Periodic Trends Periodic Law When elements are arranged










![e- Configurations of Anions & Cations Na: [Ne]3 s 1 Na+: [Ne] Ca: [Ar]4 e- Configurations of Anions & Cations Na: [Ne]3 s 1 Na+: [Ne] Ca: [Ar]4](https://slidetodoc.com/presentation_image/5983958d81a3b5489420067048af75ae/image-27.jpg)

- Slides: 31

Periodic Trends

Periodic Law When elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties.

Elements’ Electron Configuration Elements in same column have similar valence e- configurations Explains similarity of chemical properties All alkali readily give up s orbital e Noble gases have filled energy levels, therefore unreactive Transition metals are filling the d orbitals, therefore colorful and multivalanced ions Helps explains periodic trends

Effective nuclear charge Positive charge or pull felt by the electrons from the nucleus. Trend in Effective Nuclear Charge Increases as you move from left to right across a period. Stays the same as you move down a group.

Going Down a Group Distance of valence e- from nucleus increases. Therefore attraction between valence e - and protons in nucleus decreases (Coulomb's Law. ) Because of this there will be less of an attraction for electrons from other atoms and electrons that are there aren’t as tightly held on.

Reasons for the Trends Effective nuclear charge increases across a period. Attraction or Pull from the nucleus increases! Distance from nucleus increases down a group. Decreased attraction down a group due to increased distance from nucleus. (Coulomb’s Law!)

Periodic Trends Prediction of periodic atomic properties Atomic Radius Ionic radius Cations Anions Ionization energy Electronegativity Metallic Character/Reactivity Nonmetallic Character/Reactivity Oxidation States

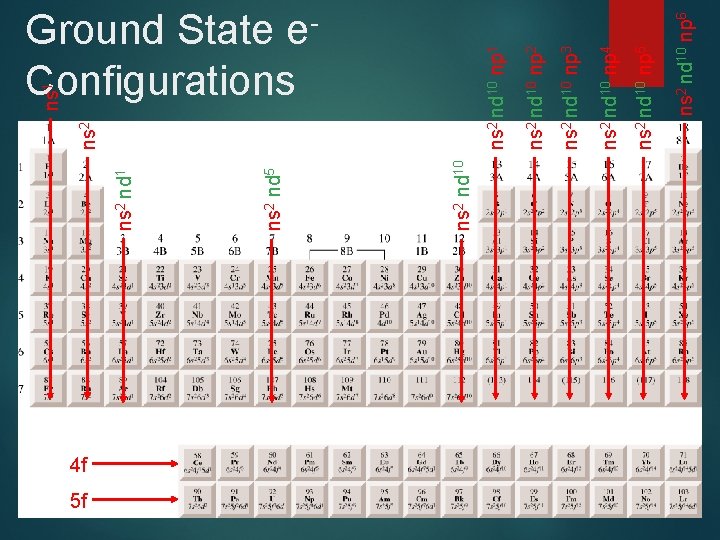
4 f 5 f ns 2 nd 10 ns 2 nd 5 ns 2 nd 10 np 6 ns 2 nd 10 np 5 ns 2 nd 10 np 4 ns 2 nd 10 np 3 ns 2 nd 10 np 2 ns 2 nd 10 np 1 ns 2 ns 1 Ground State Configurations e

