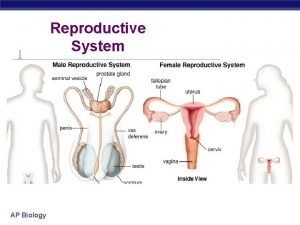
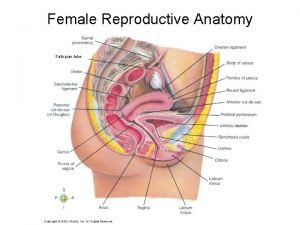
Female Reproductive System Cervix and Ovaries prof Mandira





















































- Slides: 118

Female Reproductive System Cervix and Ovaries prof Mandira Sharma

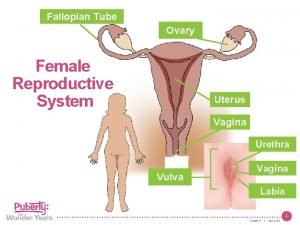
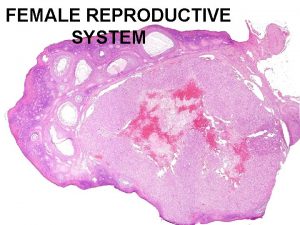
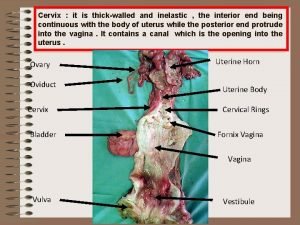
NORMAL STRUCTUR E • The cervix consists of an internal os communicating with the • endometrial cavity above, and an external os opening into • the vagina below.

CERVIX • Ectocervix (exocervix) is the part of the cervix exposed to the vagina and is lined by stratified squamous epithelium. • whereas the endocervix is continuous with the endocervical canal and is lined by a single layer of tall columnar mucus-secreting epithelium. The endocervical mucosa is thrown into folds resulting in formation of clefts and tunnels, commonly referred to as cervical glands that secrete mucus.

CERVIX • The junction of the ectocervix and endocervix—junctional mucosa, consists of gradual transition between squamous and columnar epithelia (squamo-columnar junction) and is clinically and pathologically significant landmark.

CERVIX • The cervical mucosa undergoes changes under the influence of hormones and during pregnancy. • The cervical mucus varies under the influence of oestrogen becomes thin which on drying forms fern-like pattern on glass slide. • Lesions of the cervix are rather common. Of great significance are cervicitis, certain benign and malignant tumours, dysplasia.

Acute and chronic cervicitis • Inflammation of the cervix is common and is related to constant exposure to bacterial flora in the vagina. • Many microorganisms, particularly endogenous vaginal aerobes and anaerobes, Streptococcus, Staphylococcus, and Enterococcus. • Other specific organisms include Chlamydia trachomatis, Neisseria gonorrhoeae, and occasionally herpes simplex, type 2.

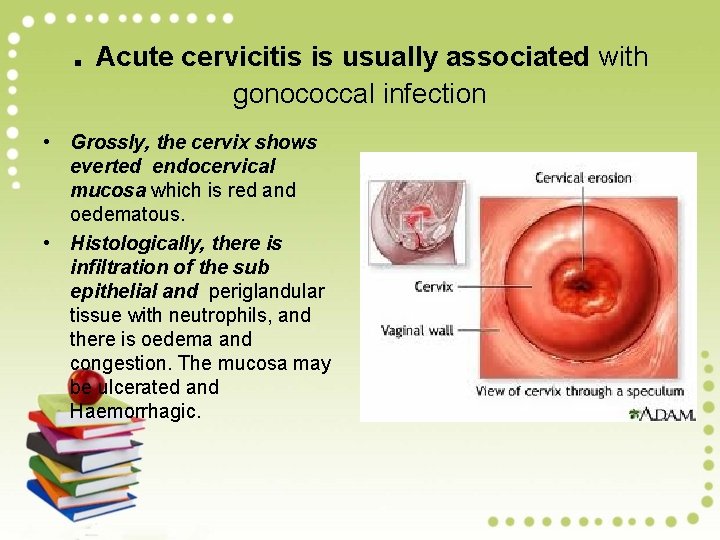
. Acute cervicitis is usually associated with gonococcal infection • Grossly, the cervix shows everted endocervical mucosa which is red and oedematous. • Histologically, there is infiltration of the sub epithelial and periglandular tissue with neutrophils, and there is oedema and congestion. The mucosa may be ulcerated and Haemorrhagic.

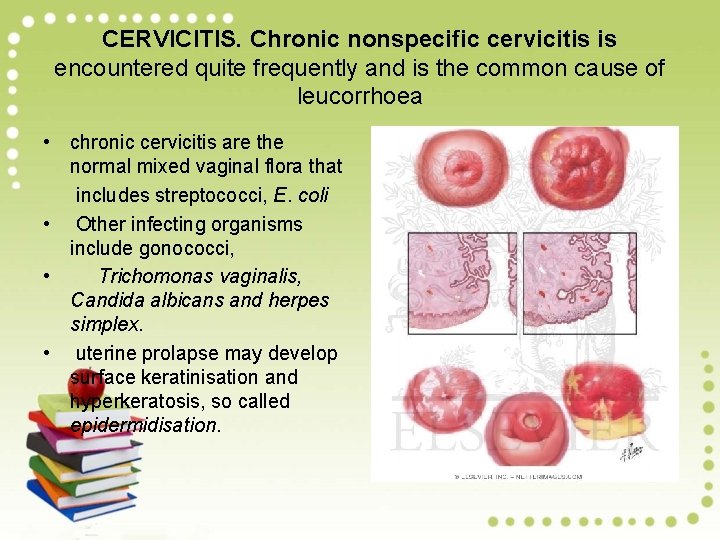
CERVICITIS. Chronic nonspecific cervicitis is encountered quite frequently and is the common cause of leucorrhoea • chronic cervicitis are the normal mixed vaginal flora that includes streptococci, E. coli • Other infecting organisms include gonococci, • Trichomonas vaginalis, Candida albicans and herpes simplex. • uterine prolapse may develop surface keratinisation and hyperkeratosis, so called epidermidisation.

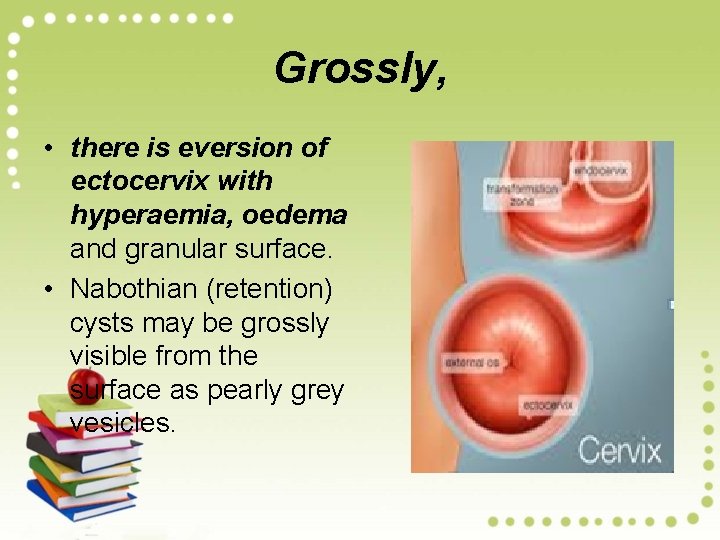
Grossly, • there is eversion of ectocervix with hyperaemia, oedema and granular surface. • Nabothian (retention) cysts may be grossly visible from the surface as pearly grey vesicles.

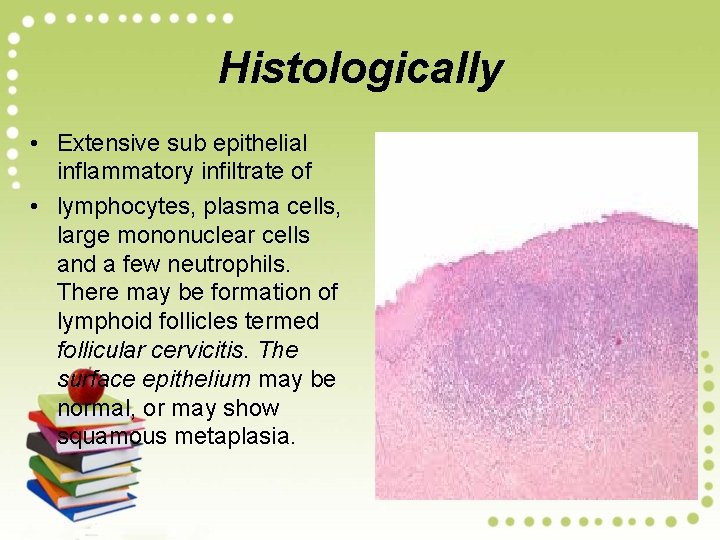
Histologically • Extensive sub epithelial inflammatory infiltrate of • lymphocytes, plasma cells, large mononuclear cells and a few neutrophils. There may be formation of lymphoid follicles termed follicular cervicitis. The surface epithelium may be normal, or may show squamous metaplasia.

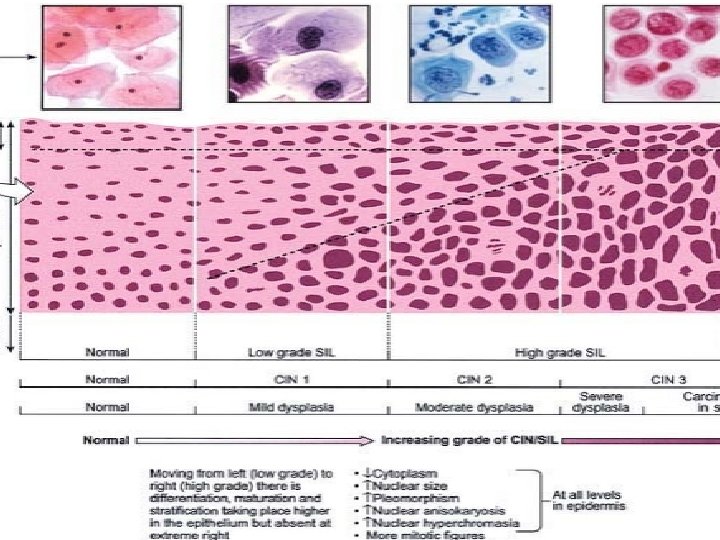
Squamous Intraepithelial Lesion (SIL) (Cervical Intraepithelial Neoplasia, CIN) • DYSPLASIA. The term ‘dysplasia’ (meaning ‘bad moulding’) • has been commonly used for atypical cytological changes in the layers of squamous epithelium, the changes being progressive. Depending upon the thickness of squamous epithelium involved by atypical cells, dysplasia is conventionally graded as mild, moderate and severe.

CIN. An alternative classification is to group various grades • Carcinoma in situ together into cervical intraepithelial Neoplasia (CIN) which is similarly graded from grade I to III.

