Polycystic ovarian syndrome and nonclassic congenital adrenal hyperplasia






















- Slides: 72

Polycystic ovarian syndrome and non-classic congenital adrenal hyperplasia: Challenges of diagnosis and management Serwa Gyamfi, MD and Sharyn Malcolm, MD, MPH Children’s National Health System Division of Adolescent and Young Adult Medicine Washington, DC American College Health Association’s Annual Meeting June 1 st 2018

Serwa Gyamfi, MD and Sharyn Malcolm, MD, MPH Have documented no financial relationships to disclose or Conflicts of Interest (COIs) to resolve.

OBJECTIVES Recognize the clinical presentations of polycystic ovarian syndrome (PCOS) Recognize the clinical presentations of non-classic congenital adrenal hyperplasia (NCAH) Compare the diagnostic criteria for PCOS and NCAH Discuss treatment modalities and their effectiveness for PCOS and NCAH

PCOS is the most common endocrine disorder of reproductive age females

PCOS OBJECTIVES • Define the entity of PCOS • List the different diagnostic criteria for PCOS • Understand the pathophysiologic theories of PCOS • Understand the approach to work-up of a patient with characteristics of PCOS • Understand the different treatment modalities in the management of PCOS and potential psychopharmacologic medication interactions

CASE • An 18 y/o freshman presents for her annual visit but is also concerned that she may be depressed. • She had menarche at age 14 and she has had menses every 2 months for the past 2 years, however has missed 4 months of her cycle most recently. • She has a BMI of 29 and in the past year has experience increased facial hair growth. She has never had acne. • She asks: “why do I have some much facial hair? ”

EPIDEMIOLOGY • PCOS occurs in 6 -10% of reproductive age women • Most common cause of excess androgen production • It is a leading cause of infertility in women • It is associated with: • Metabolic syndrome (cardiovascular disease, insulin resistance, sleepdisordered breathing and excessive daytime sleepiness, NAFLD) • Type 2 diabetes • Dysfunctional Uterine bleeding • Endometrial cancer • Psychosocial (depression, anxiety, cosmetic concerns)

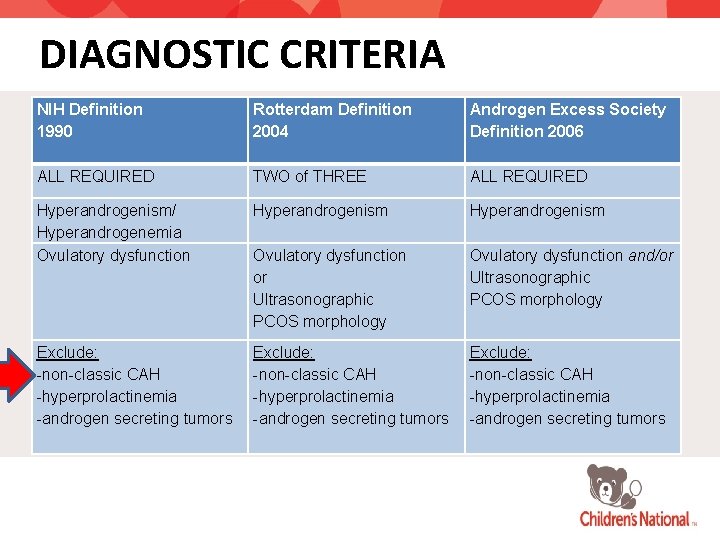
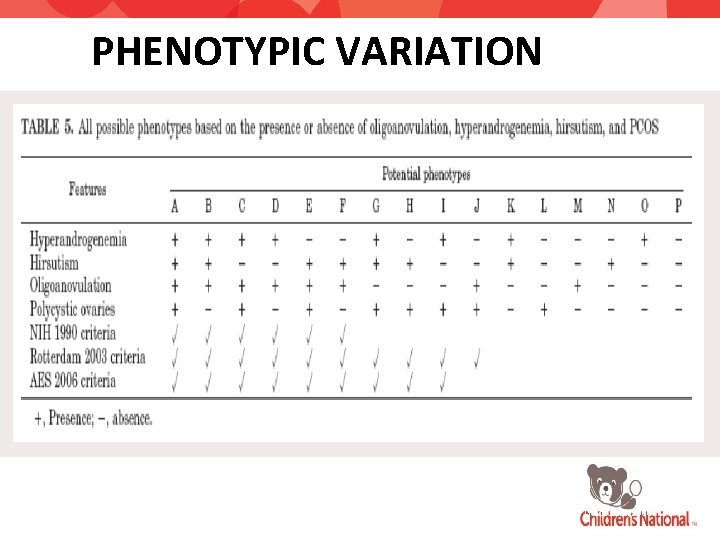
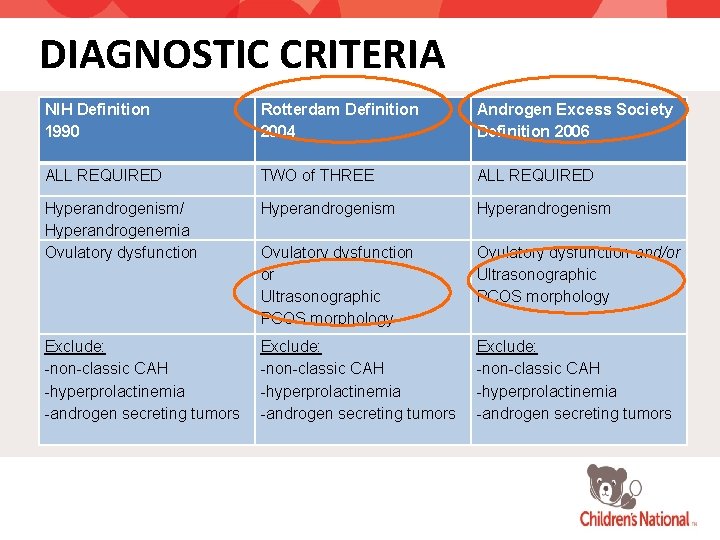
DIAGNOSTIC CRITERIA NIH Definition 1990 Rotterdam Definition 2004 Androgen Excess Society Definition 2006 ALL REQUIRED TWO of THREE ALL REQUIRED Hyperandrogenism/ Hyperandrogenemia Ovulatory dysfunction Hyperandrogenism Ovulatory dysfunction or Ultrasonographic PCOS morphology Ovulatory dysfunction and/or Ultrasonographic PCOS morphology Exclude: -non-classic CAH -hyperprolactinemia -androgen secreting tumors

OBJECTIVES • Understand the pathophysiologic theories of PCOS

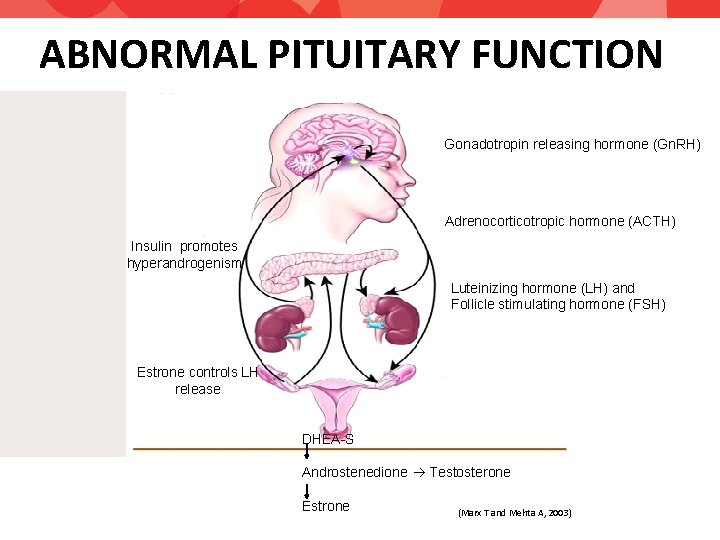
THEORIES OF PATHOPHYSIOLOGY • Abnormal pituitary function • Abnormal steroidogenesis • Ovaries • Adrenals • Insulin Resistance • Chromosome 2 p 16. 3?