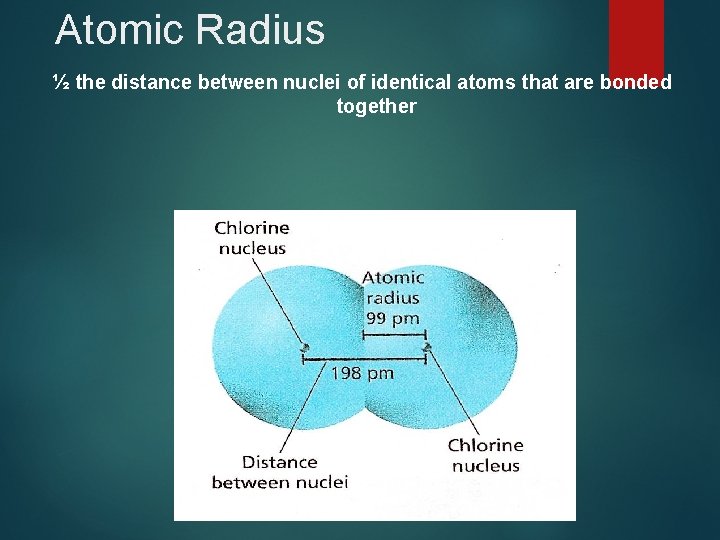
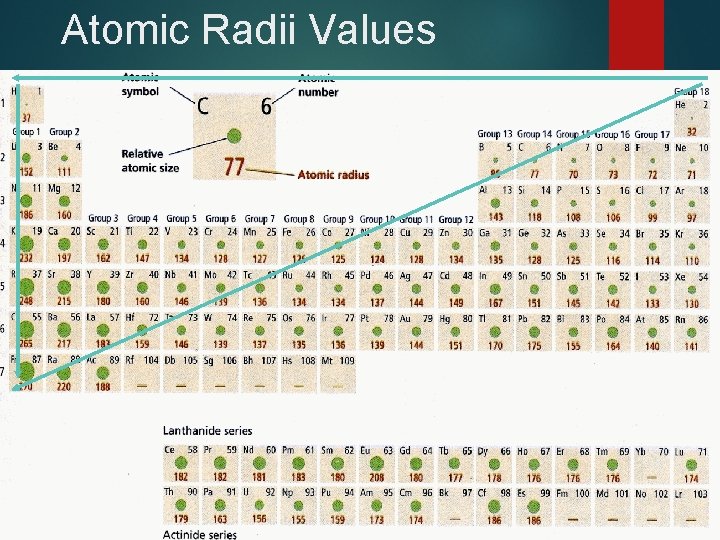
Atomic Radius ½ the distance between nuclei of identical atoms that are bonded together

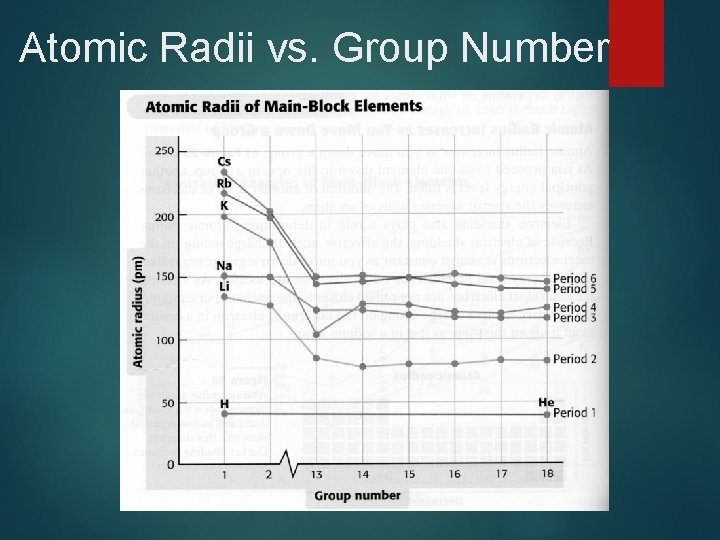
Atomic Radii Trends Across a period, radii decreases from left to right. Zeff increases thus increasing the pull from the nucleus. Down a family radii increase. The highest energy level occupied by electrons increases. Distance electrons are from nucleus increases.