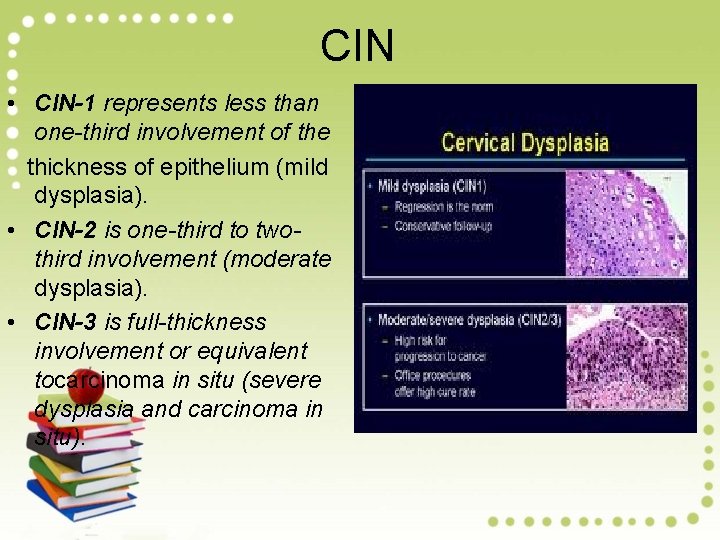
CIN • CIN-1 represents less than one-third involvement of the thickness of epithelium (mild dysplasia). • CIN-2 is one-third to twothird involvement (moderate dysplasia). • CIN-3 is full-thickness involvement or equivalent tocarcinoma in situ (severe dysplasia and carcinoma in situ).

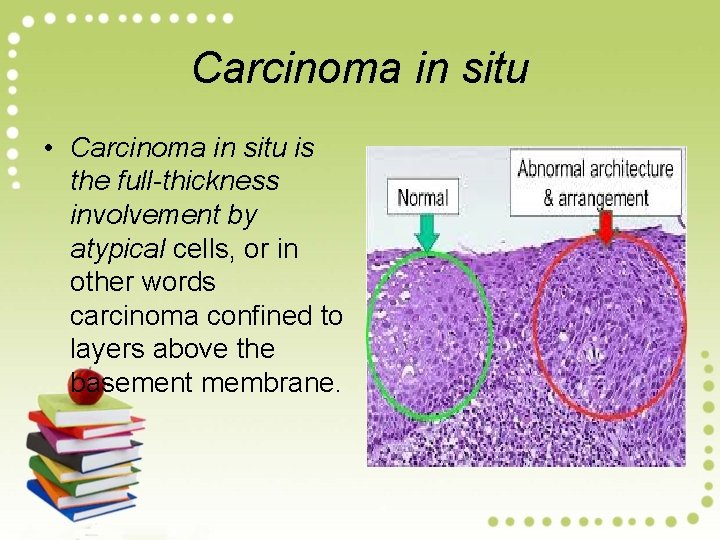
Carcinoma in situ • Carcinoma in situ is the full-thickness involvement by atypical cells, or in other words carcinoma confined to layers above the basement membrane.

HPV types implicated in their etiology • The three grades of CIN are readjusted into two grades of Squamous intraepithelial lesions • (SIL)— low-grade SIL (L-SIL) • High grade SIL (H-SIL) • L-SIL corresponds to CIN-1 and is a flat condyloma, having koilocytic atypia, usually related to HPV 6 and 11 infection (i. e. includes mild dysplasia and HPV infection).

HPV types implicated in their etiology • H-SIL corresponds to CIN-2 and 3 and has abnormal pleomorphic atypical squamous cells. • HPV 16 and 18 are implicated in the etiology of H-SIL (i. e. includes moderate dysplasia, severe dysplasia, and carcinoma in situ).

EXFOLIATIVE CYTOLOGY Smears from the female genital tract have traditionally been known as ‘Pap smears’. CELLS IN NORMAL COMBINED SMEARS. Normally, combined smear contains two types of cells: cells. delivery. epithelial and others Epithelial

EXFOLIATIVE CYTOLOGY There are 4 types of squamous epithelial cells in the cervical smear: superficial, intermediate, parabasal and basal cells. Morphological features of these cells Navicular cells are boat-shaped intermediate cells with folded cell borders. Lactation cells are parabasal cells with strongly acidophilic cytoplasm. Endocervical cells appear either as single dispersed Endometrial cells are seen up to 12 th day of menstrual cycle. They are slightly smaller than endocervical cells Trophoblastic cells are seen following abortion

APPLICATIONS OF PAP SMEAR • Cytohormonal Evaluation • Assessment of hormonal status is best carried out from lateral vaginal smears although vaginal ‘pool’ or fast smears may also be used. • Abnormal Combined Smears • In order to evolve a system acceptable to clinicians and cytopathologists, National Cancer Institute Workshop in 1988 developed the Bethesda System (TBS) for uniformity in evaluation as well as limitations of reporting in cervicovaginal cytopathology

APPLICATIONS OF PAP SMEAR 1. Specimen adequacy: 2. General categorisation: It includes categorising the smear • in one of the three broad categories: within normal limits, benign cellular changes, and epithelial cell abnormalities. • 3. Descriptive diagnosis: Final aspect of the Bethesda system includes detailed description of the benign cellular changes or epithelial cell abnormalities in the smear. Based on it, the cellular changes in cervical smears are described under • 2 headings: non-neoplastic (or benign) and • neoplastic epithelial cell abnormalities.

NON-NEOPLASTIC (BENIGN) CELLULAR CHANGES • NON-SPECIFIC INFLAMMATORY CHANGES. • Inflammatory changes not associated with any specific infection or identifiable infectious agent Acute inflammatory changes are characterised by an increase in the number of parabasal cells (due to disruption of superficial layers of the epithelium resulting in exposure of deeper layers), cytoplasmic acidophilia and vacuolisation, leucocytes migration into cytoplasm, and perinuclear halos with nuclear pyknosis or enlargement. • • Chronic inflammatory changes (Reactive changes) manifest in squamous cells as nuclear enlargement, hyperchromatism, and nucleolar prominence, with multinucleation in some instances. Endocervical cells may show reparative hyperplasia and/or squamous metaplasia.

SPECIFIC INFLAMMATORY CHANGES • SPECIFIC INFLAMMATORY CHANGES. • inflammatory changes may be associated with a variety of infectious agents, the common among which are listed in • a) Bacterial agents: • N. gonorrhoeae (the gonococcus) is a gramnegative diplococcus • Viral agents: • Human papilloma virus (HPV) is one of the commonest sexually-transmitted infectionsinclusions.

SPECIFIC INFLAMMATORY CHANGES • c) Fungal agents: • Moniliasis (infection by Candida albicans) is the commonest fungal infection of the female genital tract and is particularly associated with diabetes, pregnancy and use of antibacterial agents (which alter the vaginal flora). • d) Parasitic agents: • Up to 25% of adult women are estimated to harbour Trichomonas vaginalis in their lower genital tract.

NEOPLASTIC EPITHELIAL CELL ABNORMALITIES • Carcinoma of the uterine cervix still ranks high in the list as the most frequent cancer in developing countries of the world and is the leading cause of cancer morbidity and mortality. • Vast majority of cervical cancers are of the squamous cell type, and the diagnosis of squamous cell carcinoma of the cervix and its precursor lesions is considered as the most important application of exfoliative cytology.

NEOPLASTIC EPITHELIAL CELL ABNORMALITIES • CIN I Mild Primitive (atypical) cells, dysplasia proliferating in lower third of epithelium. • CIN II Moderate Involvement up to dysplasia middlethird of epithelium. • CIN III Severe Involvement of upper-third dysplasia of epithelium. • Carcinoma Involvement of full in situ (CIS) thickness of epithelium. • Presently, the Bethesda system divides squamous cell abnormalities into four categories: • .

NEOPLASTIC EPITHELIAL CELL ABNORMALITIES • Atypical squamous cells of undetermined significance(ASCUS) which represents cellular changes falling short of intraepithelial lesion. • Low-grade squamous intraepithelial lesion (LSIL) that includes CIN-I and cellular changes associated with HPV infection. • High-grade squamous intraepithelial lesion (HSIL) includes. CIN grade II, III and CIS. Squamous cell carcinoma.

Collection , fixation and Staining • • PREPARATION OF LATERAL VAGINAL SMEARS (LVS). The LVS is obtained by scraping the lateral walls of the upper third of the vagina (at the level of the cervical external os) with the flat surface of a wooden tongue depressor and smearing the material directly onto labelled slides. Fixation and Fixatives All material for cytological examination must be properly fixed to ensure preservation of cytomorphological details. Methods of fixation vary depending upon the type of staining Employed: Material for exfoliative cytodiagnosis is usually wet-fixed i. e. smears are immersed in fixative without allowing them to dry. These smears are then stained with Papanicolaou (Pap) or haematoxylin and eosin (H & E) stains. Sometimes, exfoliative cytology smears are air-dried for use with the Romanowsky stains as are used in haematologic studies. D. Staining of Smears Three staining procedures are commonly employed: Papanicolaou and H&E stains are used for wet-fixed smearswhile Romanowsky stains are used for air-dried smears. .


ETIOPATHOGENESIS. • The biology of CIN/SIL and its relationship to invasive carcinoma of the cervix is well understood by - epidemiologic, - virology, - molecular, immunologic and - ultra structural studies

ETIOPATHOGENESIS. • • Based on epidemiology of large population of women with cervical cancer, several risk factors have been identified which include the following 4 most important factors: i) Women having early age of sexual activity. ii) Women having multiple sexual partners. iii) Women with persistent HPV infection with high-risk types of oncogenes virus. iv) Potential role of high risk male sexual partner such as promiscuous male having previous multiple sexual partners, having history of penile condyloma, or male who had previous spouse with cervical cancer.

Virologic studies Human papilloma virus (HPV) • infection is strongly implicated in the etiology of cervical cancer. By recombinant DNA hybridisation techniques, following observations have been documented: • High-risk type HPV, most commonly of types 16 and 18 • Low-risk type HPV types 6 and 11 are found most frequently in condylomas. • Mixed high and low risk types of HPV may be found in Dysplasias.

Cervical intraepithelial Neoplasia (CIN). A. CIN-1: The cervical epithelium shows pronounced cellular atypia in the basal third.


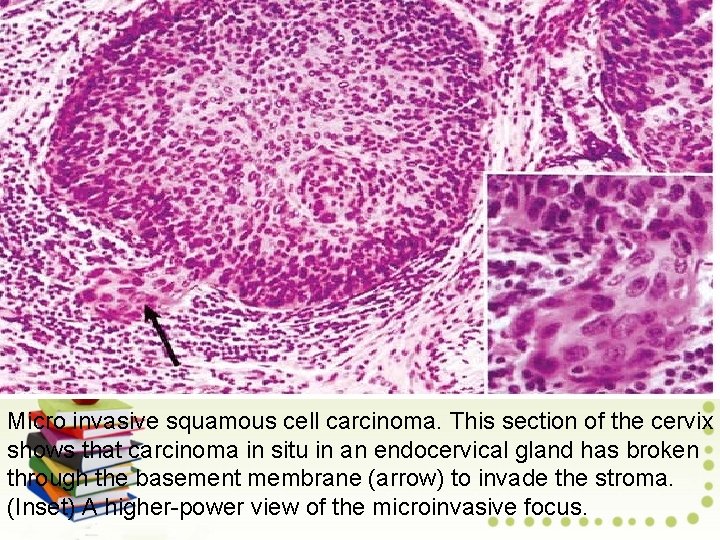
Micro invasive Squamous Cell Carcinoma • • • Earliest Stage of Invasive Cervical Cancer Micro invasive cancer features neoplastic cells that invade the stroma minimally. Small clusters of cells or solid lesions in the stroma have the following characteristics. Invasion to a depth of less than 3 mm (stage 1 A 1) or 5 mm (stage 1 A 2) below the basement membrane 7 -mm maximum lateral extension Lack of vascular invasion No lymph node metastases simple hysterectomy generally suffices to cure microinvasive cancers less than 3 mm deep.