ABNORMAL PITUITARY FUNCTION Gonadotropin releasing hormone (Gn. RH) Adrenocorticotropic hormone (ACTH) Insulin promotes hyperandrogenism Luteinizing hormone (LH) and Follicle stimulating hormone (FSH) Estrone controls LH release DHEA-S Androstenedione Testosterone Estrone (Marx T and Mehta A, 2003)

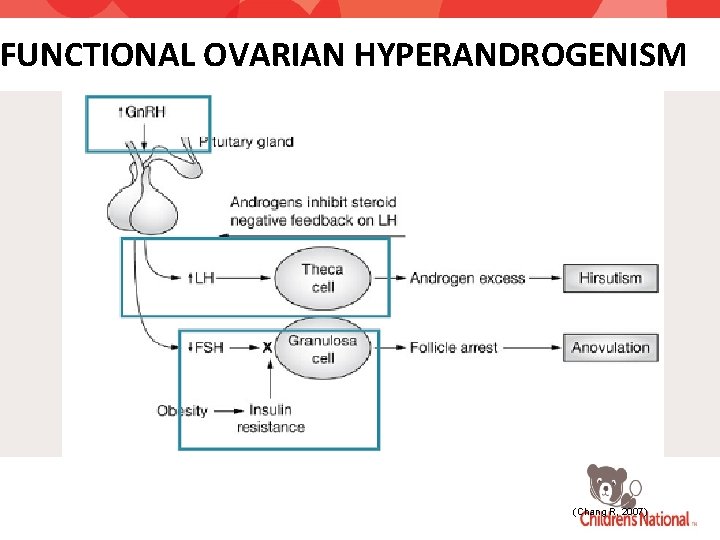
FUNCTIONAL OVARIAN HYPERANDROGENISM (Chang R, 2007)

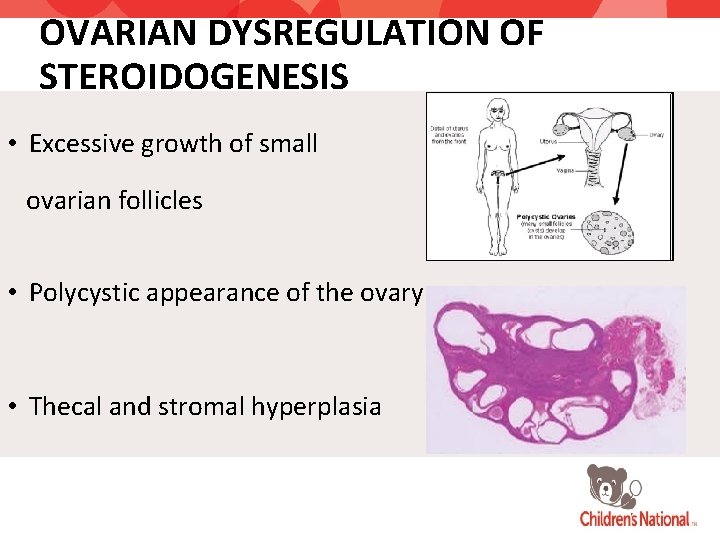
OVARIAN DYSREGULATION OF STEROIDOGENESIS • Excessive growth of small ovarian follicles • Polycystic appearance of the ovary • Thecal and stromal hyperplasia

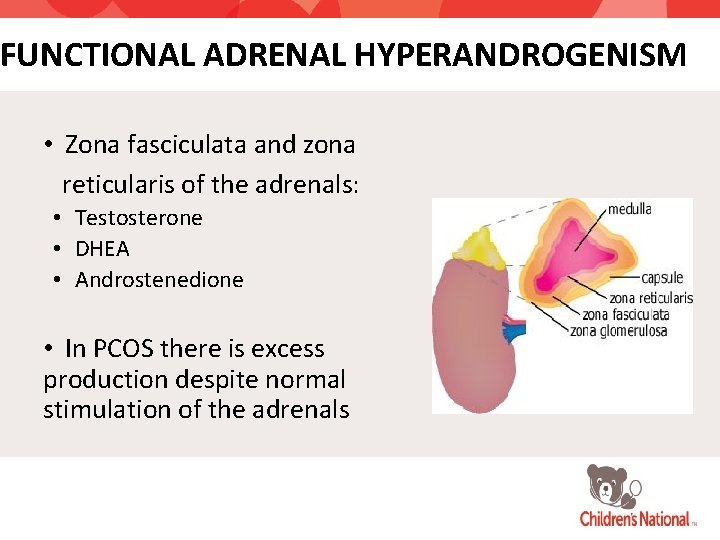
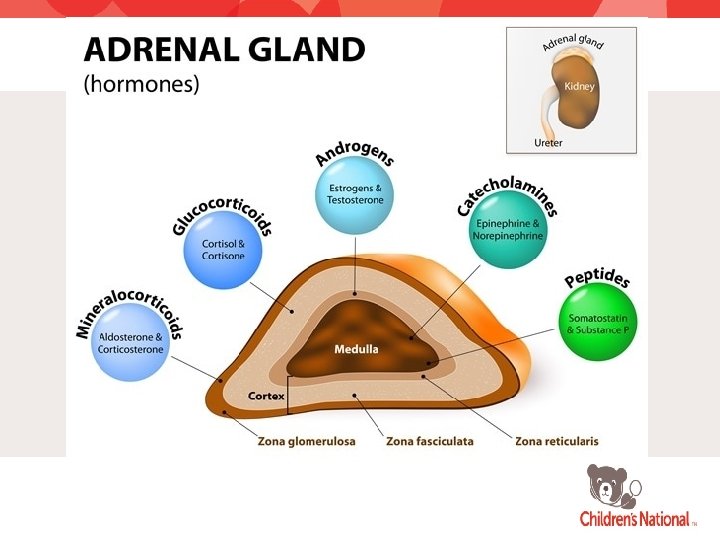
FUNCTIONAL ADRENAL HYPERANDROGENISM • Zona fasciculata and zona reticularis of the adrenals: • Testosterone • DHEA • Androstenedione • In PCOS there is excess production despite normal stimulation of the adrenals

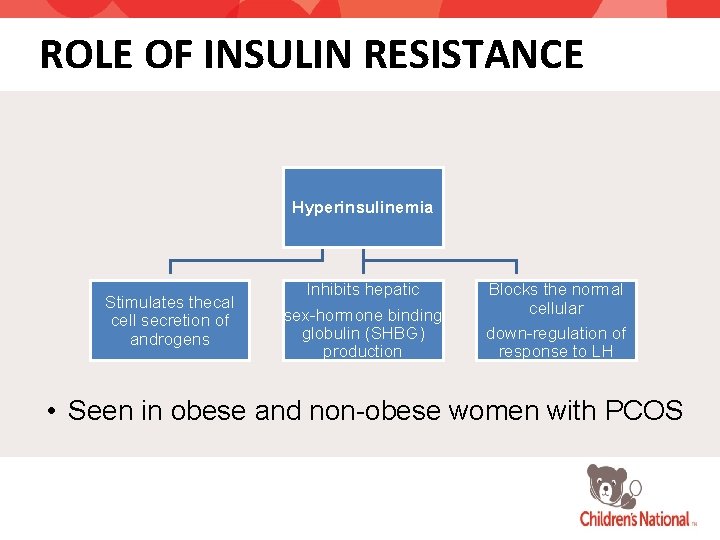
ROLE OF INSULIN RESISTANCE Hyperinsulinemia Stimulates thecal cell secretion of androgens Inhibits hepatic sex-hormone binding globulin (SHBG) production Blocks the normal cellular down-regulation of response to LH • Seen in obese and non-obese women with PCOS

OBJECTIVES • Understand the approach to work-up of a patient with characteristics of PCOS

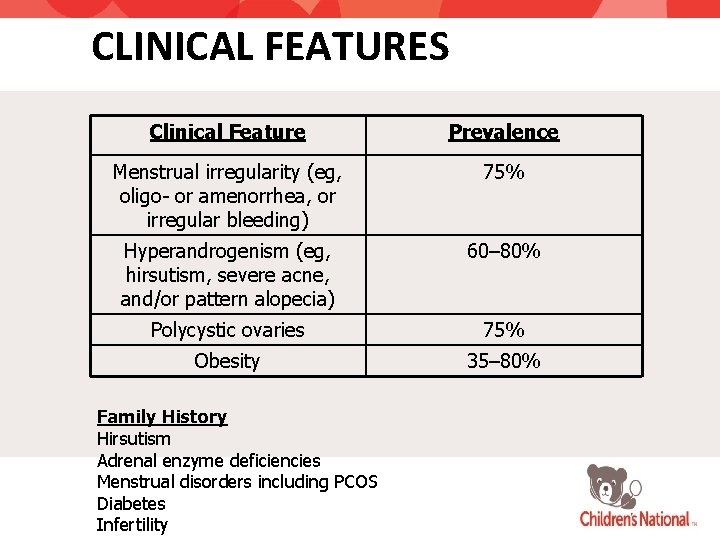
CLINICAL FEATURES Clinical Feature Prevalence Menstrual irregularity (eg, oligo- or amenorrhea, or irregular bleeding) 75% Hyperandrogenism (eg, hirsutism, severe acne, and/or pattern alopecia) 60– 80% Polycystic ovaries 75% Obesity 35– 80% Family History Hirsutism Adrenal enzyme deficiencies Menstrual disorders including PCOS Diabetes Infertility

