Pathologic Intimal Hyperplasia as a Response to Vascular

![Background • Ebolectomy catheter induced intimal thickening (accumulation of smooth muscle cells [SMCs] and Background • Ebolectomy catheter induced intimal thickening (accumulation of smooth muscle cells [SMCs] and](https://slidetodoc.com/presentation_image_h/fe3fee46c96ed9d776a3693e89e1e197/image-2.jpg)



- Slides: 26

Pathologic Intimal Hyperplasia as a Response to Vascular Injury and Reconstruction
![Background Ebolectomy catheter induced intimal thickening accumulation of smooth muscle cells SMCs and Background • Ebolectomy catheter induced intimal thickening (accumulation of smooth muscle cells [SMCs] and](https://slidetodoc.com/presentation_image_h/fe3fee46c96ed9d776a3693e89e1e197/image-2.jpg)
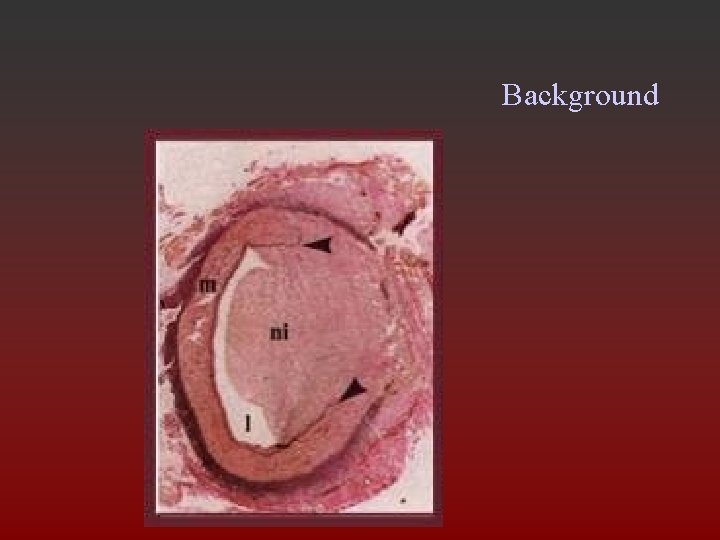
Background • Ebolectomy catheter induced intimal thickening (accumulation of smooth muscle cells [SMCs] and extracellular matrix) • Balloon angioplasty – 30% coronary arteries develop marked restenosis after 6 months – fibrous with many SMCs

Background • Carotid endarterectomy – intimal hyperplasia >50% stenosis in 10 -20%, symptomatic in 1% • Vascular grafts, reversed or in situ, autologous or synthetic

Background

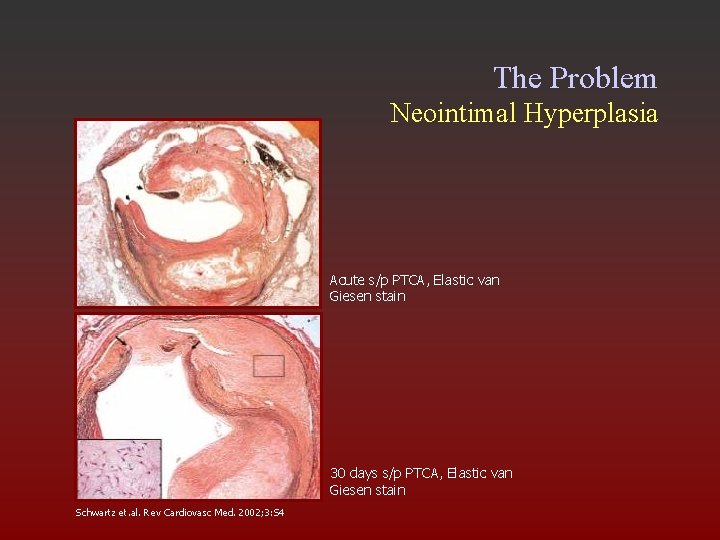
The Problem Neointimal Hyperplasia Acute s/p PTCA, Elastic van Giesen stain 30 days s/p PTCA, Elastic van Giesen stain Schwartz et. al. Rev Cardiovasc Med. 2002; 3: S 4