Micro invasive squamous cell carcinoma. This section of the cervix shows that carcinoma in situ in an endocervical gland has broken through the basement membrane (arrow) to invade the stroma. (Inset) A higher-power view of the microinvasive focus.

Invasive Squamous Cell Carcinoma Epidemiology: Squamous cell carcinoma is by far the most common type of cervical cancer. Squamous cell cervical cancer is still a major cause of cancer death. A cervical cancer vaccine has recently been approved and recommended for women between the ages of 9 and 26 which in clinical trials decreased the risk of cervical cancer by 97%. Vaccinated women developed neither HPV-associated precancer nor invasive cervical cancer.

Pathology: Early stages of cervical cancer often manifest as poorly defined, granular, eroded lesions or nodular and exophytic masses

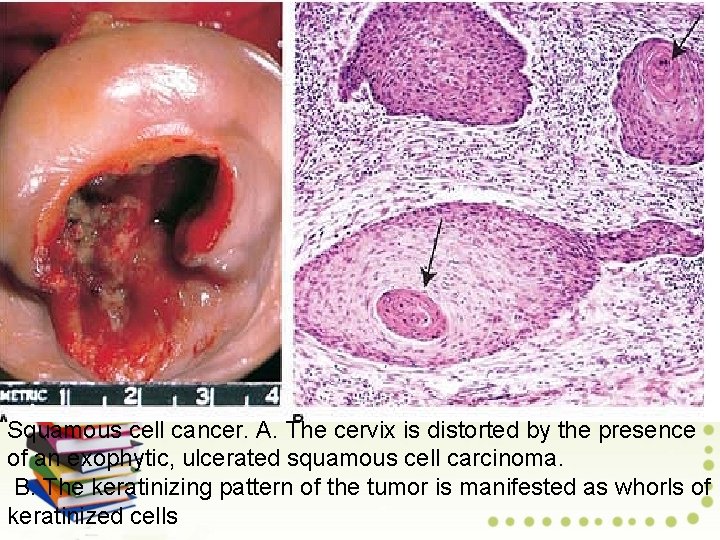
Squamous cell cancer. A. The cervix is distorted by the presence of an exophytic, ulcerated squamous cell carcinoma. B. The keratinizing pattern of the tumor is manifested as whorls of keratinized cells

Histologically On microscopic examination, most tumors display a no keratinizing pattern, characterized by solid nests of large malignant squamous cells. Occasional cancers exhibit nests of keratinized cells organized in concentric whorls, so -called keratin pearls. The least common pattern of squamous cell cancer is small cell carcinoma, which is the most aggressive type of cervical cancer and has the worst prognosis.

TYPES OF CARCINOMA 1. Epidermoid (Squamous cell) carcinoma. This type comprises vast majority of invasive cervical carcinomas (about 70%). The most common pattern (70%) is moderately differentiated. • non-keratinising large cell type and has better prognosis • Next in frequency (25%) is well-differentiated keratinising epidermoid carcinoma. • Small cell undifferentiated carcinoma (neuroendocrine or oat cell carcinoma) is less common (5%) and has a poor

2. Adenocarcinoma comprise about 20 -25% of cases. These may be well-differentiated mucus secreting adenocarcinoma, or clear cell type containing glycogen but no mucin. 3. Others. The remaining 5% cases are a variety of other patterns such as Adenosquamous carcinoma, verrucous carcinoma and undifferentiated carcinoma.


Cervical cancer spreads by direct extension and through lymphatic vessels and only rarely by the haematogenous route. Local extension into surrounding tissues (parametrium) results in urethral compression and ultimately renal failure, the most common cause of death (50% of patients). Bladder and rectal involvement may lead to fistula formation. Metastases to regional lymph nodes involve paracervical, hypo gastric, and external iliac nodes.

Clinical Features : The Pap smear remains the most reliable screening test for detecting cervical cancer. The clinical stage of cervical cancer is the best prognostic index of survival. The overall 5 -year survival rate is 60% and decreases to 10% in widely disseminated disease. Radical hysterectomy is favored for localized tumor, especially in younger women; radiation therapy or combinations of the two are used for more advanced tumors.

OVARIAN TUMORS Prof Mandira Sharma Deptt of Pathology

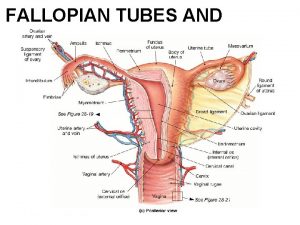
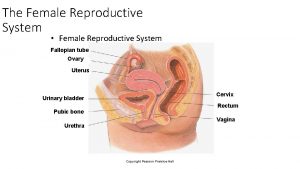
OVARIES NORMAL STRUCTURE The ovaries are paired bean-shaped organs hanging from either tube by a mesentery called the mesovarium, the lateral suspensor ligament and the ovarian ligament. The lateral suspensory ligament of the ovary contains blood vessels, lymphatics and plexuses of nerves. Histologically, the ovarian structure consists of covering by - coelomic epithelium, outer cortex inner medulla

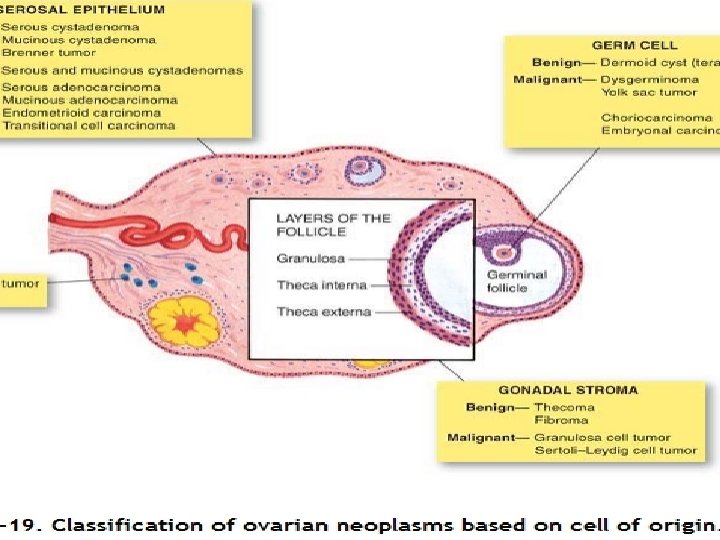
The classification of ovarian tumors identifies them by the tissue of origin. Most frequently encountered tumors arise from surface epithelium and are termed common epithelial tumors. Other important groups include Germ cell tumors, Sex cord/Stromal tumors, Steroid cell tumors, and Tumors metastatic to the ovary.


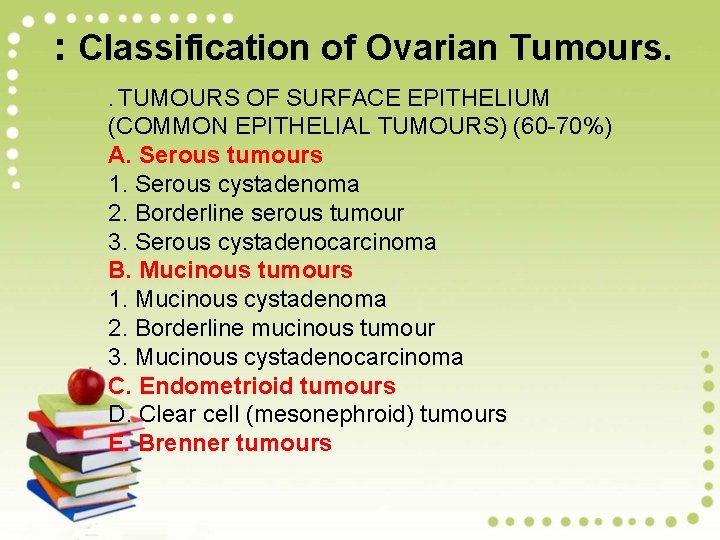
: Classification of Ovarian Tumours. . TUMOURS OF SURFACE EPITHELIUM (COMMON EPITHELIAL TUMOURS) (60 -70%) A. Serous tumours 1. Serous cystadenoma 2. Borderline serous tumour 3. Serous cystadenocarcinoma B. Mucinous tumours 1. Mucinous cystadenoma 2. Borderline mucinous tumour 3. Mucinous cystadenocarcinoma C. Endometrioid tumours D. Clear cell (mesonephroid) tumours E. Brenner tumours

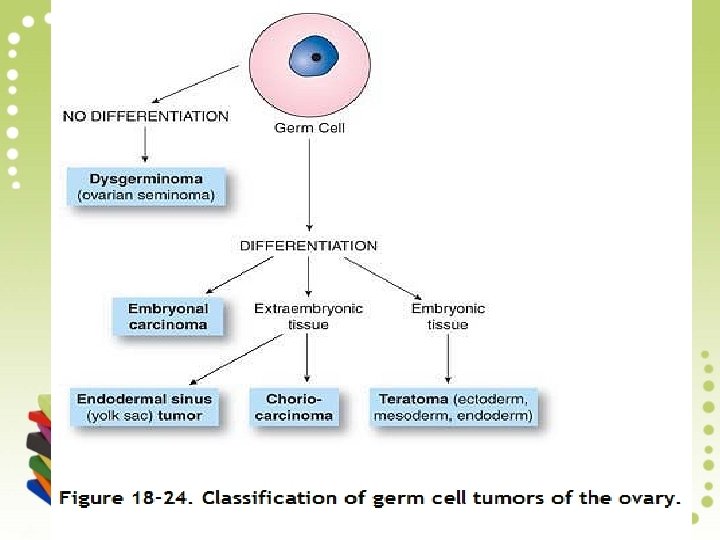
II. GERM CELL TUMOURS (15 -20%) A. Teratoma 1. Benign (mature, adult) teratoma • Benign cystic teratoma (dermoid cyst) • Benign solid teratoma 2. Malignant (immature) teratoma 3. Monodermal or specialised teratoma • Struma ovarii • Carcinoid tumour B. Dysgerminoma C. Endodermal sinus (yolk sac) tumour D. Choriocarcinoma E. Others (embryonal carcinoma, polyembryoma, mixed germ cell tumours)

III. SEX CORD-STROMAL TUMOURS (5 -10%) A. Granulosa-theca cell tumours 1. Granulosa cell tumour 2. Thecoma 3. Fibroma B. Sertoli-Leydig cell tumours (Androblastoma) C. Gynandroblastoma IV. MISCELLANEOUS TUMOURS A. Lipid cell tumours B. Gonadoblastoma V. METASTATIC TUMOURS (5%) A. Krukenberg tumour B. Others studded with multiple small (0. 5 -1. 5 cm in diameter

ETIOPATHOGENESIS Risk factors have been identified as under: 1. Null parity. 2. Heredity. About 10% cases of ovarian cancer occur in women with family history of ovarian or breast cancer. The risk in BRCA-1 carriers is higher compared to that in BRCA 2 carriers. 3. Complex genetic syndromes. i) Lynch syndrome associated with increased risk of ovarian cancer. ii) Peutz-Jeghers syndrome with ovarian sex cord-stromal tumours. iii) Gonadal dysgenesis with gonadoblastoma. iv) Nevoid basal cell carcinoma with ovarian fibromas.