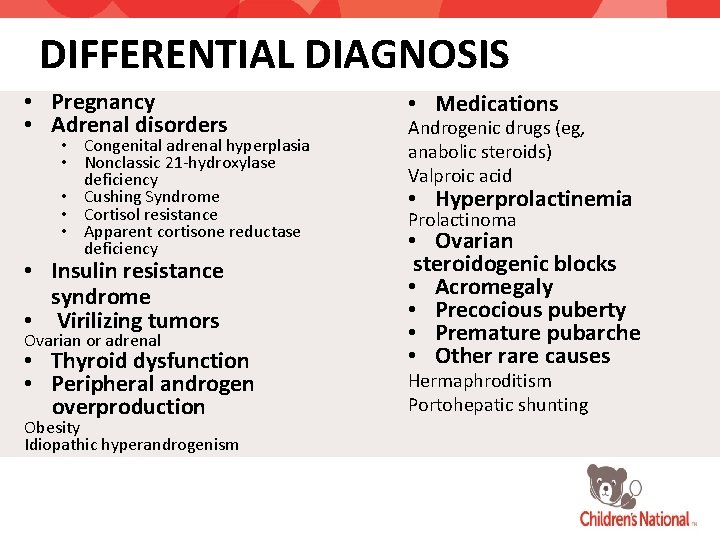
DIFFERENTIAL DIAGNOSIS • Pregnancy • Adrenal disorders • Congenital adrenal hyperplasia • Nonclassic 21 -hydroxylase deficiency • Cushing Syndrome • Cortisol resistance • Apparent cortisone reductase deficiency • Insulin resistance syndrome • Virilizing tumors Ovarian or adrenal • Thyroid dysfunction • Peripheral androgen overproduction Obesity Idiopathic hyperandrogenism • Medications Androgenic drugs (eg, anabolic steroids) Valproic acid • Hyperprolactinemia Prolactinoma • Ovarian steroidogenic blocks • Acromegaly • Precocious puberty • Premature pubarche • Other rare causes Hermaphroditism Portohepatic shunting

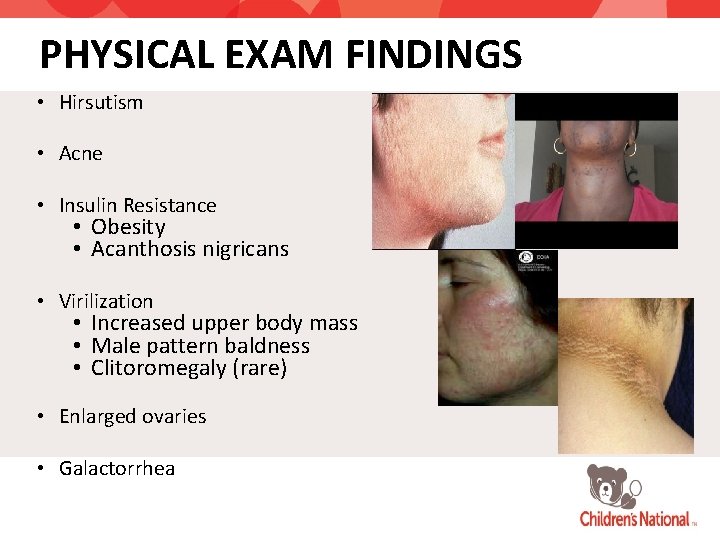
PHYSICAL EXAM FINDINGS • Hirsutism • Acne • Insulin Resistance • Obesity • Acanthosis nigricans • Virilization • Increased upper body mass • Male pattern baldness • Clitoromegaly (rare) • Enlarged ovaries • Galactorrhea

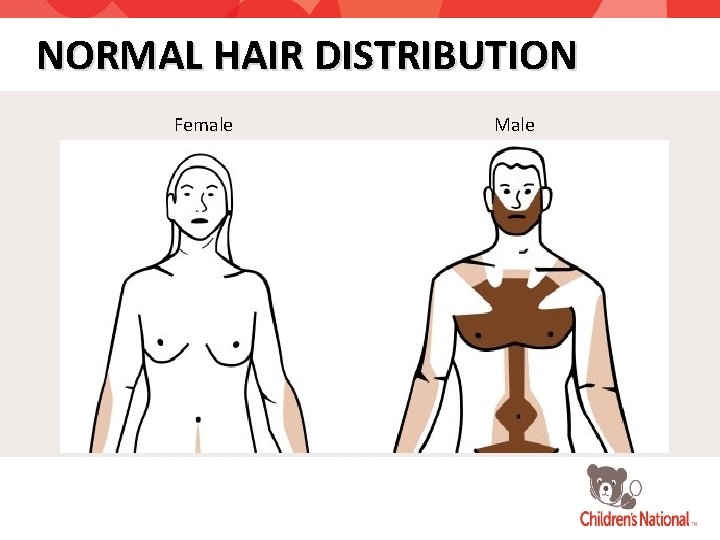
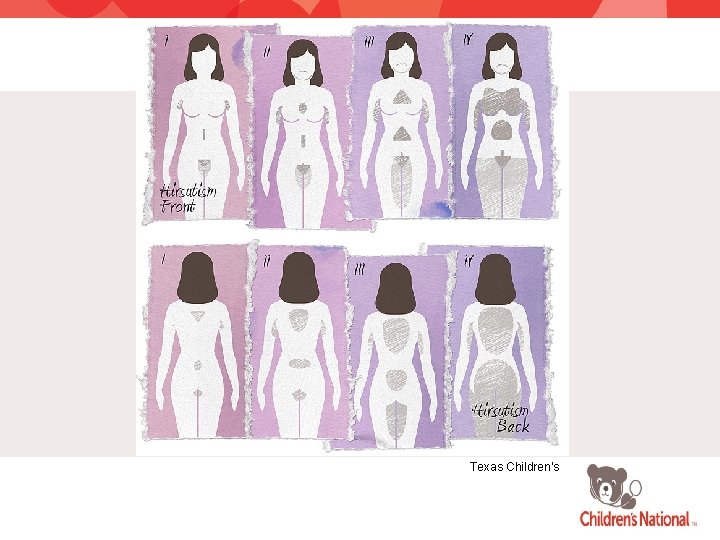
NORMAL HAIR DISTRIBUTION Female Male

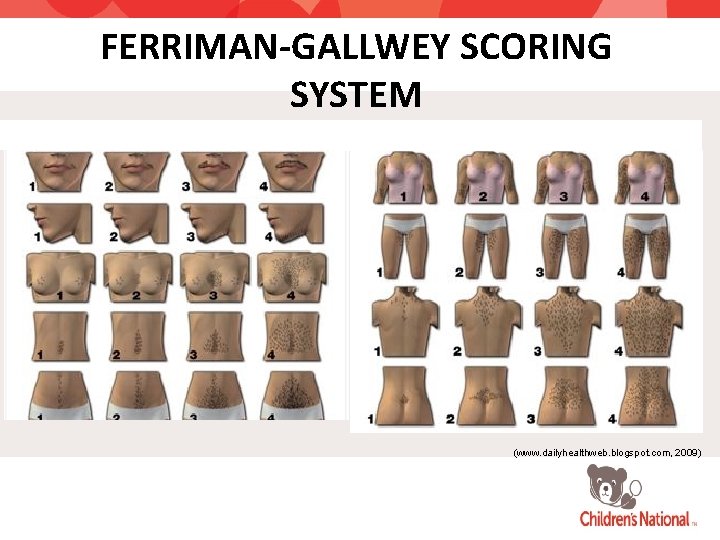
FERRIMAN-GALLWEY SCORING SYSTEM (www. dailyhealthweb. blogspot. com, 2009)

PHENOTYPIC VARIATION Androgen Excess Society Guidelines, 2006.

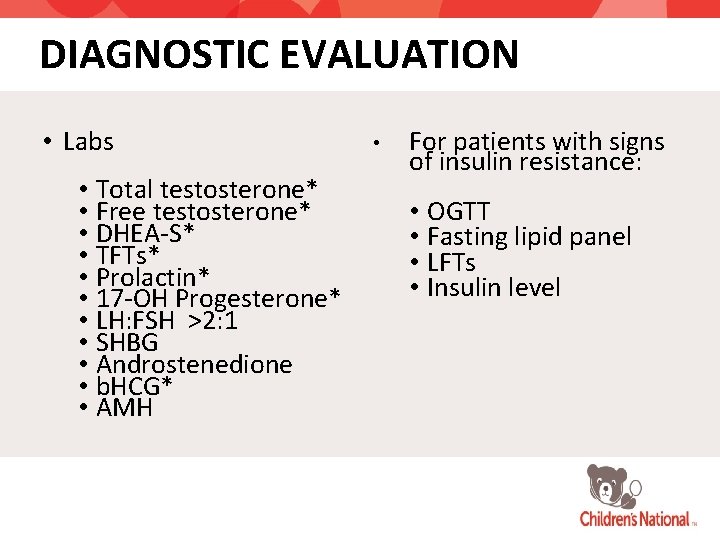
DIAGNOSTIC EVALUATION • Labs • Total testosterone* • Free testosterone* • DHEA-S* • TFTs* • Prolactin* • 17 -OH Progesterone* • LH: FSH >2: 1 • SHBG • Androstenedione • b. HCG* • AMH • For patients with signs of insulin resistance: • OGTT • Fasting lipid panel • LFTs • Insulin level