Atomic Radii vs. Group Number

Atomic Radii Values

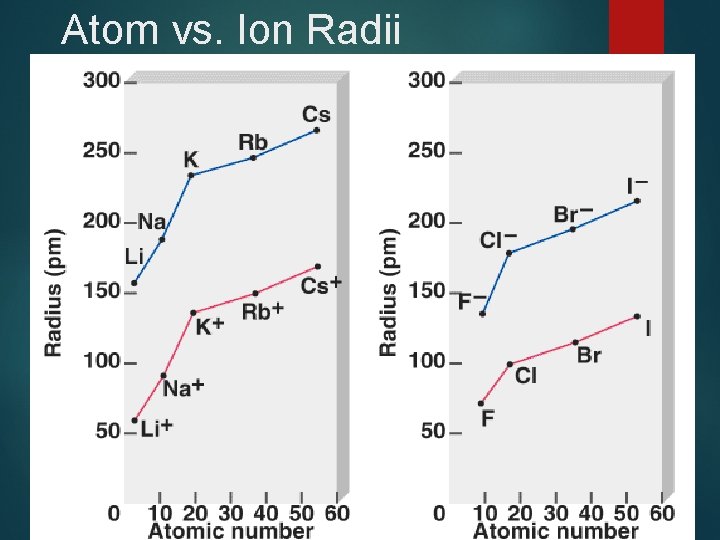
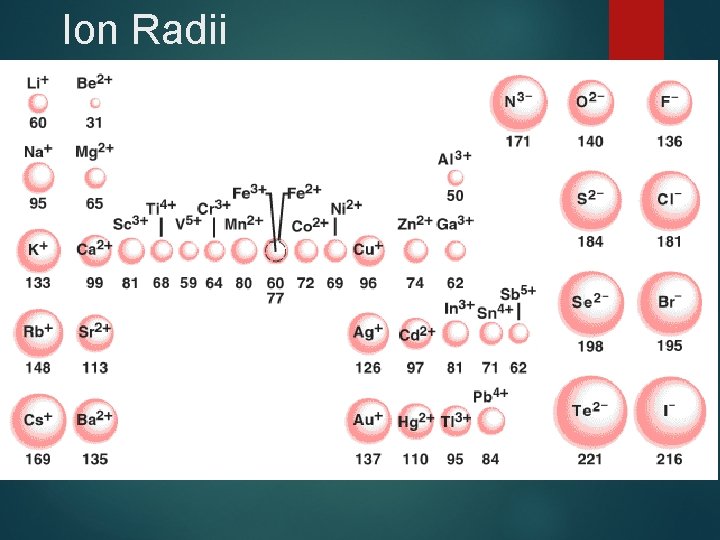
Ion size - Cations Ø Ø Ø Cations are smaller than parent atom Cations sizes generally decrease from left to right as Zeff increases Cations sizes increase down a family as valence electrons distance from nucleus increases

Ion Size - Anions are larger than parent element For isoelectronic anions Ionic radii decrease across row from left to right as Zeff increases. Ionic radii increase down a family as valence electrons distance from nucleus increases.