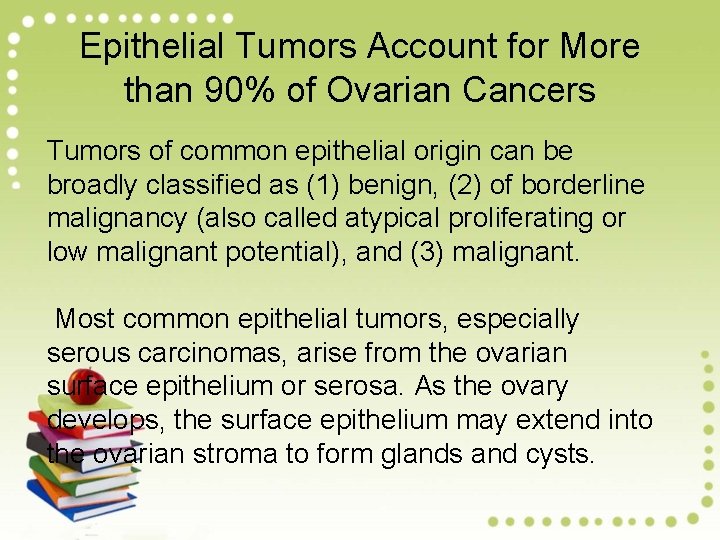
Epithelial Tumors Account for More than 90% of Ovarian Cancers Tumors of common epithelial origin can be broadly classified as (1) benign, (2) of borderline malignancy (also called atypical proliferating or low malignant potential), and (3) malignant. Most common epithelial tumors, especially serous carcinomas, arise from the ovarian surface epithelium or serosa. As the ovary develops, the surface epithelium may extend into the ovarian stroma to form glands and cysts.

Pathology: In order of decreasing frequency, the common epithelial tumors are: Serous tumors, which resemble the epithelium of the fallopian tube Mucinous tumors, which mimic the mucosa of the endocervix Endometrioid tumors, which are similar to the glands of the endometrium Clear cell tumors, which display glycogen-rich cells that resemble endometrial glands in pregnancy Transitional cell tumors, which resemble the mucosa of the bladder

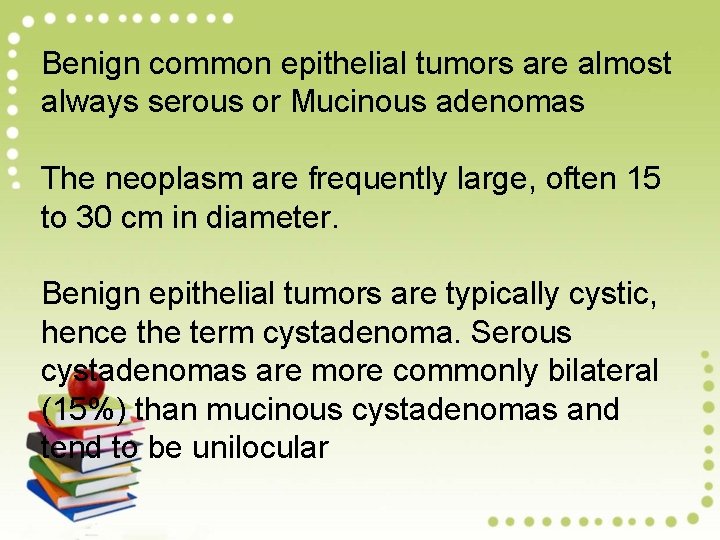
Benign common epithelial tumors are almost always serous or Mucinous adenomas The neoplasm are frequently large, often 15 to 30 cm in diameter. Benign epithelial tumors are typically cystic, hence the term cystadenoma. Serous cystadenomas are more commonly bilateral (15%) than mucinous cystadenomas and tend to be unilocular

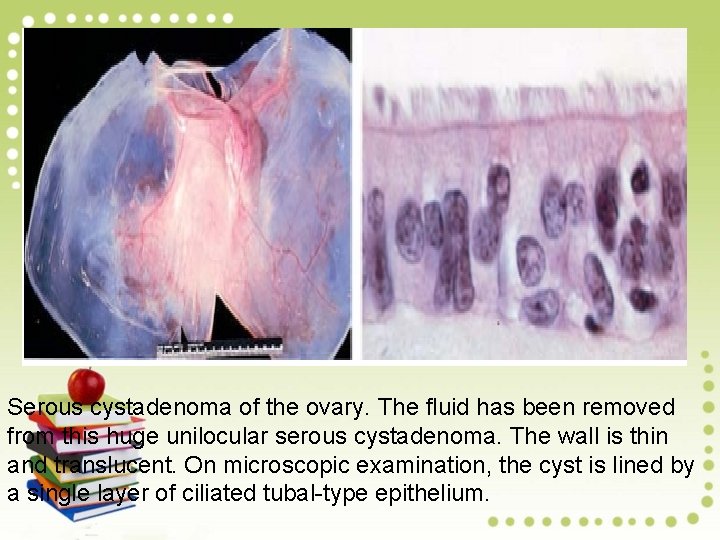
Serous cystadenoma of the ovary. The fluid has been removed from this huge unilocular serous cystadenoma. The wall is thin and translucent. On microscopic examination, the cyst is lined by a single layer of ciliated tubal-type epithelium.

Mucinous tumors characteristically show hundreds of small cysts (locules) benign ovarian epithelial tumors tend to have thin walls and lack solid areas. Microscopically, one layer of tall columnar epithelium lines the cysts. Papillae, when present, consist of a fibrovascular core covered by a single layer of tall columnar epithelium identical to that of the cyst lining.

Mucinous cystadenoma of the ovary. A. The tumor is characterized by numerous cysts filled with thick, viscous fluid. B. A single layer of mucinous epithelial cells lines the cyst.

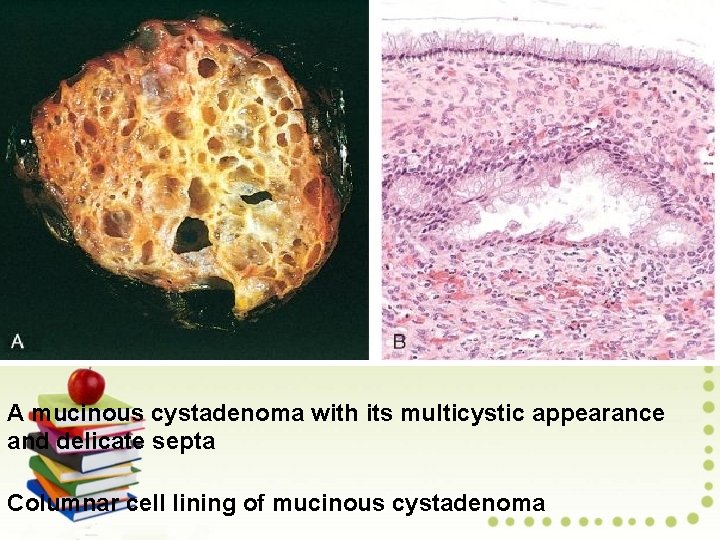
A mucinous cystadenoma with its multicystic appearance and delicate septa Columnar cell lining of mucinous cystadenoma

Borderline Tumors (Tumors of Low Malignant Potential) Borderline tumor� comprise a well-defined group of ovarian tumors that share an excellent prognosis, despite histologic features suggesting cancer. Microscopically, these structures resemble papillary fronds in benign cystadenomas, but they are distinguished from them by epithelial stratification, nuclear atypia, and mitotic activity.

The same criteria apply to borderline mucinous tumors, - although papillary projections are less conspicuous -. By definition, the presence of more than focal microinvasion - which is defined as discrete nests of epithelial cells that invade less than 3 mm into the ovarian stoma identifies a tumor as frankly malignant, rather than borderline

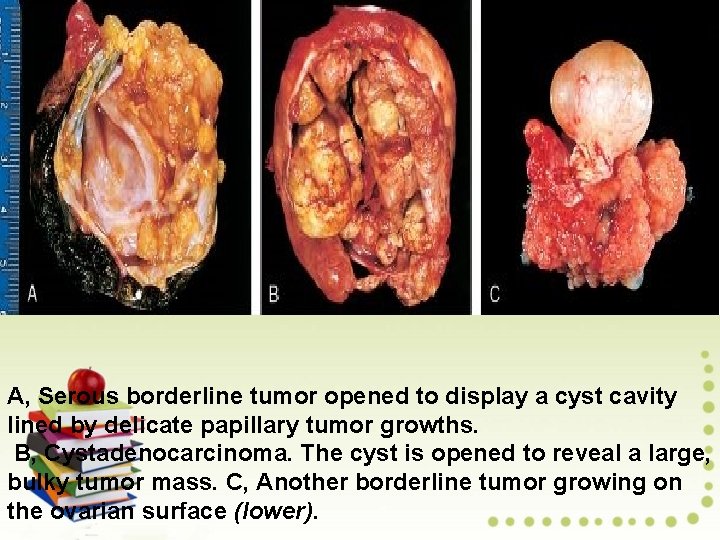
A, Serous borderline tumor opened to display a cyst cavity lined by delicate papillary tumor growths. B, Cystadenocarcinoma. The cyst is opened to reveal a large, bulky tumor mass. C, Another borderline tumor growing on the ovarian surface (lower).

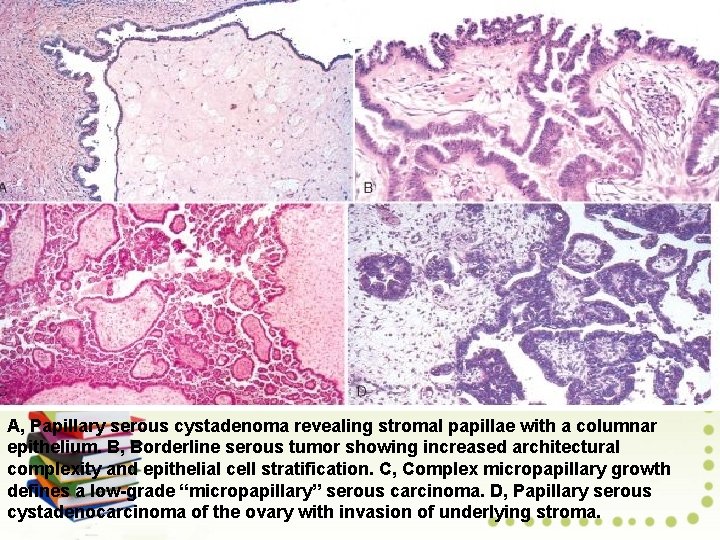
A, Papillary serous cystadenoma revealing stromal papillae with a columnar epithelium. B, Borderline serous tumor showing increased architectural complexity and epithelial cell stratification. C, Complex micropapillary growth defines a low-grade “micropapillary” serous carcinoma. D, Papillary serous cystadenocarcinoma of the ovary with invasion of underlying stroma.