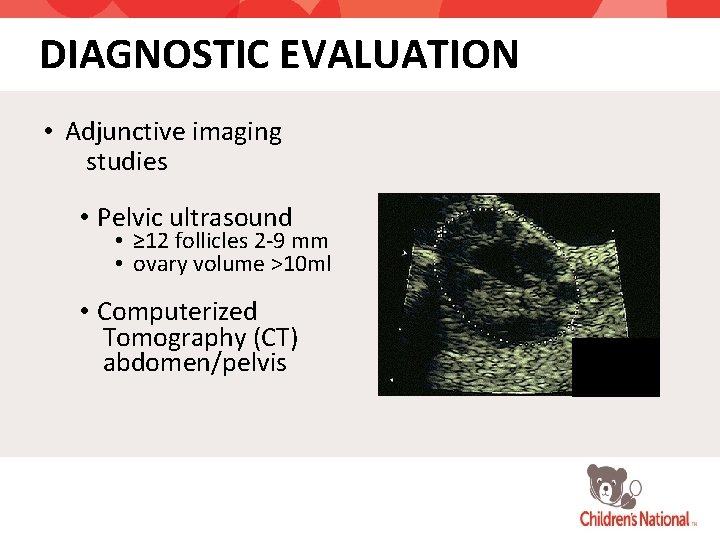
DIAGNOSTIC EVALUATION • Adjunctive imaging studies • Pelvic ultrasound • ≥ 12 follicles 2 -9 mm • ovary volume >10 ml • Computerized Tomography (CT) abdomen/pelvis

DIAGNOSTIC CRITERIA NIH Definition 1990 Rotterdam Definition 2004 Androgen Excess Society Definition 2006 ALL REQUIRED TWO of THREE ALL REQUIRED Hyperandrogenism/ Hyperandrogenemia Ovulatory dysfunction Hyperandrogenism Ovulatory dysfunction or Ultrasonographic PCOS morphology Ovulatory dysfunction and/or Ultrasonographic PCOS morphology Exclude: -non-classic CAH -hyperprolactinemia -androgen secreting tumors

The Challenge: Adolescents & Young Adults • Challenges in diagnosing PCOS in adolescents: • Difficult to distinguish physiologic anovulation/oligomenorrhea from that of PCOS • Diagnosis can only be made 2 yrs after menarche • Multifollicular ovaries can be normal • Polycystic ovaries may be missed by transabdominal ultrasound • Acne and mild hirsutism are common • No standardized Ferriman-Gallwey scoring system in adolescents and young adults

OBJECTIVES • Understand the different treatment modalities in the management of PCOS and potential psychopharmacologic medication interactions

TREATMENT • Lifestyle changes • (Domecq P et al. , 2013) • Medications • Cosmetic treatment

TREATMENT • Menstrual irregularities • Oral contraceptives • Other contraceptive methods • Hyperandrogenism • Spironolactone • Hyperinsulinemia • Metformin • Other oral diabetic medications

TREATMENT • Hirsutism • Depilatory (Hair removal methods) • Chemical • Mechanical • Hormonal (Eflornithine 13. 9%, Vaniqa®) • Infertility • Metformin • Clomiphene Citrate

ORAL CONTRACEPTIVES • Most efficient means of androgen suppression (ovarian as well as adrenal) (Goodman, N et al. , 2015 current best practices guide) • First-line treatment for PCOS and menstrual irregularity • In patients with cutaneous signs of androgen excess • OCPs reduce hirsutism and acne

METFORMIN • Obese adolescents 15 -18 yrs • Received Metformin for 6 months • Found to have increased ovulatory menstrual periods, reduction in testosterone androstenedione and free testosterone. (Bridger T et al. , 2006) Morin P et al. 2004

OCP vs. Metformin • 60 PCOS patients (25 obese, 35 non-obese) enrolled in a prospective clinical study. • Patients randomized to receive for 3 months • OCP Diane 35 (35 mcg ethinyl estradiol plus 2 mg cyproterone acetate), • Metformin • Combination of Diane 35/metformin RESULTS: • Testosterone levels decreased in all three groups • Menstrual regularity was restored in all PCOS patients treated with OCP vs. 28% of those receiving metformin • Metformin alone significantly decreased fasting insulin concentrations, and increased the insulin sensitivity in both obese and non-obese PCOS patients (Wu J et al. , 2008) (Al Khalifah R et al. , 2016)

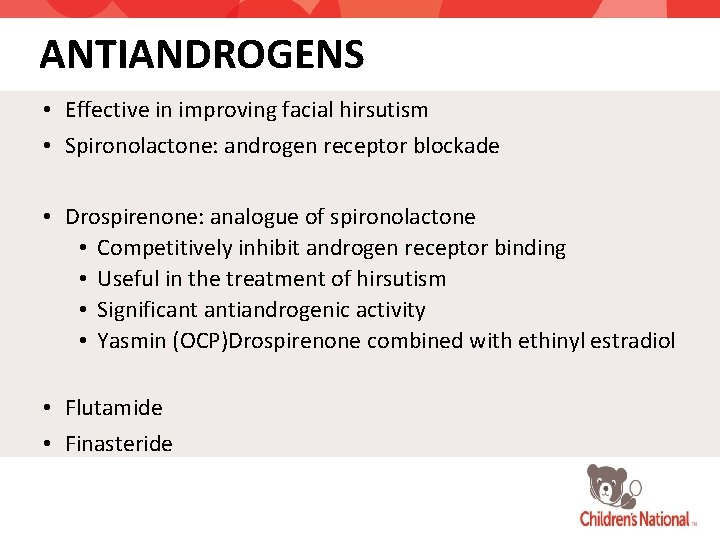
ANTIANDROGENS • Effective in improving facial hirsutism • Spironolactone: androgen receptor blockade • Drospirenone: analogue of spironolactone • Competitively inhibit androgen receptor binding • Useful in the treatment of hirsutism • Significant antiandrogenic activity • Yasmin (OCP)Drospirenone combined with ethinyl estradiol • Flutamide • Finasteride

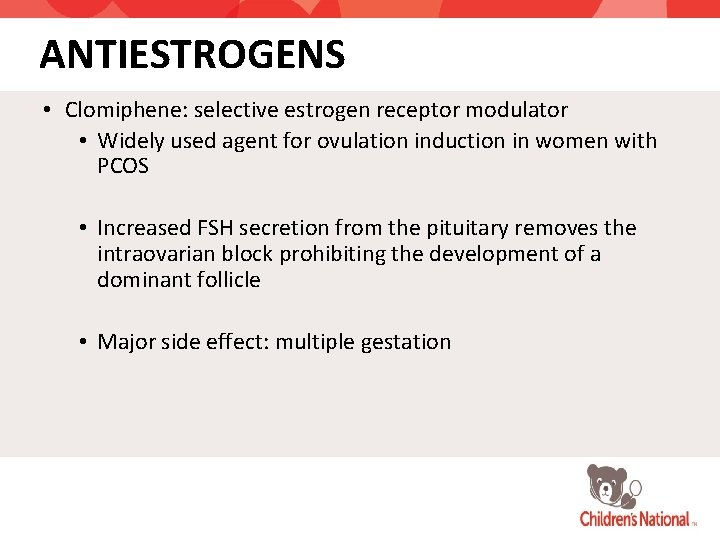
ANTIESTROGENS • Clomiphene: selective estrogen receptor modulator • Widely used agent for ovulation induction in women with PCOS • Increased FSH secretion from the pituitary removes the intraovarian block prohibiting the development of a dominant follicle • Major side effect: multiple gestation

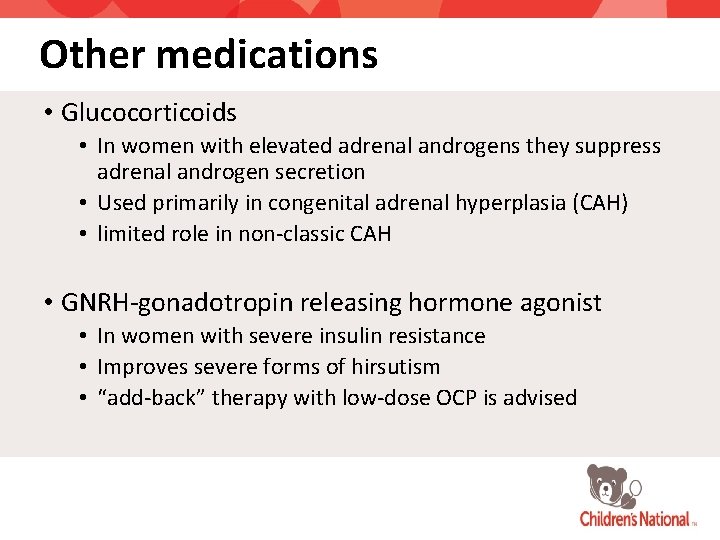
Other medications • Glucocorticoids • In women with elevated adrenal androgens they suppress adrenal androgen secretion • Used primarily in congenital adrenal hyperplasia (CAH) • limited role in non-classic CAH • GNRH-gonadotropin releasing hormone agonist • In women with severe insulin resistance • Improves severe forms of hirsutism • “add-back” therapy with low-dose OCP is advised

BACK TO THE CASE • An 18 y/o freshman presents for her annual visit but is also concerned that she may be depressed. • She had menarche at age 14 and she has had menses every 2 months for the past 2 years, however has missed 4 months of her cycle most recently. • She has a BMI of 29 and in the past year has experience increased facial hair growth. She has never had acne. • She asks: “why do I have some much facial hair? ”