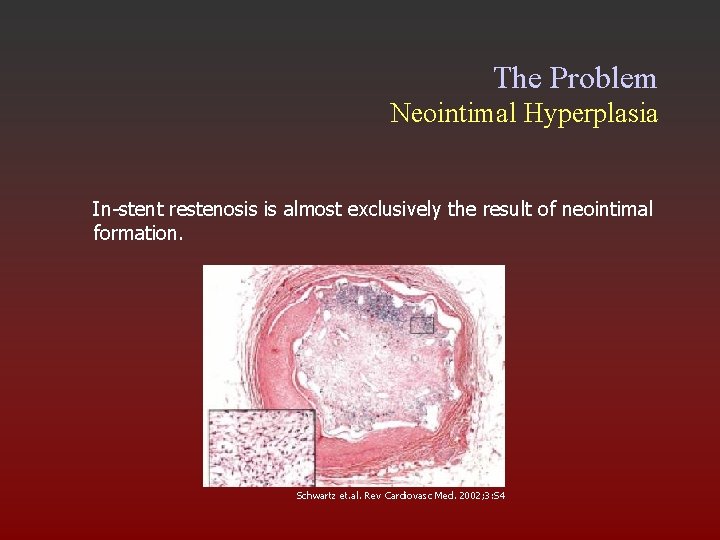
The Problem Neointimal Hyperplasia In-stent restenosis is almost exclusively the result of neointimal formation. Schwartz et. al. Rev Cardiovasc Med. 2002; 3: S 4

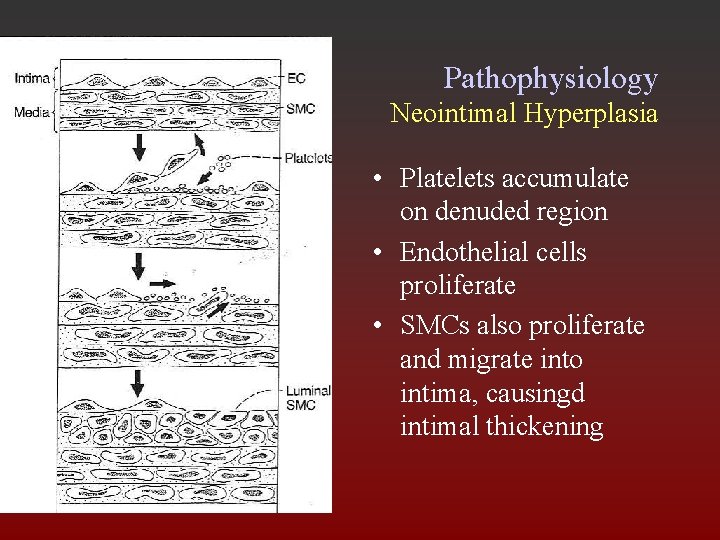
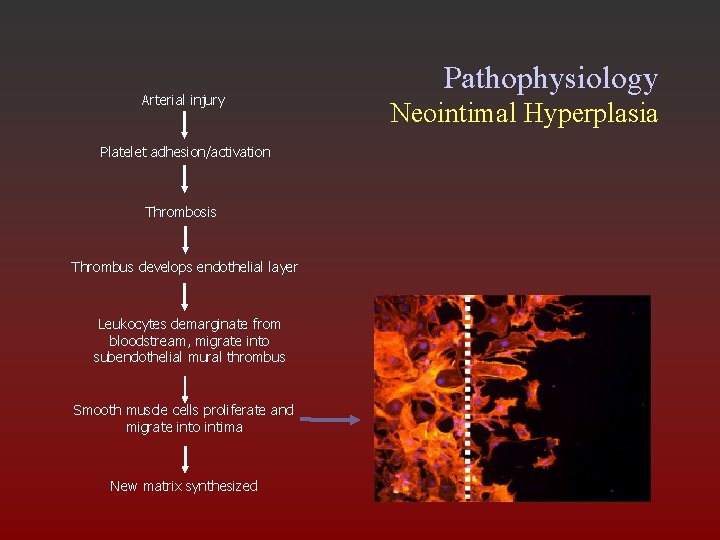
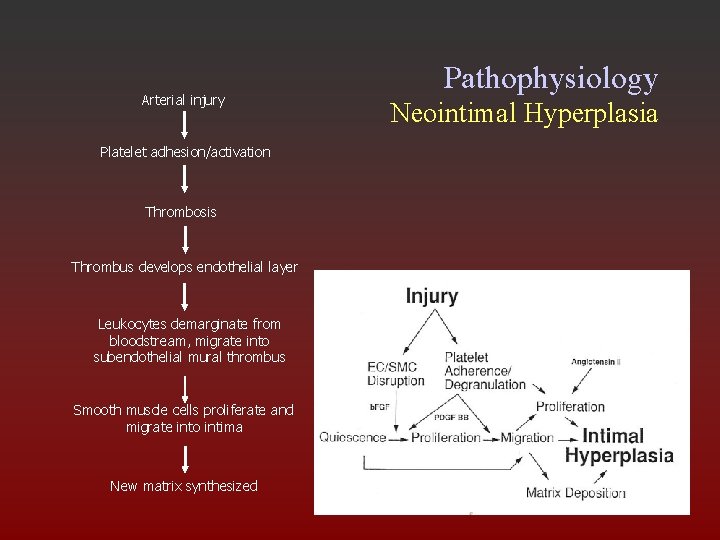
Pathophysiology Neointimal Hyperplasia • Platelets accumulate on denuded region • Endothelial cells proliferate • SMCs also proliferate and migrate into intima, causingd intimal thickening