Malignant Epithelial Tumors Malignant epithelial tumors of the ovary are most common between 40 and 60 years of age and are rare under the age of 35. By the time an ovarian cancer has reached 10 to 15 cm, it has often spread beyond the ovary and seeded the peritoneum.

Serous Adenocarcinoma This tumor is the most common malignancy of the ovary, accounting for one third of all ovarian cancers On gross examination, serous tumors tend to be uniform throughout and are usually uniloculated or pauci loculated, - with soft, delicate papillae lining the entire surface. -- Solid areas, often with necrosis and hemorrhage.

Microscopically, serous adenocarcinomas vary from well differentiated to poorly differentiated. - In the latter case, the papillary pattern may be inconspicuous, with most areas composed of solid sheets of malignant cells. -- Stromal and capsular invasion by the tumor cells is evident. - Laminated calcified concretions, referred to as psammoma bodies, are present in one third of cases

Mucinous Adenocarcinoma: Mucinous cystadenocarcinoma constitutes about 10% of ovarian cancers. Mucinous cancers are typically multilocular, with hundreds to thousands of small cysts. Primary ovarian mucinous tumors often contain both solid areas with clearly malignant features and cystic areas with papillary projections.

Microscopically, - The mucinous tumor may display a full range of appearances from well to poorly differentiated. - Well-differentiated mucinous tumors contain neoplastic glands lined by tall columnar, mucinproducing cells, usually with some solid or cribriform areas. - Poorly differentiated mucinous adenocarcinoma exhibit irregular nests and cords of tumor cells and numerous mitoses. - Stromal invasion is the rule, and infiltration of the serosa is common.

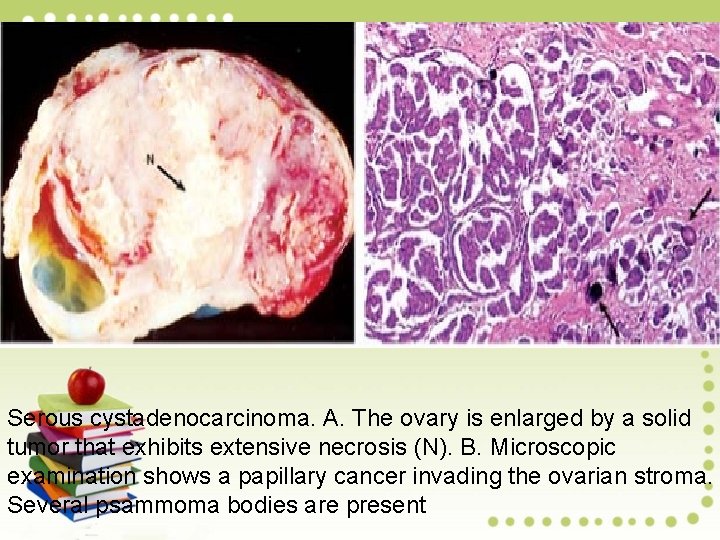
Serous cystadenocarcinoma. A. The ovary is enlarged by a solid tumor that exhibits extensive necrosis (N). B. Microscopic examination shows a papillary cancer invading the ovarian stroma. Several psammoma bodies are present

Mucinous cystadenocarcinoma. The malignant glands are arranged in a cribriform pattern and are composed of mucinproducing columnar cells.

Endometrioid Adenocarcinoma: Endometrioid adenocarcinoma histologically resembles its endometrial counterpart, may include areas of squamous differentiation. The tumor occurs most commonly after menopause. In contrast to serous and mucinous neoplasm, most endometrioid tumors are malignant.

On gross examination, endometrioid carcinomas vary in size from 2 cm to more than 30 cm. Most are largely solid and exhibit necrotic areas, although they may be cystic. Microscopically, they are graded according to the same scheme used for endometrial adenocarcinoma. As with all malignant epithelial tumors of the ovary, the prognosis depends on the stage.

Clear Cell Adenocarcinoma It constitutes 5% to 10% of all ovarian cancers, usually occurring after menopause. The size ranges from 2 to 30 cm in diameter, and 40% are bilateral. Most of these tumors are partially cystic and exhibit necrosis and hemorrhage in the solid areas. Microscopically, clear cell ovarian adenocarcinoma displays sheets or tubules of malignant cells with clear cytoplasm. In its tubular form, malignant cells often display bulbous nuclei that protrude into the lumen of the tubule.

Brenner Tumor Brenner tumors are classified as adenofibromas in which the epithelial component consists of nests of transitional-type epithelial cells resembling those lining the urinary bladder. Morphology. These neoplasm may be solid or cystic, are usually unilateral and vary in size -The fibrous stroma, resembling that of the normal ovary, is marked by sharply demarcated nests of epithelial cells resembling the epithelium of the urinary tract, often with mucinous glands in their centre. - Most Brenner tumors are benign, but borderline (proliferative Brenner tumor) and malignant counterparts have been reported.


Germ Cell Tumors derived from germ cells constitute one fourth of all ovarian tumors. In adult females, germ cell tumors are virtually all benign (mature cystic teratoma, dermoid cyst), whereas in children and young adults, they are largely cancerous. In children, germ cell tumors are the most common type of ovarian cancer (60%); they are rare after menopause.


Dysgerminoma is the ovarian counterpart of testicular seminoma and is composed of primordial germ cells. It accounts for less than 2% of all ovarian cancers. Grossly, Dysgerminomas are often large and firm and have a bosselated external surface. The cut surface is soft and fleshy.

Microscopic examination - Reveals large nests of monotonously uniform tumor cells, which have a clear glycogen-filled cytoplasm and irregularly flattened central nuclei. - Fibrous septa containing lymphocytes traverse the tumor. Dysgerminoma is treated surgically, The tumor is highly radiosensitive and also responsive to chemotherapy.


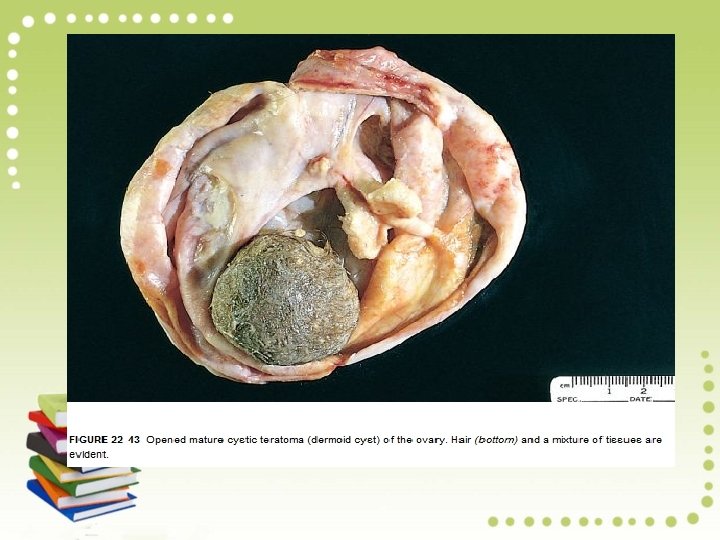
Teratoma is a tumor of germ cell origin that differentiates toward somatic structures. Most teratomas contain tissues from at least two and usually all three embryonic layers. Mature Teratoma (Mature Cystic Teratoma, Dermoid Cyst): This benign neoplasm accounts for one fourth of all ovarian tumors, with a peak incidence in the third decade.

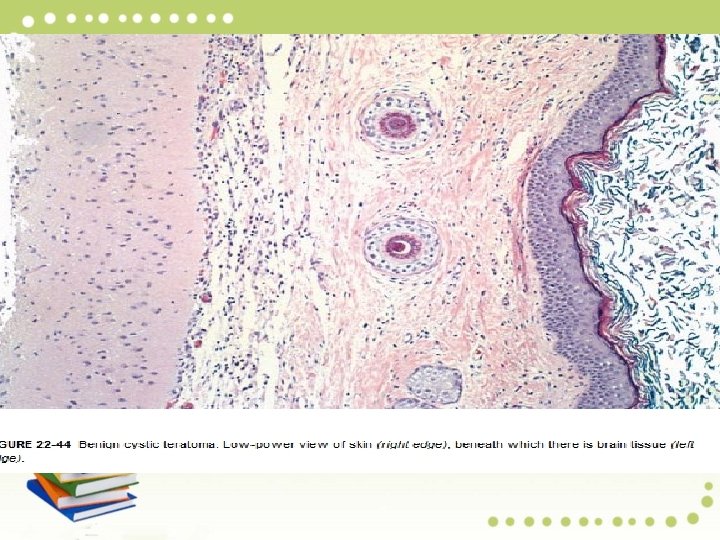
Pathology: Mature teratomas are cystic and more than 90% contain skin, sebaceous glands, and hair follicles. Half exhibit smooth muscle, sweat glands, cartilage, bone, teeth, and respiratory tract epithelium. Tissues such as gut, thyroid, and brain are seen less frequently. Struma ovarii refers to a cystic lesion composed predominantly of thyroid tissue

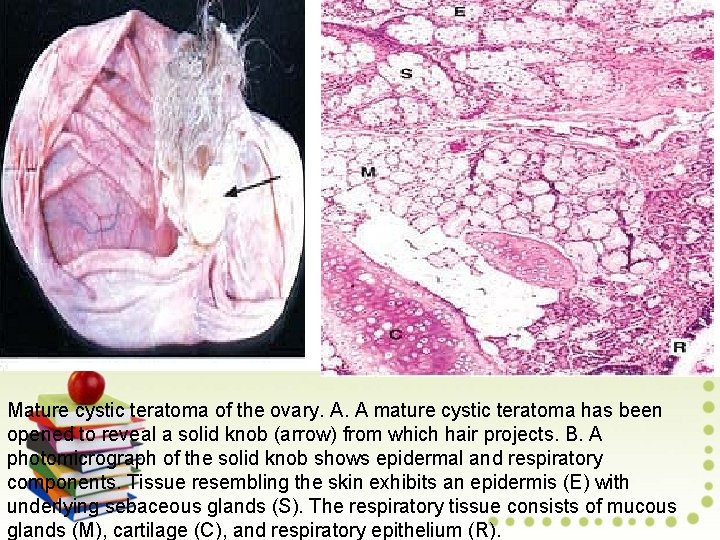
Mature cystic teratoma of the ovary. A. A mature cystic teratoma has been opened to reveal a solid knob (arrow) from which hair projects. B. A photomicrograph of the solid knob shows epidermal and respiratory components. Tissue resembling the skin exhibits an epidermis (E) with underlying sebaceous glands (S). The respiratory tissue consists of mucous glands (M), cartilage (C), and respiratory epithelium (R).