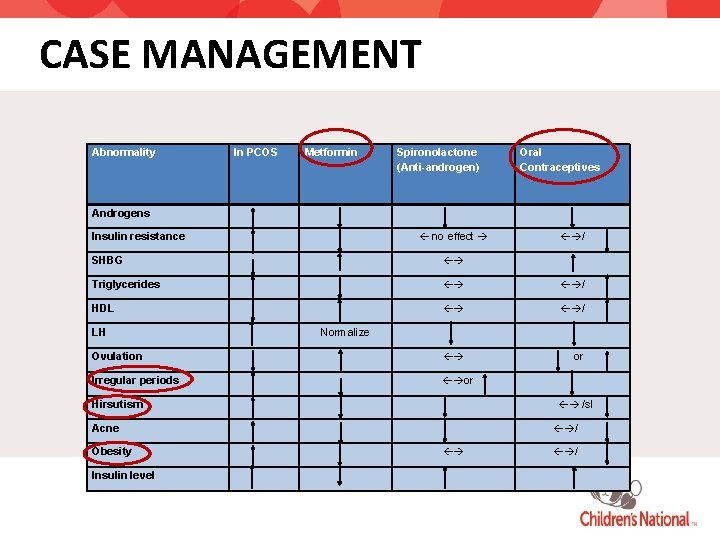
CASE MANAGEMENT Abnormality In PCOS Metformin Spironolactone (Anti-androgen) Oral Contraceptives Androgens no effect Insulin resistance / SHBG Triglycerides / HDL / Ovulation or Irregular periods or LH Normalize /sl Hirsutism Acne Obesity Insulin level / /

Non-classic Congenital Adrenal Hyperplasia (NCAH)

NCAH Objectives • List the diagnostic criteria for NCAH • Understand the pathophysiology of NCAH • Understand the approach to work-up of a patient with characteristics of NCAH • Understand the different treatment modalities in the management of NCAH

CASE M. R. • 19 year old female referred to Adolescent Medicine for menstrual irregularity • Menarche 13 years old • Initially menses would skip a month • 4 -5 months between menses now • Menses last 5 days requiring 3 -4 pads/day • Endorses hair growth on • upper lip, chin, and back

CASE M. R. Physical examination • BMI 44 • Genital exam: Tanner V, no evidence of clitoromegaly • Skin: • face notable for sideburns and hair above the upper lip and the chin • diffuse hair on the chest, lower abdomen extending from umbilicus to the pubis, and legs and arms • acne throughout the back and acanthosis nigricans of the neck Initial laboratory evaluation obtained and started on Provera


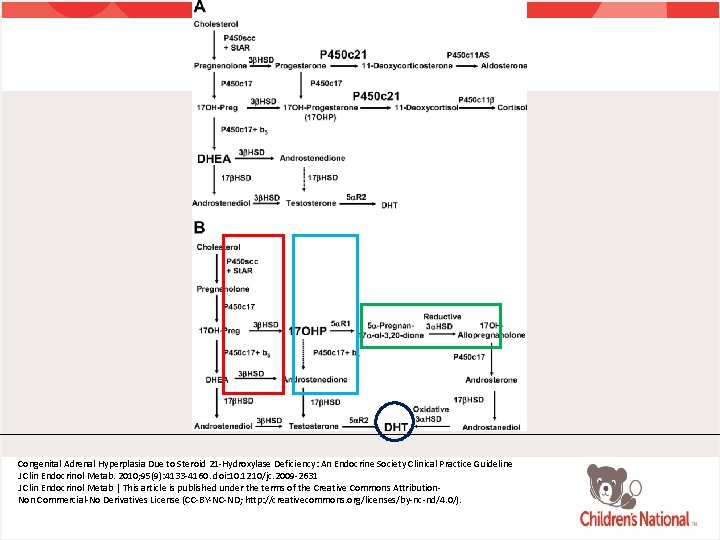
Classic Congenital Adrenal Hyperplasia • Autosomal recessive disorders resulting in impaired cortisol production due to an enzyme deficiency in the pathway of cortisol synthesis • Elevation in ACTH and subsequent hormones before the deficient enzyme • 90% cases due to mutations in CYP 21 A 1 gene • Encodes 21 -hydroxylase enzyme


Classic Congenital Adrenal Hyperplasia • 21 -hydroxylase is an essential enzyme for the production of aldosterone and cortisol • Impairment of 21 -hydroxylase results in overproduction of hormonal products • 17 -OH progesterone • Three pathways exist for the production of androgens in the absence of 21 -hydroxylase

Congenital Adrenal Hyperplasia Due to Steroid 21 -Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline J Clin Endocrinol Metab. 2010; 95(9): 4133 -4160. doi: 10. 1210/jc. 2009 -2631 J Clin Endocrinol Metab | This article is published under the terms of the Creative Commons Attribution. Non Commercial-No Derivatives License (CC-BY-NC-ND; http: //creativecommons. org/licenses/by-nc-nd/4. 0/).

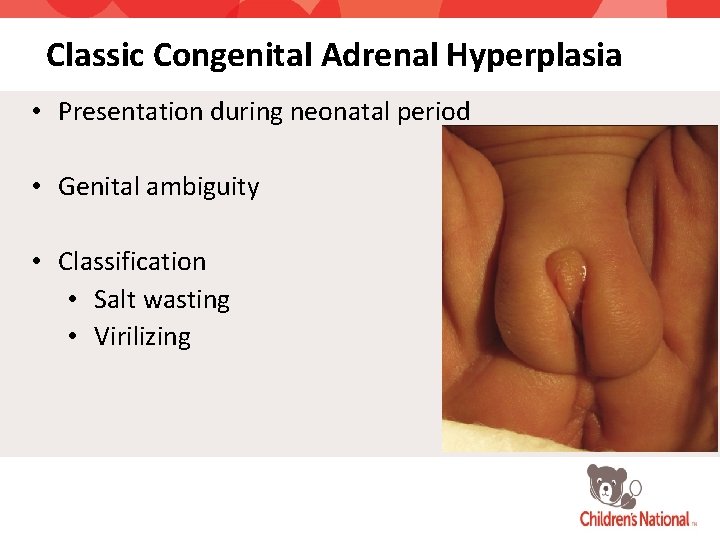
Classic Congenital Adrenal Hyperplasia • Presentation during neonatal period • Genital ambiguity • Classification • Salt wasting • Virilizing

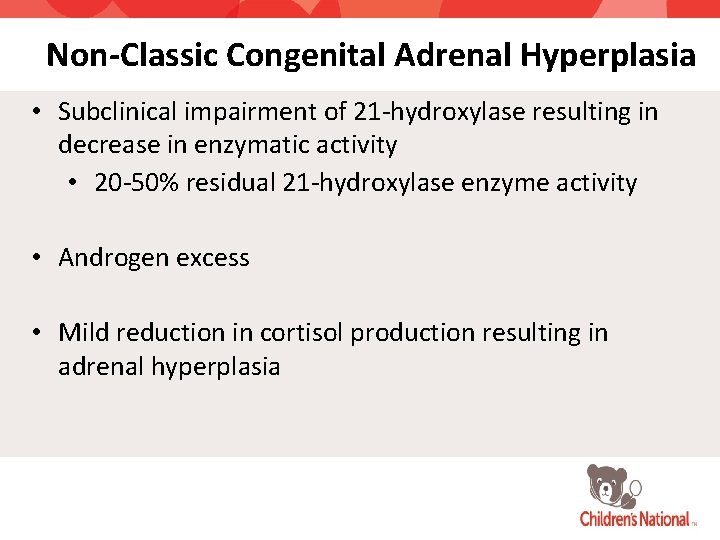
Non-Classic Congenital Adrenal Hyperplasia • Subclinical impairment of 21 -hydroxylase resulting in decrease in enzymatic activity • 20 -50% residual 21 -hydroxylase enzyme activity • Androgen excess • Mild reduction in cortisol production resulting in adrenal hyperplasia

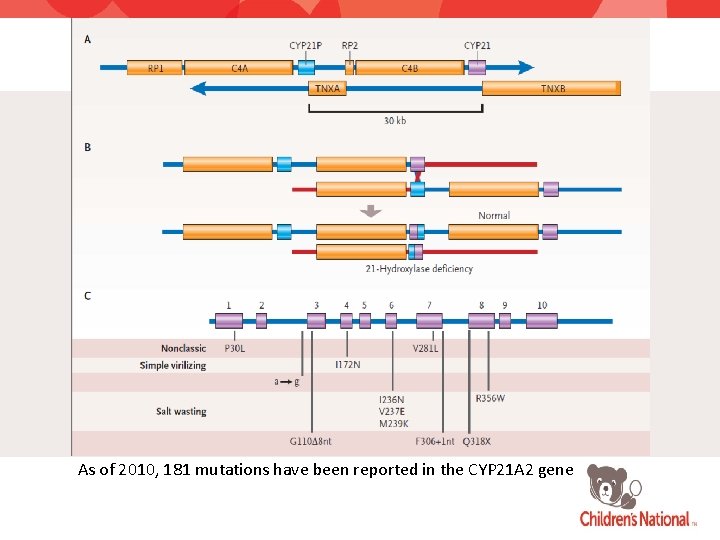
As of 2010, 181 mutations have been reported in the CYP 21 A 2 gene