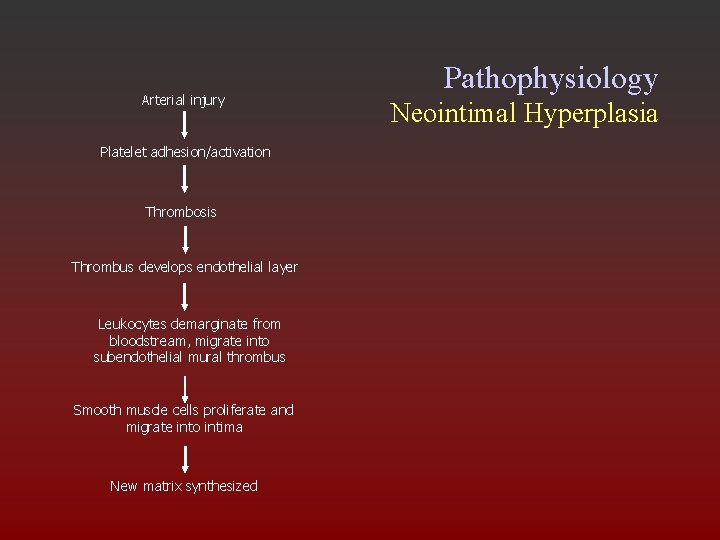
Arterial injury Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Pathophysiology Neointimal Hyperplasia

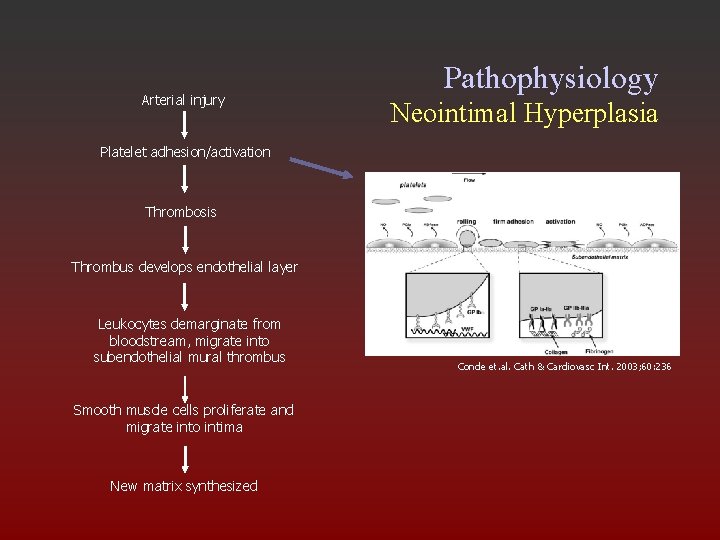
Arterial injury Pathophysiology Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Conde et. al. Cath & Cardiovasc Int. 2003; 60: 236

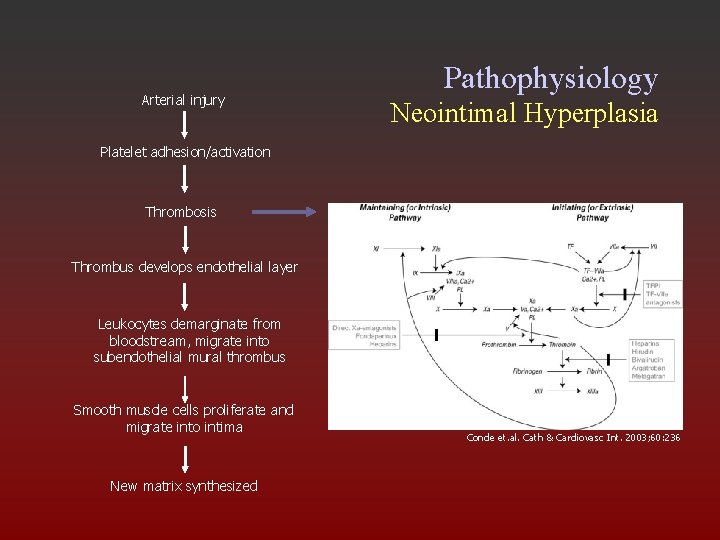
Arterial injury Pathophysiology Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Conde et. al. Cath & Cardiovasc Int. 2003; 60: 236