Immature Teratoma Immature teratomas of the ovary are composed of elements derived from the three germ layers. However, unlike mature cystic teratoma, the immature variety contains embryonal tissues. Pathology: Immature teratoma is predominantly solid and lobulated, with numerous small cysts. Solid areas may contain grossly recognizable immature bone and cartilage.

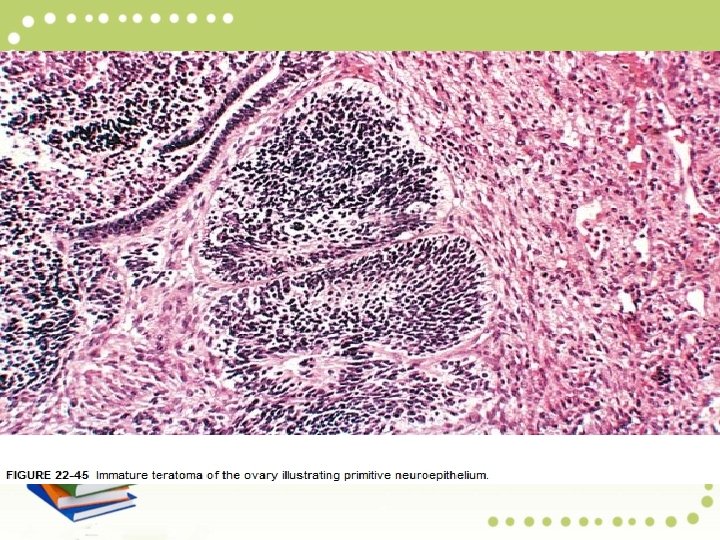
Microscopically, -Multiple tumor components are usually found, including those differentiating toward nerve (neuroepithelial rosettes and immature glia) glands and other structures found in mature cystic teratomas. - Survival correlates with tumor grade.


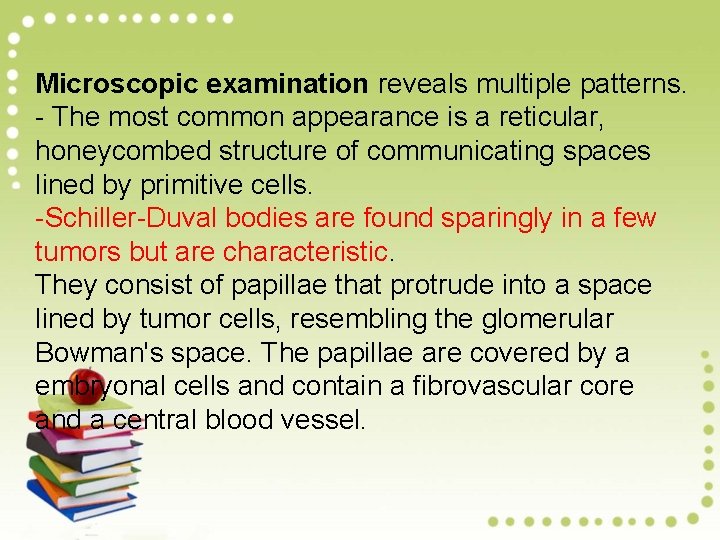
Yolk Sac Tumor Yolk sac tumor is a highly malignant tumor of women under the age of 30 years that histological resembles the mesenchyme of the primitive yolk sac. It is the second most common malignant germ cell tumor and is almost always unilateral. Pathology: Typically, the neoplasm is large and displays extensive necrosis and hemorrhage. Yolk sack tumors secrete α-fetoprotein The neoplasm was previously nearly always fatal but with chemotherapy, the 5 -year survival rate for stage I yolk sac tumors exceeds 80%.

Microscopic examination reveals multiple patterns. - The most common appearance is a reticular, honeycombed structure of communicating spaces lined by primitive cells. -Schiller-Duval bodies are found sparingly in a few tumors but are characteristic. They consist of papillae that protrude into a space lined by tumor cells, resembling the glomerular Bowman's space. The papillae are covered by a embryonal cells and contain a fibrovascular core and a central blood vessel.


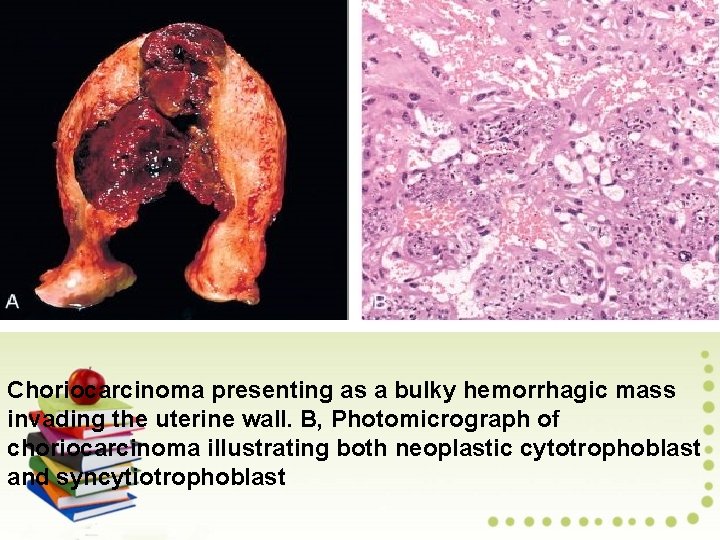
Choriocarcinoma of the ovary is a rare tumor that mimics the epithelial covering of placental villi, namely, cytotrophoblast and syncytiotrophoblast. Choriocarcinoma of germ cell origin manifests in young girls as precocious sexual development, menstrual irregularities, or rapid breast enlargement.

Pathology: Choriocarcinoma is unilateral, solid, and widely hemorrhagic. Microscopically, it shows a mixture of malignant cytotrophoblast and syncytiotrophoblast. The syncytial cells secrete h. CG, which accounts for the frequent finding of a positive pregnancy test result. Serial serum h. CG determinations are useful both for diagnosis and follow-up. The tumor is highly aggressive but responds to chemotherapy.

Choriocarcinoma presenting as a bulky hemorrhagic mass invading the uterine wall. B, Photomicrograph of choriocarcinoma illustrating both neoplastic cytotrophoblast and syncytiotrophoblast

Sex Cord/Stromal Tumors of the sex cord and stroma originate from either primitive sex cords or from mesenchymal stroma of the developing gonad. The tumors range from benign to low-grade malignant and may differentiate toward female (granulosa and theca cells) or male (Sertoli and Leydig cells) structures.

Ovarian Fibromas account for 75% of all stromal tumors and 7% of all ovarian tumors. They occur at all ages, with a peak in the perimenopausal period and are virtually always benign. Pathology: Fibromas are solid, firm, and white. Microscopically, the cells resemble the stroma of the normal ovarian cortex, appearing as welldifferentiated spindle cells embedded in variable amounts of collagen.

Large bisected fibroma of the ovary apparent as a white, firm mass (right). The fallopian tube is attached.

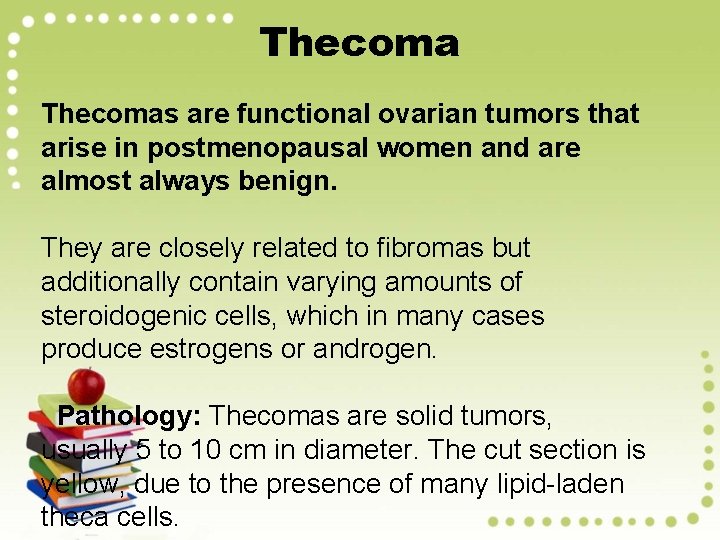
Thecomas are functional ovarian tumors that arise in postmenopausal women and are almost always benign. They are closely related to fibromas but additionally contain varying amounts of steroidogenic cells, which in many cases produce estrogens or androgen. Pathology: Thecomas are solid tumors, usually 5 to 10 cm in diameter. The cut section is yellow, due to the presence of many lipid-laden theca cells.

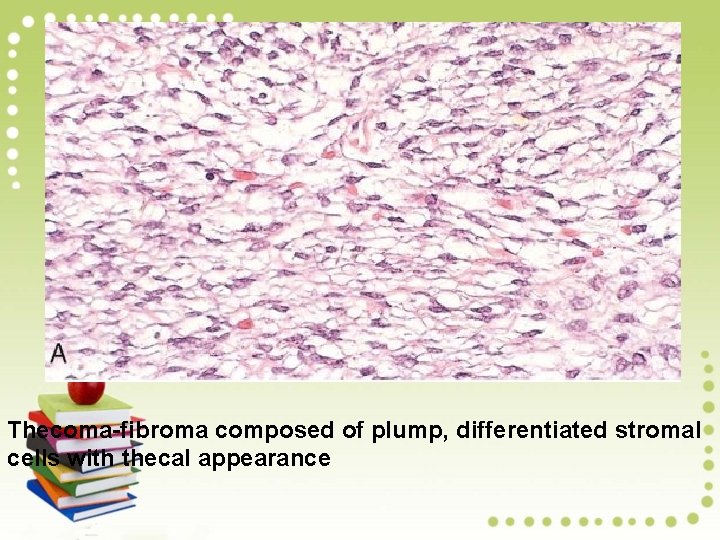
Microscopically, - The cells are large and oblong to round, with a vacuolated cytoplasm that contains lipid. - Bands of hyalinized collagen separate nests of theca cells. - Because of estrogen output by the tumor, thecomas in premenopausal women commonly cause irregularity in menstrual cycles and breast enlargement. - Endometrial hyperplasia and cancer are wellrecognized complications.

Thecoma-fibroma composed of plump, differentiated stromal cells with thecal appearance

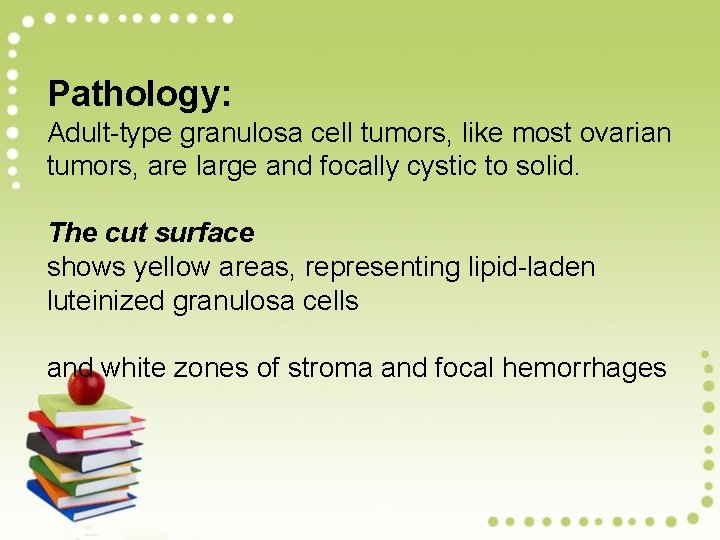
Granulosa Cell Tumor Granulosa cell tumor is the functional neoplasm of the ovary associated with estrogen secretion. - This tumor should be considered malignant because of its potential for local spread and the rare occurrence of distant metastases. Pathogenesis: Most granulosa cell tumors occur after menopause (adult form). The juvenile form, which occurs in children and young women, has distinct clinical and pathologic features (hyperestrinism and precocious puberty).