NCAH: Prevalence • 0. 1– 0. 2% in the general Caucasian population • 1– 2% among Eastern European (Ashkenazi) Jews • 1 -2% among general Hispanic population • One of the most common autosomal recessive diseases • Frequency of heterozygote carriers 1: 60

NCAH: Presentation • Presentation during childhood • Premature pubarche • Clitoromegaly • Advanced bone age • Presentation during adolescence or adulthood • Hirsutism • Acne vulgaris • Menstrual irregularity • Asymptomatic with normal growth, puberty, and reproductive function

Texas Children’s

NCAH vs. PCOS • PCOS physiology may develop due to chronic androgen excess and impact on H-P-A axis and ovaries • ¼ of NCAH females have polycystic ovaries on ultrasound • The clinical presentation of NCAH and PCOS can be identical • Mandatory to exclude NCAH prior to the diagnosis of PCOS

NCAH: Genetic Testing • Genotyping recommended for genetic counseling and confirmation of the diagnosis • Risk of child with classic CAH

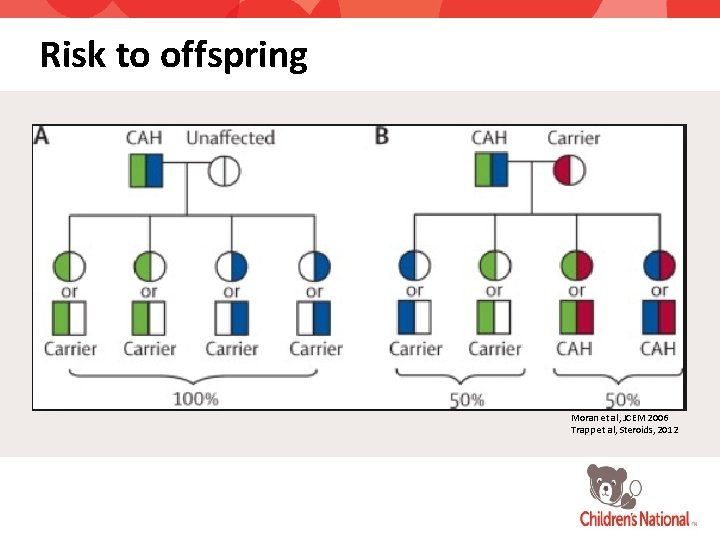
Risk to offspring Moran et al, JCEM 2006 Trapp et al, Steroids, 2012

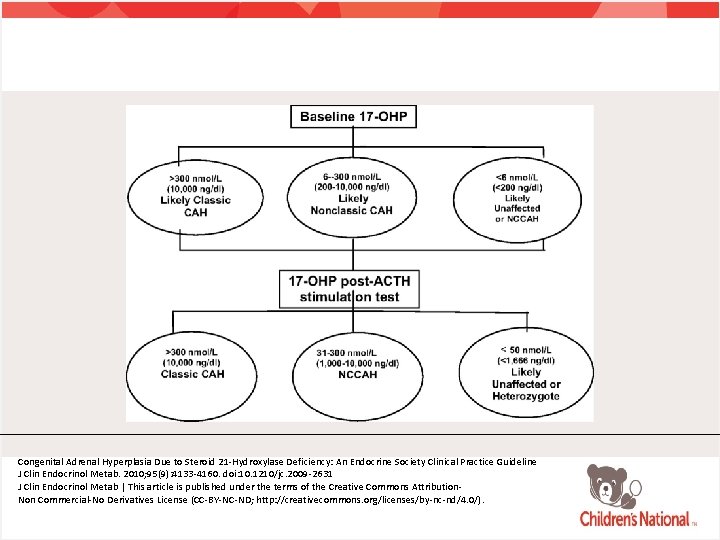
NCAH: Testing • NCAH usually not detected on newborn screen • Early morning (0730 -0830) 17 -OH progesterone sample during the follicular phase in menstruating females • Random sample if patient amenorrheic • ACTH stimulation test • Gold standard to diagnosis • Genetic testing for CYP 21 A 2

ACTH Stimulation Testing • Measurement of basal 17 -OHP and at 60 minutes • Consider measurement of basal and post-ACTH stimulation cortisol • Early morning follicular phase 17 -OHP levels of > 200 ng/dl should prompt further evaluation (will capture 90% of NCAH)

Congenital Adrenal Hyperplasia Due to Steroid 21 -Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline J Clin Endocrinol Metab. 2010; 95(9): 4133 -4160. doi: 10. 1210/jc. 2009 -2631 J Clin Endocrinol Metab | This article is published under the terms of the Creative Commons Attribution. Non Commercial-No Derivatives License (CC-BY-NC-ND; http: //creativecommons. org/licenses/by-nc-nd/4. 0/).

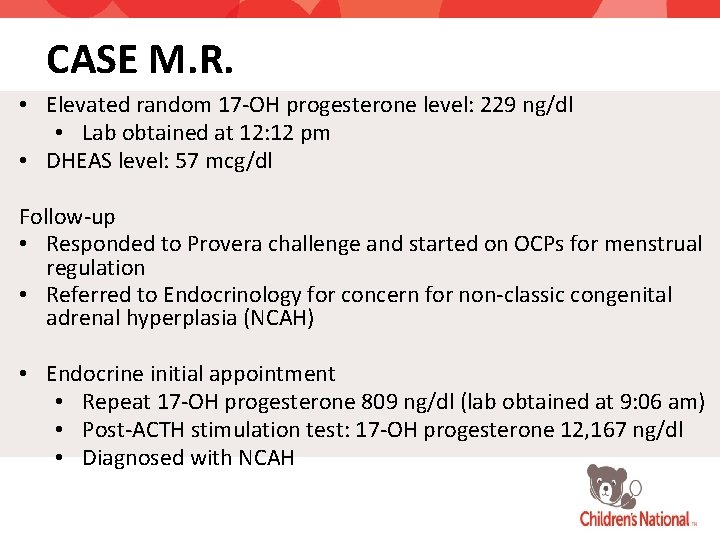
CASE M. R. • Elevated random 17 -OH progesterone level: 229 ng/dl • Lab obtained at 12: 12 pm • DHEAS level: 57 mcg/dl Follow-up • Responded to Provera challenge and started on OCPs for menstrual regulation • Referred to Endocrinology for concern for non-classic congenital adrenal hyperplasia (NCAH) • Endocrine initial appointment • Repeat 17 -OH progesterone 809 ng/dl (lab obtained at 9: 06 am) • Post-ACTH stimulation test: 17 -OH progesterone 12, 167 ng/dl • Diagnosed with NCAH

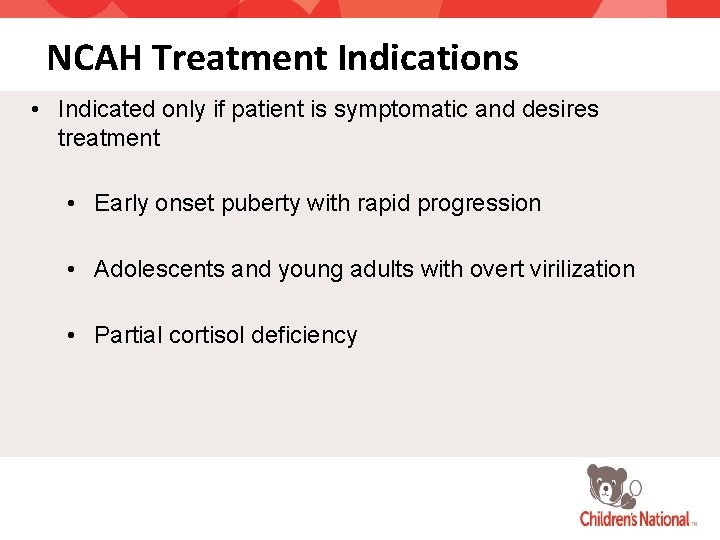
NCAH Treatment Indications • Indicated only if patient is symptomatic and desires treatment • Early onset puberty with rapid progression • Adolescents and young adults with overt virilization • Partial cortisol deficiency

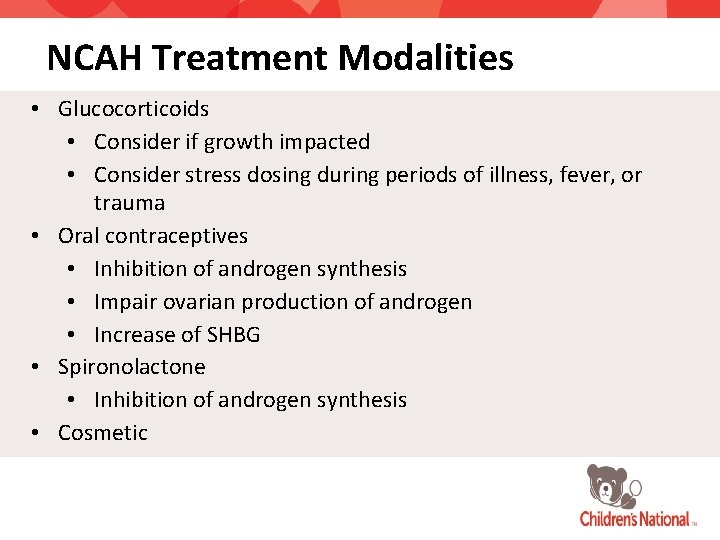
NCAH Treatment Modalities • Glucocorticoids • Consider if growth impacted • Consider stress dosing during periods of illness, fever, or trauma • Oral contraceptives • Inhibition of androgen synthesis • Impair ovarian production of androgen • Increase of SHBG • Spironolactone • Inhibition of androgen synthesis • Cosmetic

