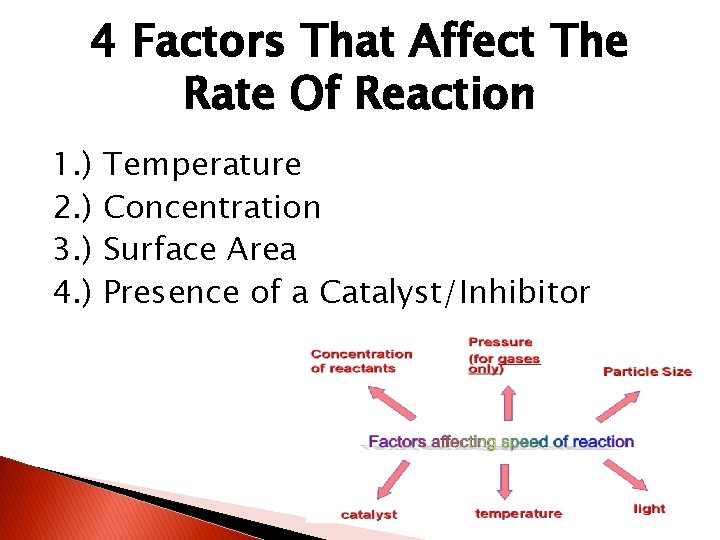
4 Factors That Affect The Rate Of Reaction 1. ) 2. ) 3. ) 4. ) Temperature Concentration Surface Area Presence of a Catalyst/Inhibitor

Temperature �An increase in temperature increases the rate of a reaction. �Particles of reactants will move faster and collide more frequently and with more energy.

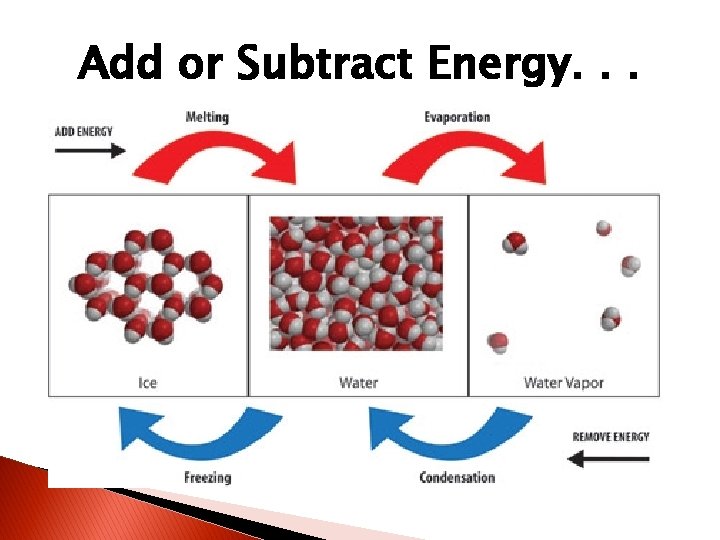
Solid + Energy = ? �When energy is added to solids, they become liquids! �Examples?

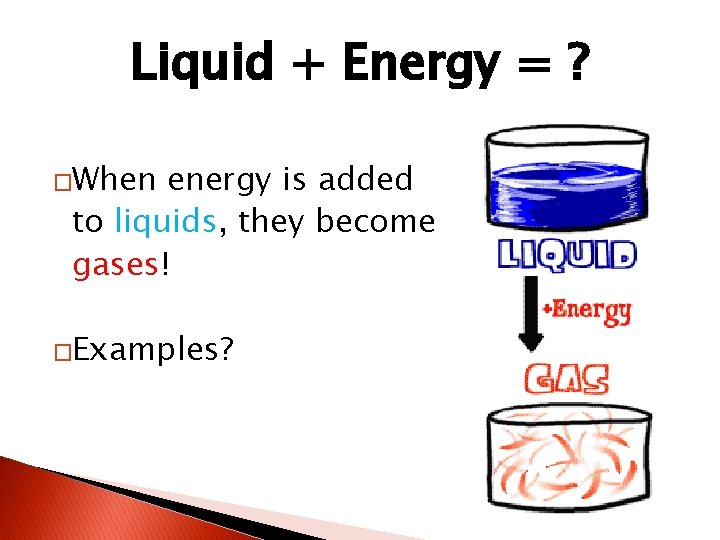
Liquid + Energy = ? �When energy is added to liquids, they become gases! �Examples?