Arterial injury Pathophysiology Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima Conde et. al. Cath & Cardiovasc Int. 2003; 60: 236 New matrix synthesized

Arterial injury Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Pathophysiology Neointimal Hyperplasia

Arterial injury Pathophysiology Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized • Factors from platelets, leukocytes, smooth muscle cells, and extracellular matrix interact and regulate the process of intimal hyperplasia, making each step a potential therapeutic target

Arterial injury Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Pathophysiology Neointimal Hyperplasia

Treatment Vascular reconstruction • Principal way to salvage failing grafts • Based on assumption that renewed or continued intimal thickening is unlikely • With regular follow-up, stenoses in vein grafts may be discovered prior to graft thrombosis • If these lesions are reconstructed in time, long-term outcome is generally good

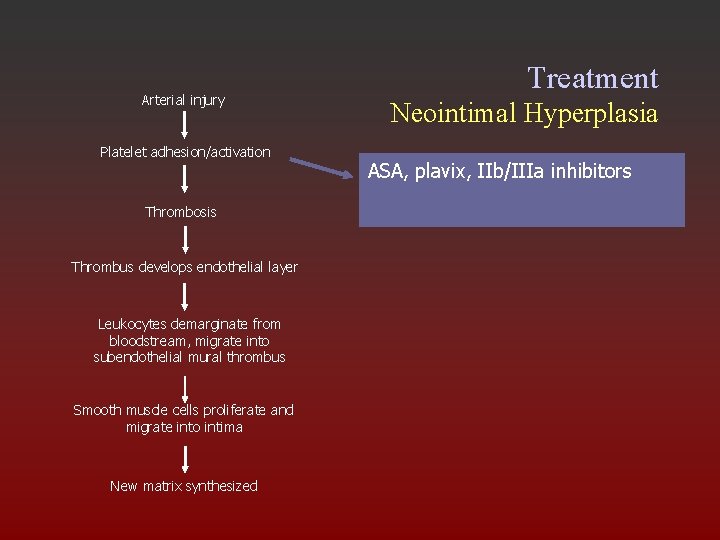
Arterial injury Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Treatment Neointimal Hyperplasia ASA, plavix, IIb/IIIa inhibitors

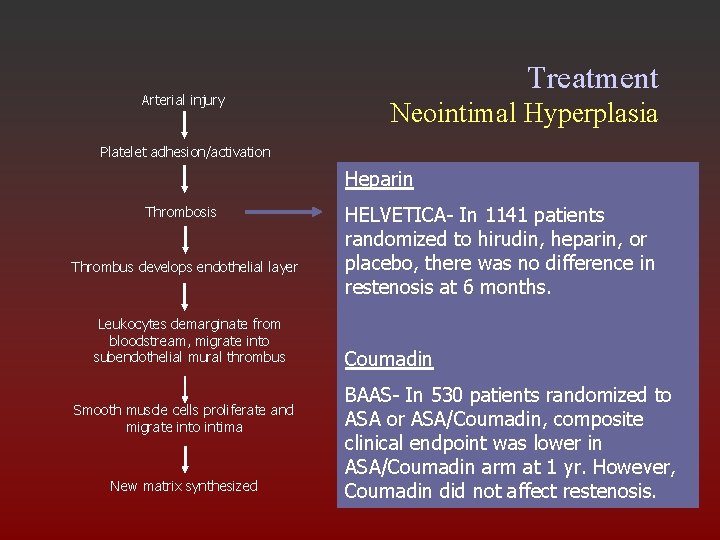
Arterial injury Treatment Neointimal Hyperplasia Platelet adhesion/activation Heparin Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized HELVETICA- In 1141 patients randomized to hirudin, heparin, or placebo, there was no difference in restenosis at 6 months. Coumadin BAAS- In 530 patients randomized to ASA or ASA/Coumadin, composite clinical endpoint was lower in ASA/Coumadin arm at 1 yr. However, Coumadin did not affect restenosis.