Atom vs. Ion Radii

Ion Radii

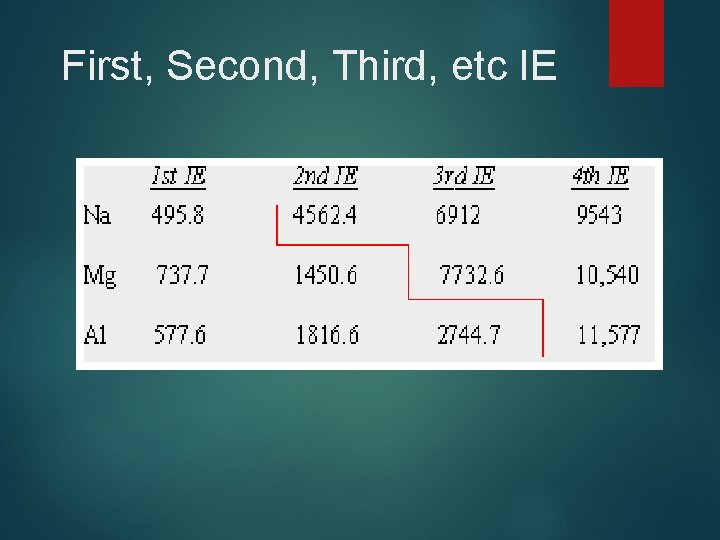
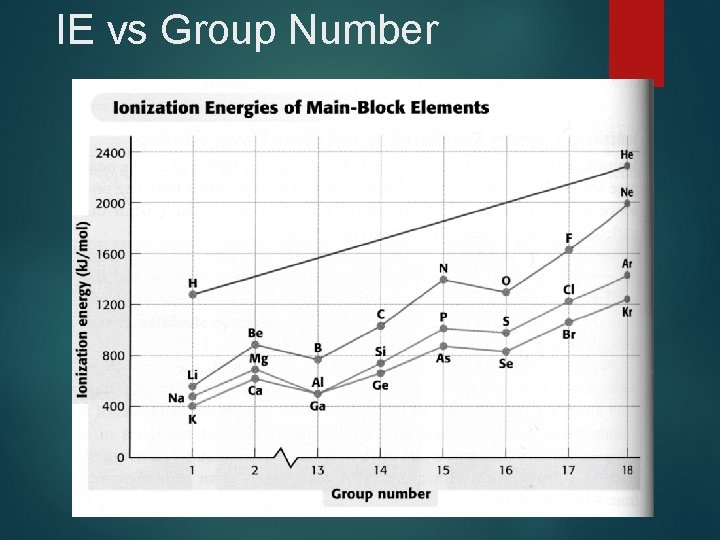
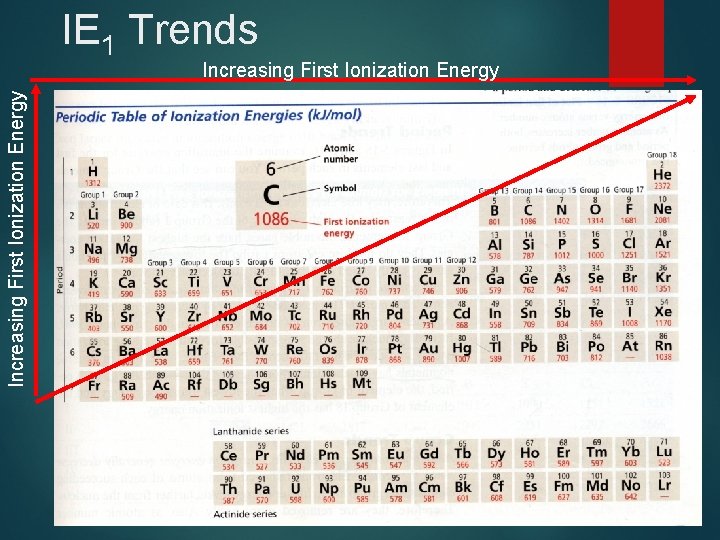
First Ionization Energy, IE 1 Energy necessary to remove the outermost efrom a gaseous atom n IE 1 increases generally from left to right because of increasing Zeff n IE 1 decreases down a group as valence eget farther from the nucleus (distance increases)

First Ionization Energy, IE 1 Energy necessary to remove the outermost efrom a gaseous atom n IE 1 increases generally from left to right because of increasing Zeff n IE 1 decreases down a group as valence eget farther from the nucleus (distance increases)