Pathology: Adult-type granulosa cell tumors, like most ovarian tumors, are large and focally cystic to solid. The cut surface shows yellow areas, representing lipid-laden luteinized granulosa cells and white zones of stroma and focal hemorrhages

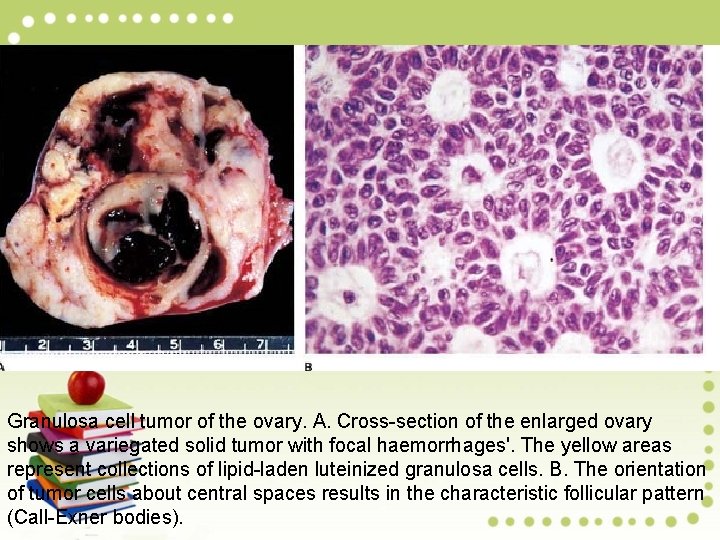
Microscopically, Granulosa cell tumors display an array of growth patterns: (1) diffuse (sarcomatoid); (2) insular (islands of cells); (3) trabecular (anastomotic bands of granulosa cells). Haphazard orientation of nuclei about a central degenerative space results in a characteristic follicular pattern (Call-Exner bodies). Tumor cells are typically spindle-shaped and commonly have a cleaved, elongated nucleus (coffee bean appearance).

Granulosa cell tumor of the ovary. A. Cross-section of the enlarged ovary shows a variegated solid tumor with focal haemorrhages'. The yellow areas represent collections of lipid-laden luteinized granulosa cells. B. The orientation of tumor cells about central spaces results in the characteristic follicular pattern (Call-Exner bodies).

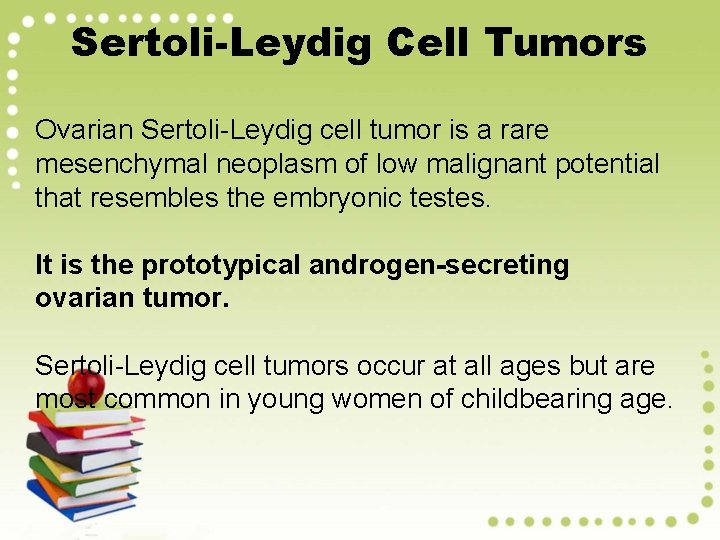
Sertoli-Leydig Cell Tumors Ovarian Sertoli-Leydig cell tumor is a rare mesenchymal neoplasm of low malignant potential that resembles the embryonic testes. It is the prototypical androgen-secreting ovarian tumor. Sertoli-Leydig cell tumors occur at all ages but are most common in young women of childbearing age.

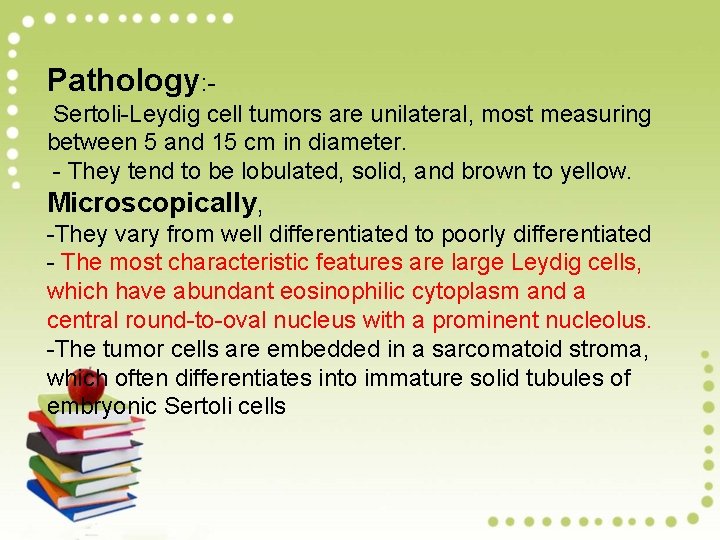
Pathology: Sertoli-Leydig cell tumors are unilateral, most measuring between 5 and 15 cm in diameter. - They tend to be lobulated, solid, and brown to yellow. Microscopically, -They vary from well differentiated to poorly differentiated - The most characteristic features are large Leydig cells, which have abundant eosinophilic cytoplasm and a central round-to-oval nucleus with a prominent nucleolus. -The tumor cells are embedded in a sarcomatoid stroma, which often differentiates into immature solid tubules of embryonic Sertoli cells

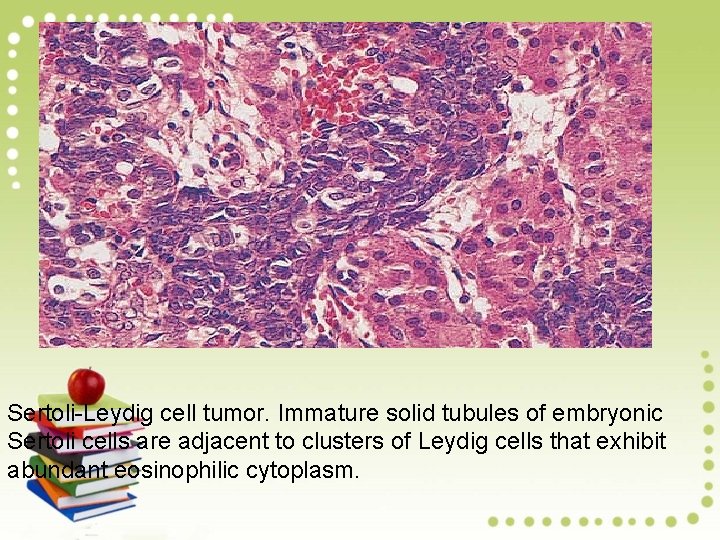
Sertoli-Leydig cell tumor. Immature solid tubules of embryonic Sertoli cells are adjacent to clusters of Leydig cells that exhibit abundant eosinophilic cytoplasm.


Tumors Metastatic to the Ovary May Mimic a Primary Tumor Metastatic tumors large enough to manifest clinically, the colon is the most frequent site of origin. The tumor cells usually stimulate the ovarian stroma to differentiate into hormonally active cells (luteinized stromal cells), thereby inducing androgenic and sometimes estrogenic symptoms. Krukenberg tumors are ovarian metastases in which the tumor appears as nests of mucin-filled within a cellular stroma derived from the ovary. The stomach is the primary site in 75% of cases, and most of the other Krukenberg tumors are from the colon.

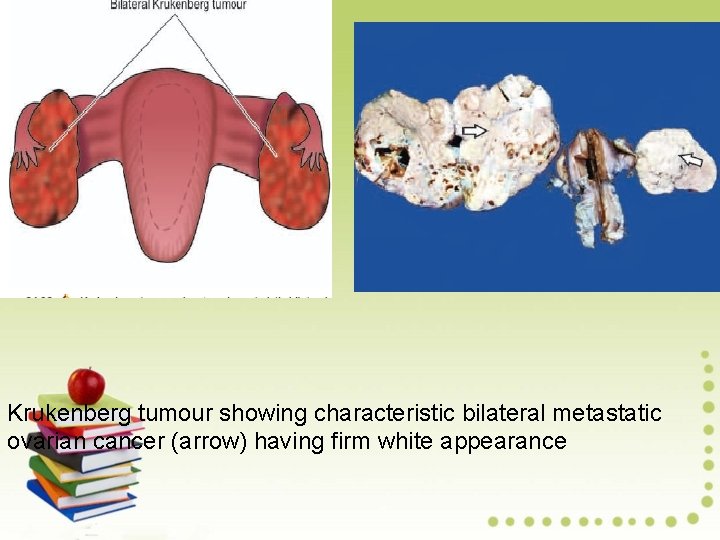
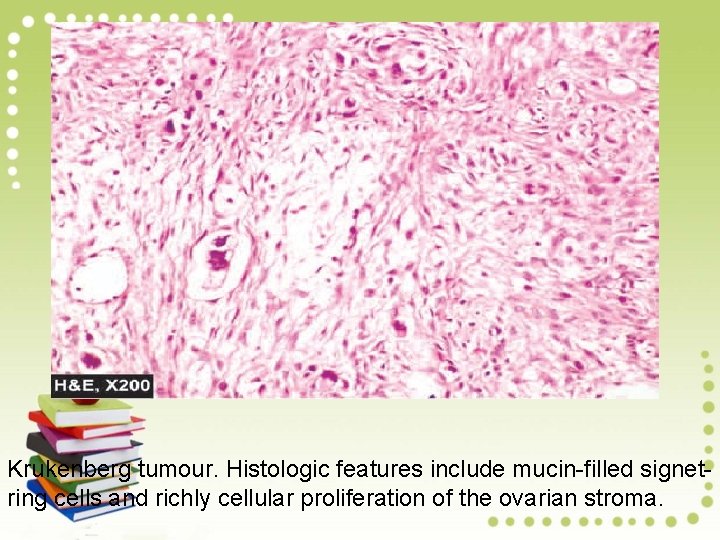
Krukenberg tumour showing characteristic bilateral metastatic ovarian cancer (arrow) having firm white appearance

Krukenberg tumour. Histologic features include mucin-filled signetring cells and richly cellular proliferation of the ovarian stroma.