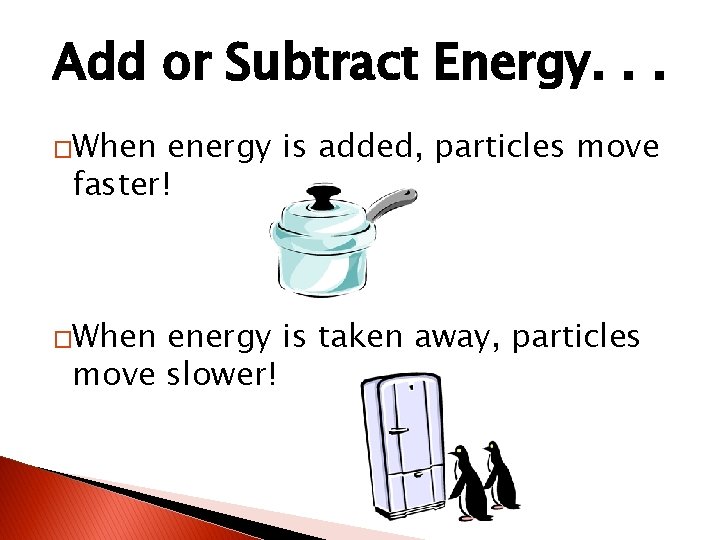
Add or Subtract Energy. . . �When energy is added, particles move faster! �When energy is taken away, particles move slower!

Add or Subtract Energy. . .

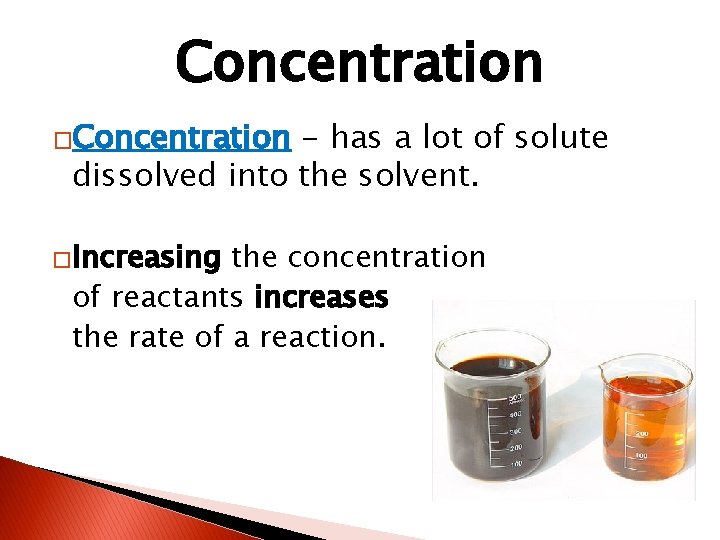
Concentration �Concentration - has a lot of solute dissolved into the solvent. �Increasing the concentration of reactants increases the rate of a reaction.

Surface Area �Increasing the surface area of the solid increases the chances of collision taking place. �The more finely divided the solid is, the faster the reaction happens.

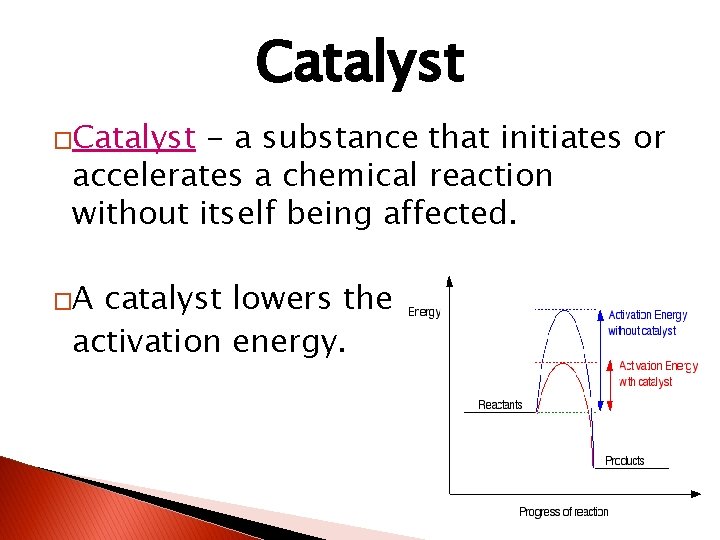
Catalyst �Catalyst - a substance that initiates or accelerates a chemical reaction without itself being affected. �A catalyst lowers the activation energy.