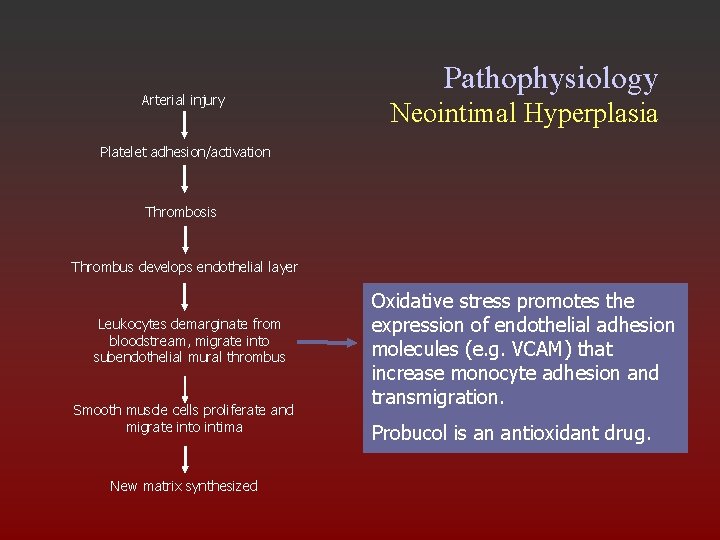
Arterial injury Pathophysiology Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Oxidative stress promotes the expression of endothelial adhesion molecules (e. g. VCAM) that increase monocyte adhesion and transmigration. Probucol is an antioxidant drug.

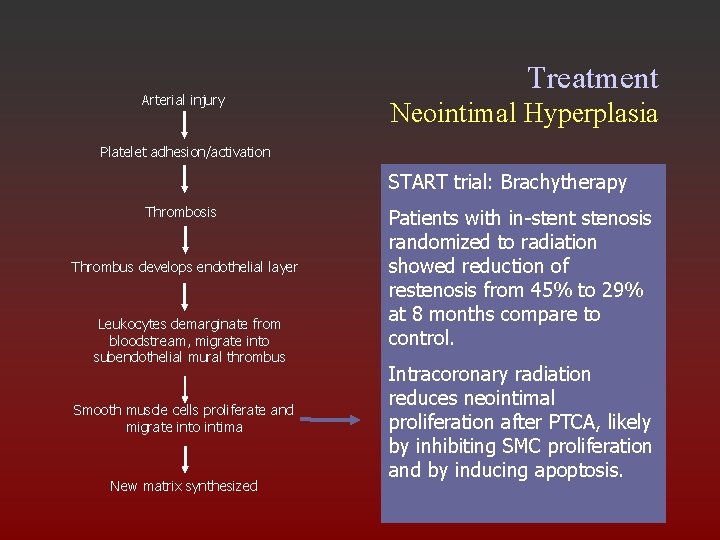
Arterial injury Treatment Neointimal Hyperplasia Platelet adhesion/activation START trial: Brachytherapy Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized Patients with in-stent stenosis randomized to radiation showed reduction of restenosis from 45% to 29% at 8 months compare to control. Intracoronary radiation reduces neointimal proliferation after PTCA, likely by inhibiting SMC proliferation and by inducing apoptosis.

Arterial injury Treatment Neointimal Hyperplasia Platelet adhesion/activation Thrombosis Thrombus develops endothelial layer Leukocytes demarginate from bloodstream, migrate into subendothelial mural thrombus Smooth muscle cells proliferate and migrate into intima New matrix synthesized MCAM is an Ig superfamily adhesion molecule expressed on endothelial cells and circulating endothelial precursors. It mediates cell adhesion and intracellular signal transduction.

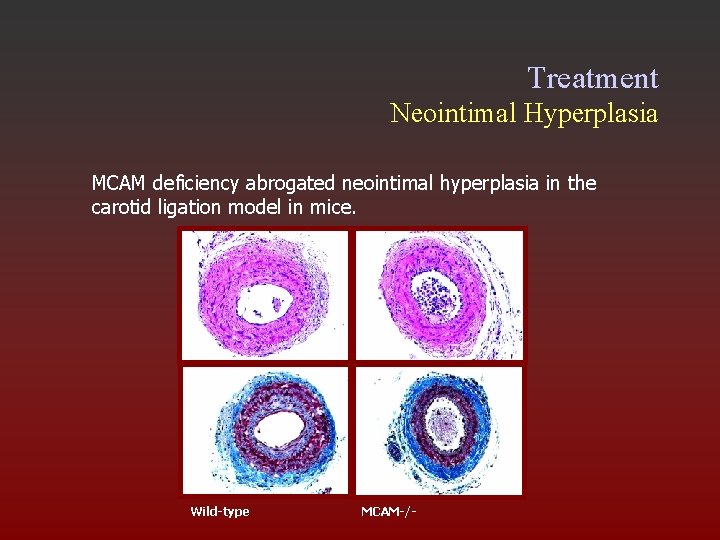
Treatment Neointimal Hyperplasia MCAM deficiency abrogated neointimal hyperplasia in the carotid ligation model in mice. ? ? ? Wild-type MCAM-/-