Gestational Trophoblastic Disease The term gestational trophoblastic disease is a spectrum of disorders with abnormal trophoblast proliferation and maturation, as well as neoplasms derived from trophoblast. Complete Hydatidiform Mole Does Not Contain an Embryo Complete hydatidiform mole is a placenta with grossly swollen chorionic villi resembling bunches of grapes and showing varying degrees of trophoblastic proliferation. Villi are enlarged, often exceeding 5 mm in diameter

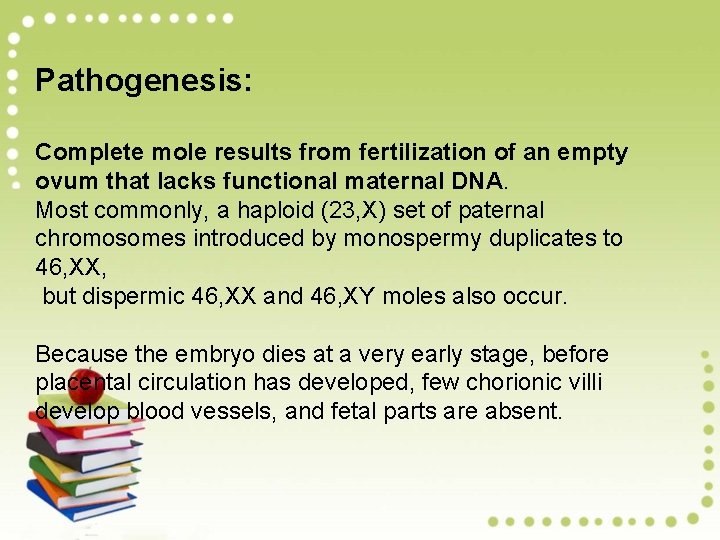
Pathogenesis: Complete mole results from fertilization of an empty ovum that lacks functional maternal DNA. Most commonly, a haploid (23, X) set of paternal chromosomes introduced by monospermy duplicates to 46, XX, but dispermic 46, XX and 46, XY moles also occur. Because the embryo dies at a very early stage, before placental circulation has developed, few chorionic villi develop blood vessels, and fetal parts are absent.

Complete hydatidiform mole. A. Complete mole in which the entire uterine cavity is filled with swollen villi. B. The villi are each 1 to 3 mm in diameter and appear grape-like. C. Individual molar villi, many of which have cavitated central cisterns, exhibit considerable trophoblastic hyperplasia and atypia. The blood vessels of the villi have atrophied and disappeared.

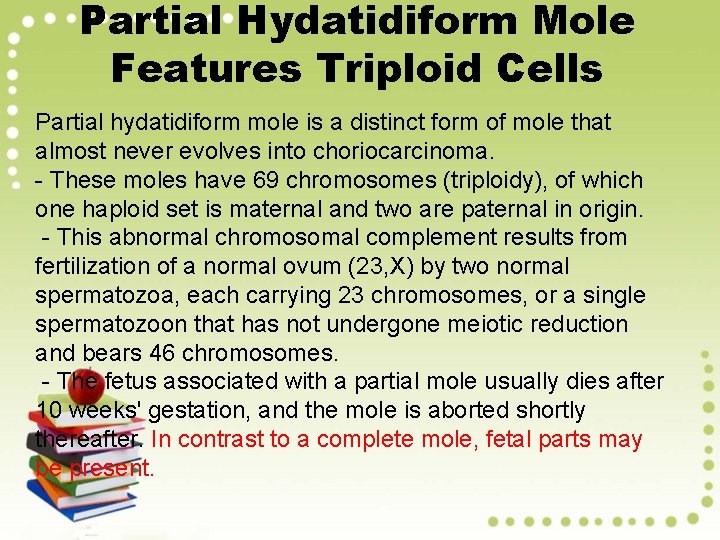
Partial Hydatidiform Mole Features Triploid Cells Partial hydatidiform mole is a distinct form of mole that almost never evolves into choriocarcinoma. - These moles have 69 chromosomes (triploidy), of which one haploid set is maternal and two are paternal in origin. - This abnormal chromosomal complement results from fertilization of a normal ovum (23, X) by two normal spermatozoa, each carrying 23 chromosomes, or a single spermatozoon that has not undergone meiotic reduction and bears 46 chromosomes. - The fetus associated with a partial mole usually dies after 10 weeks' gestation, and the mole is aborted shortly thereafter. In contrast to a complete mole, fetal parts may be present.

Pathology: Partial moles have two populations of chorionic villi. Some are normal, whereas others are enlarged by hydropic swelling and show central cavitation. Trophoblastic proliferation is focal and less pronounced than in a complete mole. Blood vessels are typically found within chorionic villi and contain fetal (nucleated) erythrocy


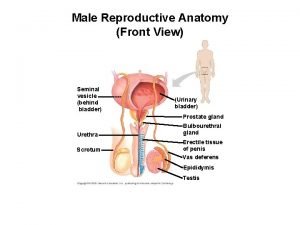
 Figure of reproductive system
Figure of reproductive system Female body parts diagram
Female body parts diagram Development of female reproductive system
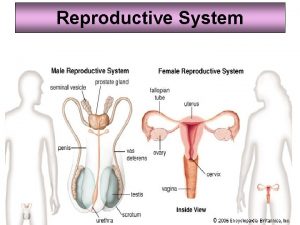
Development of female reproductive system Female and male reproductive system
Female and male reproductive system Similarities between male and female reproductive system
Similarities between male and female reproductive system Function of reproductive organs
Function of reproductive organs Parts of and functions of female reproductive system
Parts of and functions of female reproductive system What are primary sexual characteristics
What are primary sexual characteristics 90/2
90/2 Similarities between female and male reproductive system
Similarities between female and male reproductive system Cartilaginous fish vs bony fish
Cartilaginous fish vs bony fish Female reproductive system with baby
Female reproductive system with baby Femaleanatomy
Femaleanatomy Epilization
Epilization Lesson 3 the female reproductive system
Lesson 3 the female reproductive system Ovary diagram
Ovary diagram Female reproductive system functions
Female reproductive system functions Uterus is the part of which system in pila...?
Uterus is the part of which system in pila...? Male fetal pig reproductive system labeled
Male fetal pig reproductive system labeled Figure 28-1 the male reproductive system
Figure 28-1 the male reproductive system Female reproductive organs sagittal section
Female reproductive organs sagittal section Chapter 16 lesson 3 the female reproductive system
Chapter 16 lesson 3 the female reproductive system Female cow reproductive system
Female cow reproductive system Ram reproductive system
Ram reproductive system Female reproductive system internal
Female reproductive system internal Corpus vagina
Corpus vagina Lesson 3 the female reproductive system
Lesson 3 the female reproductive system Female reproductive system colour
Female reproductive system colour Female reproductive system bones
Female reproductive system bones Chapter 8 female reproductive system
Chapter 8 female reproductive system Conclusion of female reproductive system
Conclusion of female reproductive system Mesosalpinx
Mesosalpinx Female reproductive system pathology
Female reproductive system pathology Male reproductive system labeled
Male reproductive system labeled Cow reproductive anatomy
Cow reproductive anatomy Female reproductive system pathology
Female reproductive system pathology Female reproductive system pathology
Female reproductive system pathology Female reproductive system
Female reproductive system Abortion types
Abortion types Uterus seal
Uterus seal Bovine female reproductive system
Bovine female reproductive system Female reproductive system anatomy
Female reproductive system anatomy Female reproductive system pathology
Female reproductive system pathology Figure 28-1 the male reproductive system
Figure 28-1 the male reproductive system Diagram of male reproductive organ
Diagram of male reproductive organ Vagina diagram labeled
Vagina diagram labeled Female reproductive system
Female reproductive system Female reproductive system
Female reproductive system Female external genitalia
Female external genitalia Female reproductive system external
Female reproductive system external Female reproductive system
Female reproductive system A hormone
A hormone Clitoris structure
Clitoris structure Animal female reproductive system diagram
Animal female reproductive system diagram Chapter 16 lesson 3 the female reproductive system
Chapter 16 lesson 3 the female reproductive system Poem about female reproductive system
Poem about female reproductive system Sperm fructose
Sperm fructose Female reproductive system se-6
Female reproductive system se-6 Inside ovary diagram
Inside ovary diagram Female reproductive system conclusion
Female reproductive system conclusion Female reproductive system
Female reproductive system Female reproductive system
Female reproductive system The functions of ovary
The functions of ovary Internal anatomy of squid
Internal anatomy of squid Mesovariu
Mesovariu Inguinal
Inguinal Labia minora glands
Labia minora glands Compylotropous ovules are found in
Compylotropous ovules are found in Functions of ovary
Functions of ovary Fallopian tube
Fallopian tube Endocrine system and reproductive system
Endocrine system and reproductive system Dysplasi i cervix uteri
Dysplasi i cervix uteri Cin
Cin Function of the vagina
Function of the vagina Average size of cervix
Average size of cervix Physiology of cervix
Physiology of cervix Portio anatomie
Portio anatomie Benign tumors in the uterus
Benign tumors in the uterus Apex vesicae
Apex vesicae Bishop's score
Bishop's score Puerperiu
Puerperiu Cervix nedir
Cervix nedir Cervixconisatie
Cervixconisatie Acrimon
Acrimon Uterus cervix
Uterus cervix Interior cervix
Interior cervix Papsmear
Papsmear Gestationssæk
Gestationssæk Clitorus
Clitorus Cervix da vaca
Cervix da vaca Cervix da vaca
Cervix da vaca Cervix da vaca
Cervix da vaca Theca interna
Theca interna Cervix anatomy
Cervix anatomy Obstetrical outlet
Obstetrical outlet Magnesium sulfate for preterm labor
Magnesium sulfate for preterm labor Cervix fibonacci
Cervix fibonacci Birth process stages
Birth process stages Tricomonase vaginalis
Tricomonase vaginalis Reproductive hygiene
Reproductive hygiene Chapter 17 reproductive system diseases and disorders
Chapter 17 reproductive system diseases and disorders Functions of testis
Functions of testis Excretory and reproductive system
Excretory and reproductive system Chapter 16 the reproductive system
Chapter 16 the reproductive system Anatomy of the reproductive system exercise 42
Anatomy of the reproductive system exercise 42 Corpus albicans vs corpus luteum
Corpus albicans vs corpus luteum Epidiymitis
Epidiymitis Reproductive system jeopardy
Reproductive system jeopardy Graham munson
Graham munson Luteinizing hormone in male reproductive system
Luteinizing hormone in male reproductive system Exterior and interior of an egg
Exterior and interior of an egg Reproduction in human
Reproduction in human Art-labeling activity: the male reproductive system, part 1
Art-labeling activity: the male reproductive system, part 1 Poultry reproductive system
Poultry reproductive system Male reproductive system information
Male reproductive system information Where is sperm stored in the male body
Where is sperm stored in the male body Seminal vesicles
Seminal vesicles Male reproductive system plants
Male reproductive system plants Function of male reproductive system
Function of male reproductive system