First Ionization Energy Graph

First, Second, Third, etc IE

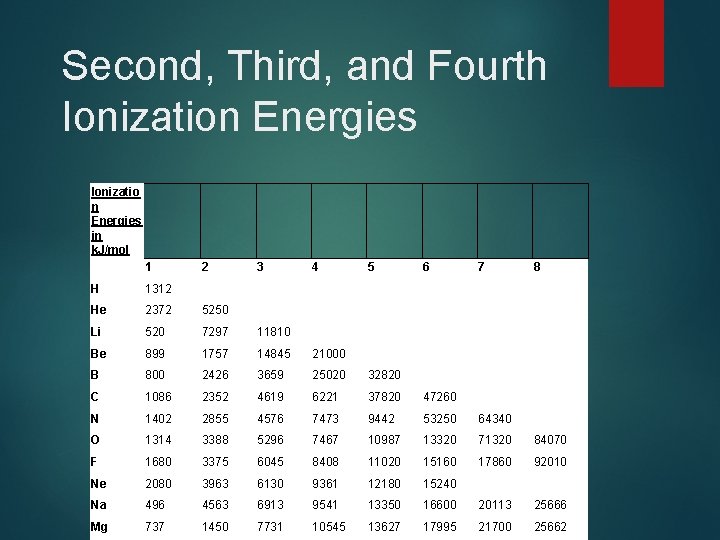
Second, Third, and Fourth Ionization Energies Ionizatio n Energies in k. J/mol 1 2 3 4 5 6 7 8 H 1312 He 2372 5250 Li 520 7297 11810 Be 899 1757 14845 21000 B 800 2426 3659 25020 32820 C 1086 2352 4619 6221 37820 47260 N 1402 2855 4576 7473 9442 53250 64340 O 1314 3388 5296 7467 10987 13320 71320 84070 F 1680 3375 6045 8408 11020 15160 17860 92010 Ne 2080 3963 6130 9361 12180 15240 Na 496 4563 6913 9541 13350 16600 20113 25666 Mg 737 1450 7731 10545 13627 17995 21700 25662

IE vs Group Number

IE 1 Trends Increasing First Ionization Energy

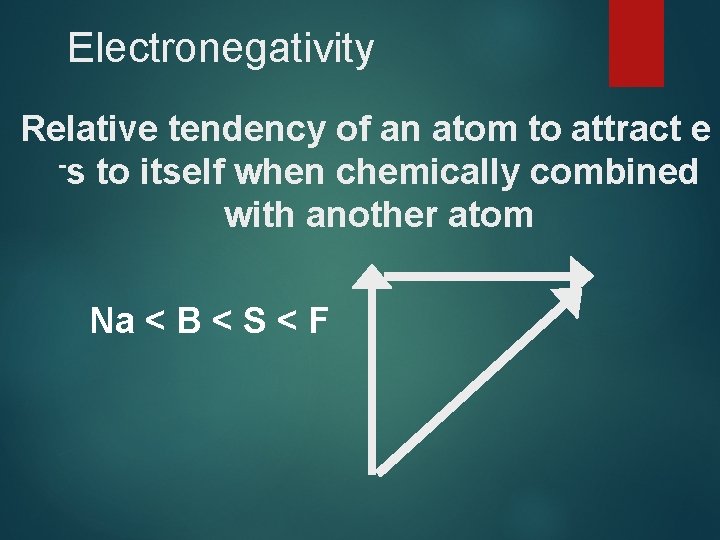
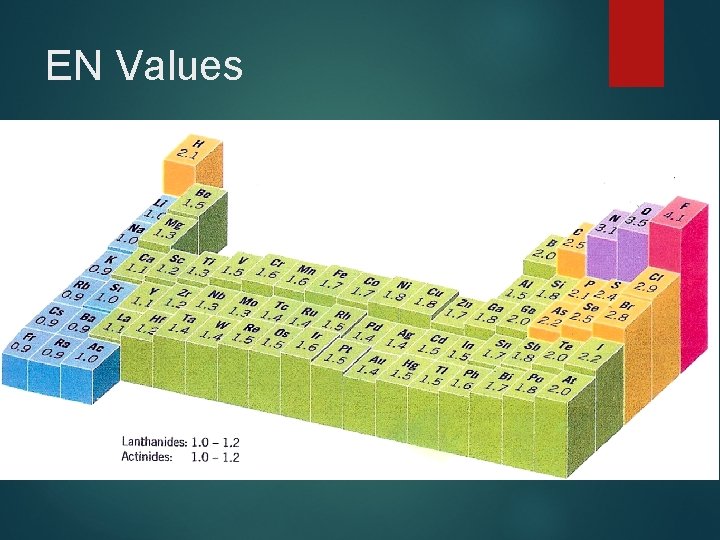
Electronegativity Relative tendency of an atom to attract e -s to itself when chemically combined with another atom Na < B < S < F