Treatment Restenosis and Drug-Eluting Stents A. Rapamycin (Sirolimus): -A macrolide produced by Streptomyces hygroscopicus -Found in soil from Easter Island in 1974 -Inhibits migration and proliferation of vascular smooth muscle cells -Anti-inflammatory properties

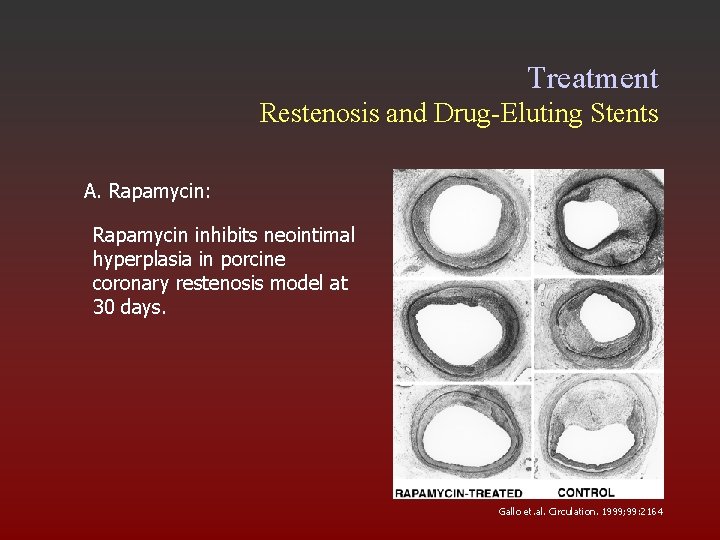
Treatment Restenosis and Drug-Eluting Stents A. Rapamycin: Rapamycin inhibits neointimal hyperplasia in porcine coronary restenosis model at 30 days. Gallo et. al. Circulation. 1999; 99: 2164

Treatment Restenosis and Drug-Eluting Stents B. Paclitaxel -An anti-neoplastic drug derived from Pacific yew tree. -Inhibits migration and proliferation by enhancing microtubule assembly.

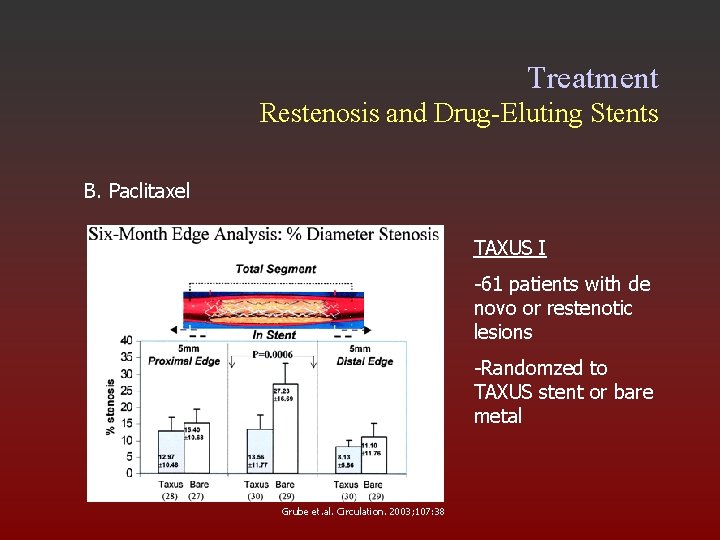
Treatment Restenosis and Drug-Eluting Stents B. Paclitaxel TAXUS I -61 patients with de novo or restenotic lesions -Randomzed to TAXUS stent or bare metal Grube et. al. Circulation. 2003; 107: 38

Conclusion Intimal hyperplasia is a complex response to injury. Vascular reconstruction is effective in salvage of vein grafts if performed in time Antiplatelet agents are effective if given at or withing a short time of surgery Development of effective therapy requires an understanding of the underlying pathophysiology.