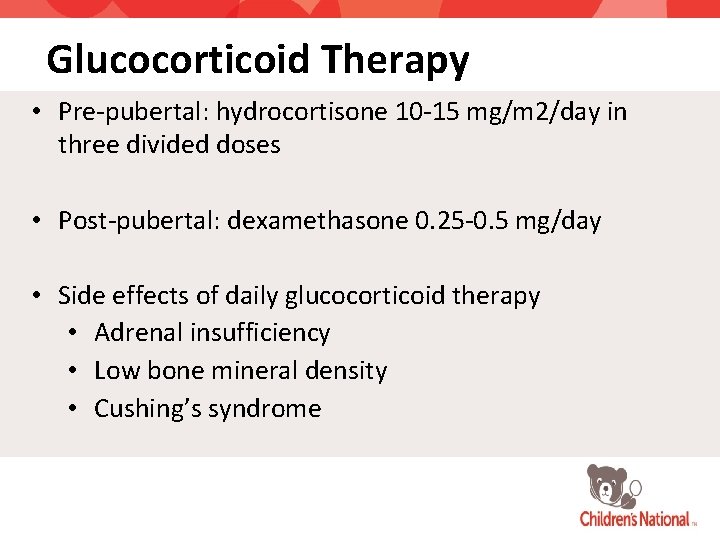
Glucocorticoid Therapy • Pre-pubertal: hydrocortisone 10 -15 mg/m 2/day in three divided doses • Post-pubertal: dexamethasone 0. 25 -0. 5 mg/day • Side effects of daily glucocorticoid therapy • Adrenal insufficiency • Low bone mineral density • Cushing’s syndrome

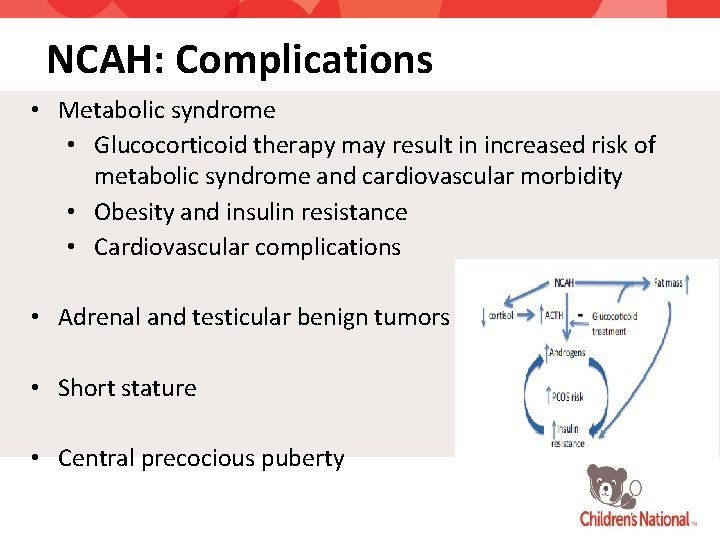
NCAH: Complications • Metabolic syndrome • Glucocorticoid therapy may result in increased risk of metabolic syndrome and cardiovascular morbidity • Obesity and insulin resistance • Cardiovascular complications • Adrenal and testicular benign tumors • Short stature • Central precocious puberty

Comparing and Contrasting PCOS & NCAH

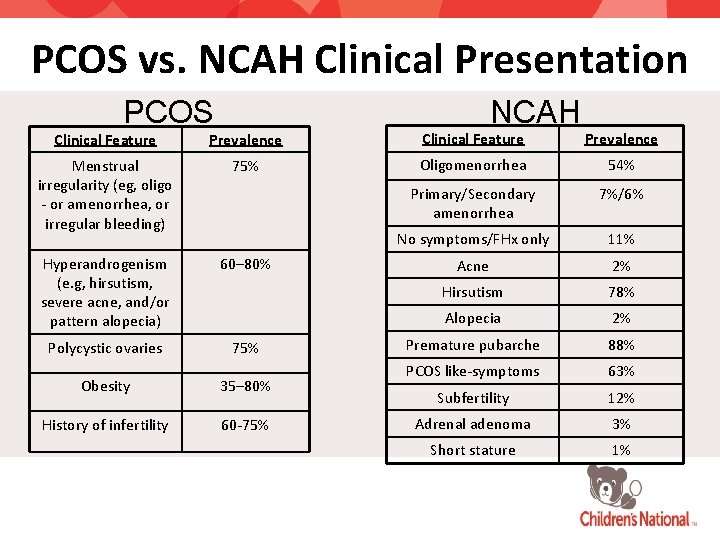
PCOS vs. NCAH Clinical Presentation PCOS NCAH Clinical Feature Prevalence Menstrual irregularity (eg, oligo - or amenorrhea, or irregular bleeding) 75% Oligomenorrhea 54% Primary/Secondary amenorrhea 7%/6% No symptoms/FHx only 11% Hyperandrogenism (e. g, hirsutism, severe acne, and/or pattern alopecia) 60– 80% Acne 2% Hirsutism 78% Alopecia 2% Polycystic ovaries 75% Premature pubarche 88% Obesity 35– 80% PCOS like-symptoms 63% Subfertility 12% History of infertility 60 -75% Adrenal adenoma 3% Short stature 1%

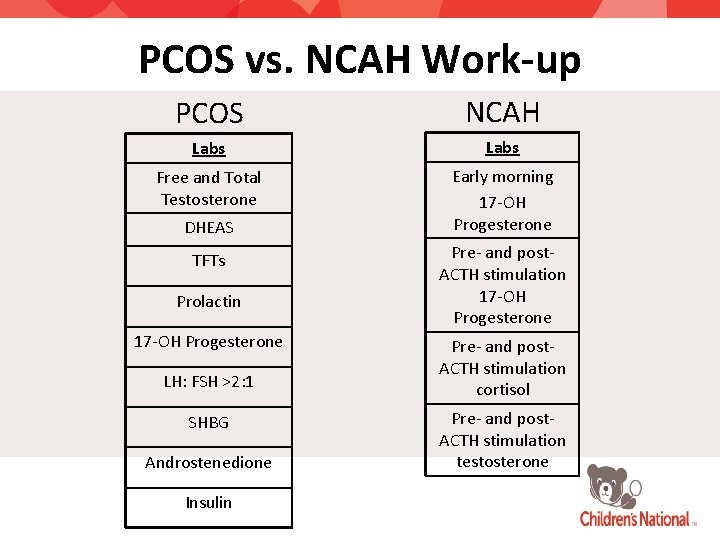
PCOS vs. NCAH Work-up PCOS NCAH Labs Free and Total Testosterone Early morning 17 -OH Progesterone DHEAS TFTs Prolactin 17 -OH Progesterone LH: FSH >2: 1 SHBG Androstenedione Insulin Pre- and post. ACTH stimulation 17 -OH Progesterone Pre- and post. ACTH stimulation cortisol Pre- and post. ACTH stimulation testosterone

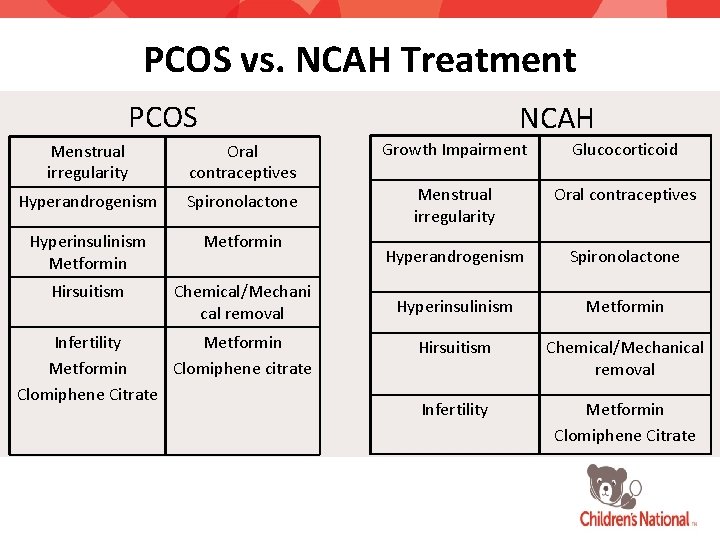
PCOS vs. NCAH Treatment PCOS NCAH Menstrual irregularity Oral contraceptives Growth Impairment Glucocorticoid Hyperandrogenism Spironolactone Menstrual irregularity Oral contraceptives Hyperinsulinism Metformin Hyperandrogenism Spironolactone Hirsuitism Chemical/Mechani cal removal Hyperinsulinism Metformin Hirsuitism Chemical/Mechanical removal Infertility Metformin Clomiphene Citrate Infertility Metformin Clomiphene citrate Clomiphene Citrate