Inhibitor �Inhibitor is a substance that slows down or stops a chemical reaction.

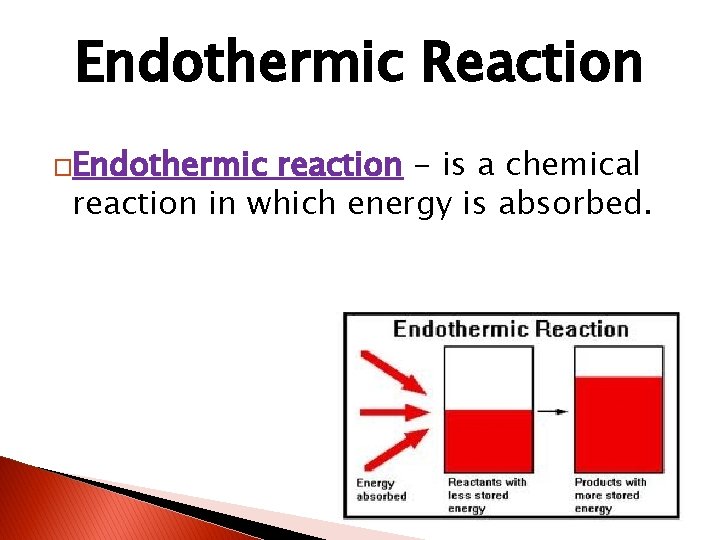
Endothermic Reaction �Endothermic reaction - is a chemical reaction in which energy is absorbed.

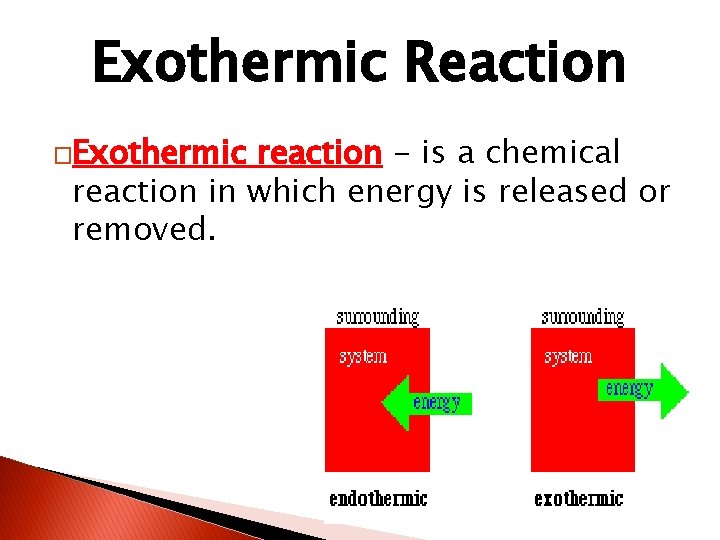
Exothermic Reaction �Exothermic reaction - is a chemical reaction in which energy is released or removed.

� Endo vs. Exo � http: //www. youtube. com/watch? v=L-G 7 p. Luf. XAo � http: //www. youtube. com/watch? v=zns. Pa 1 BSa. IM � http: //www. youtube. com/watch? v=My. Azj. Sdc 3 Fc&feature=related � https: //prezi. com/yn 4 sef 5 oddss/exothermic-and- endothermic-reactions/
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Examples of redox reaction
Examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Factors that affect the rate of reactions
Factors that affect the rate of reactions Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry Types of chemical reactions
Types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions





