References • • • • • Azziz, R et al. Androgen Excess Society. (2006). Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. The Journal of Clinical Endocrinology &Metabolism, (91)11, 4237 -4245. Barry, J. , Kuczmierczyk, A & Hardiman, P. (2011). Anxiety and depression in polycystic ovary syndrome: a systematic review and metaanalysis. Human Reproduction, 26(9): 2442– 2451. Buggs, C. & Rosenfield, R. (2005). Polycystic ovary syndrome in adolescence. Endocrinol. Metab Clin North Am, 34, 677– 705. Chandra, A et al. (2013). Infertility and Impaired Fecundity in the United States, 1982– 2010: Data From the National Survey of Family Growth. Retrieved from http: //www. cdc. gov/nchs/nsfg. htm Corona, M. , Mc. Carty, C. , & Richardson, L. (2013). Screening adolescents for depression. Retrieved from http: //www. agencymeddirectors. wa. gov/Files/depressoverview. pdf Creatsas, G. , Koliopoulos, C. , & Mastorakos, G. (2000). Combined oral contraceptive treatment of adolescent girls with polycystic ovary syndrome. Lipid profile. Ann N Y Acad Sci, 900, 245 -52. Ehrmann, D. (2004). Medical progress: polycystic ovary syndrome. N Engl J Med, 352, 1223– 1236. Goodman, N et al. , (2015). AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME--PART 1. Endocr Pract, 21(11): 1291 -300. Ghazeeri, G et al. (2013). Anxiety, Cognitive, and Depressive Assessment in Adolescents with Polycystic Ovarian Syndrome: A Pilot Study. Journal of Pediatric and Adolescent Gynecology, 26(5): 269 -273. Khalifah, R et al. (2016). Metformin or Oral Contraceptives for Adolescents With Polycystic Ovarian Syndrome: A Meta-analysis. Pediatrics, 137(5). Kroenke, K. , & Spitzer, R. (2002). The PHQ-9: a new depression and diagnostic severity measure. Psychiatric Annals, 32, 509 -521. Laggari, V et al. , (2009). Anxiety and depression in adolescents with polycystic ovary syndrome and Mayer-Rokitansky-Küster-Hauser syndrome. J Psychosom Obstet Gynaecol, 30(2): 83 -8. Michelmore, K. , Balen, A. , & Dunger, D. (2001). Polycystic ovaries and eating disorders: Are they related? Hum Reprod, 16(4): 765 -9. National Institutes of Health. (2012). Evidence-based Methodology Workshop on Polycystic Ovary Syndrome. Executive Summary. Retrieved from https: //prevention. nih. gov/programs-events/pathways-to-prevention/previous-workshops/pcos Rosenfield, R. (2012). Definition, pathogenesis, and etiology of polycystic ovary syndrome in adolescents. Retrieved from: www. Up. To. Date. com. Last updated Dec 13, 2012 Sirmans, S. , & Pate, K. (2014). Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical Epidemiology, 6, 1– 13. Trent, M. , Rich, M. , Austin, S. , & Gordon, C. (2003). Fertility Concerns and Sexual Behavior in Adolescent Girls with Polycystic Ovary Syndrome: Implications for Quality of Life. Journal of Pediatric and Adolescent Gynecology, 16(1), 33– 37. Trent, M. , Rich, M. , Austin, S. , & Gordon, C. (2001). Polycystic ovary syndrome in adolescent girls: Fertility concerns and sexual behavior. Journal of Pediatric and Adolescent Gynecology, 14(31), 144.

References • • • Falhammar, H. , & Nordenström, A. (2015). Nonclassic congenital adrenal hyperplasia due to 21 -hydroxylase deficiency: Clinical presentation, diagnosis, treatment, and outcome. Endocrine, 50(1), 32 -50. Merke, D. P. , & Poppas, D. P. (2013). Management of adolescents with congenital adrenal hyperplasia. Lancet Diagetes Endocrinology, 1, 341 -352. Trapp, C. M. , & Oberfield, S. E. (2012). Recommendations for treatment of nonclassic congenital adrenal hyperplasia (NCAH): An update. Steroids, 77(4), 342 -346. White, P. C. (2000). Congenital Adrenal Hyperplasia due to 21 -Hydroxylase Deficiency. Endocrine Reviews, 21(3), 245 -291. Pall , M. , Azziz, R. , Beires, J. , & Pignatelli , D. (2010). The phenotype of hirsute women: a comparison of polycystic ovary syndrome and 21 -hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 94(2), 684 -9

Thank you! Dr. David Mc. Bride Dr. Kim Shimy

Questions? Contact information Serwa Gyamfi, MD sgyamfi@childrensnational. org Sharyn Malcolm, MD, MPH smalcolm@childrensnational. org
 Non classical adrenal hyperplasia
Non classical adrenal hyperplasia Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Site:slidetodoc.com
Site:slidetodoc.com Vaginal agensis
Vaginal agensis Raas system
Raas system 17 hydroxyprogesterone levels
17 hydroxyprogesterone levels Infantile polycystic kidney
Infantile polycystic kidney Symptomatic polycystic kidney disease
Symptomatic polycystic kidney disease Congenital rubella syndrome
Congenital rubella syndrome Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital rubella syndrome triad
Congenital rubella syndrome triad Hyperplasia and hypertrophy difference
Hyperplasia and hypertrophy difference It projektov�� mana����r
It projektov�� mana����r Sebaceous hyperplasia
Sebaceous hyperplasia Fundus mirigy hyperplasia
Fundus mirigy hyperplasia Intimal hyperplasia
Intimal hyperplasia Seminal vesicle
Seminal vesicle Intimal hyperplasia
Intimal hyperplasia Dianne zwicke
Dianne zwicke Gingival hyperplasia
Gingival hyperplasia Ebolectomy
Ebolectomy Muscle layer
Muscle layer Types of cell injury
Types of cell injury Corpora amylacea
Corpora amylacea The functions of ovary
The functions of ovary Femaleanatomy
Femaleanatomy Ovarian cancer marker
Ovarian cancer marker Leiomyomas
Leiomyomas Duct system of female reproductive system
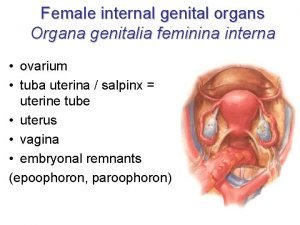
Duct system of female reproductive system Organa genitalia feminina externa
Organa genitalia feminina externa Ovarian cancer causes
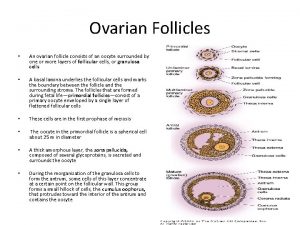
Ovarian cancer causes Ovarian follicle
Ovarian follicle Immature teratoma
Immature teratoma Ovarian cycle
Ovarian cycle Ovarian teratoma
Ovarian teratoma Ovarian cycle phases
Ovarian cycle phases Ovarian cancer brca
Ovarian cancer brca Hrd score ovarian cancer
Hrd score ovarian cancer Spermiogenesis
Spermiogenesis Ovarian ligament
Ovarian ligament Ovarian benign tumor
Ovarian benign tumor Sru consensus ovarian cysts
Sru consensus ovarian cysts Benjamin cummings
Benjamin cummings The ovarian cycle
The ovarian cycle Ovarian ligament.
Ovarian ligament. Follicles in ovary
Follicles in ovary Ovarian ligament
Ovarian ligament Corpus luteum vs corpus albicans histology
Corpus luteum vs corpus albicans histology Pathoma slides
Pathoma slides Slide
Slide Nerve ganglia
Nerve ganglia Adrenal bez histolojisi
Adrenal bez histolojisi кагами-огата
кагами-огата Congenital pneumonia
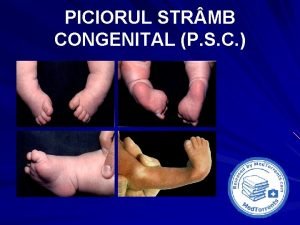
Congenital pneumonia Picior stramb congenital
Picior stramb congenital Trabeculodysgenesis
Trabeculodysgenesis Hipotálamo-hipófise-adrenal
Hipotálamo-hipófise-adrenal Eisenmenger syndrome
Eisenmenger syndrome Congenital malformations
Congenital malformations Congenital voice disorders
Congenital voice disorders Hypothyroidism
Hypothyroidism Cyanotic vs acyanotic
Cyanotic vs acyanotic Egg on a string heart
Egg on a string heart Congenital fibrosis of the extraocular muscles
Congenital fibrosis of the extraocular muscles Polyrhinia
Polyrhinia Most common congenital anomalies
Most common congenital anomalies Estadiamento de tanner
Estadiamento de tanner 3 beta hidroksisteroid dehidrogenaz
3 beta hidroksisteroid dehidrogenaz Adrenal gland relations
Adrenal gland relations Zona reticularis gonadocorticoids
Zona reticularis gonadocorticoids What causes addisons disease
What causes addisons disease Endocardial cushion defect
Endocardial cushion defect Adrenal yetmezlik acilci
Adrenal yetmezlik acilci