EN Values

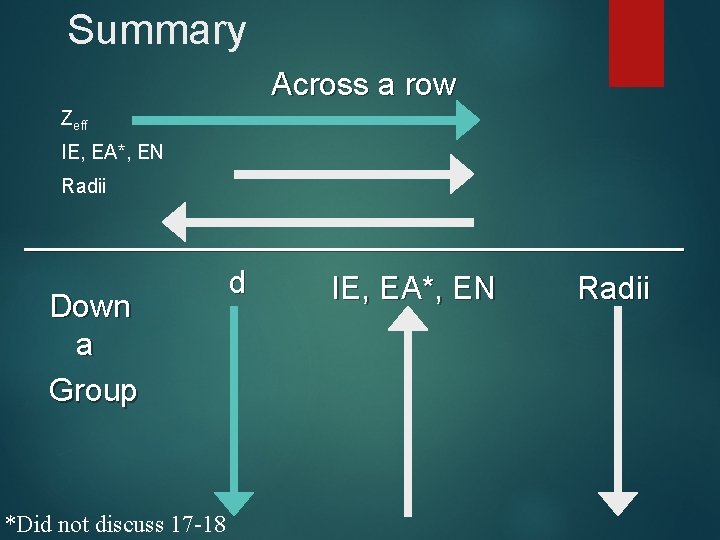
Summary Across a row Zeff IE, EA*, EN Radii ___________________ d IE, EA*, EN Radii Down a Group *Did not discuss 17 -18
![e Configurations of Anions Cations Na Ne3 s 1 Na Ne Ca Ar4 e- Configurations of Anions & Cations Na: [Ne]3 s 1 Na+: [Ne] Ca: [Ar]4](https://slidetodoc.com/presentation_image/5983958d81a3b5489420067048af75ae/image-27.jpg)
e- Configurations of Anions & Cations Na: [Ne]3 s 1 Na+: [Ne] Ca: [Ar]4 s 2 Ca 2+: [Ar] Al: [Ne]3 s 23 p 1 Al 3+: [Ne] Non-metals gain electrons so that anion has a noble-gas outer electron configuration. Metals lose electrons so that cation has a noble-gas outer electron configuration. H 1 s 1 H- 1 s 2 or [He] F 1 s 22 p 5 F- 1 s 22 p 6 or [Ne] O 1 s 22 p 4 O 2 - 1 s 22 p 6 or [Ne] N 1 s 22 p 3 N 3 - 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, N 3 -, are isoelectronic with Ne

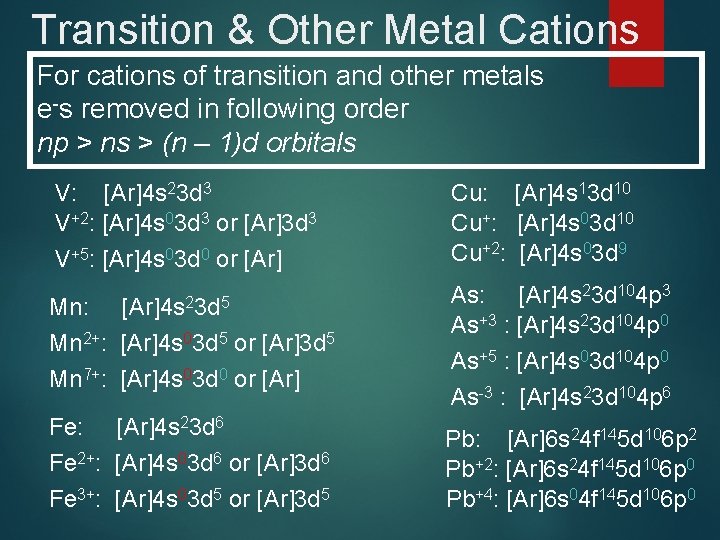
Transition & Other Metal Cations For cations of transition and other metals e-s removed in following order np > ns > (n – 1)d orbitals V: [Ar]4 s 23 d 3 V+2: [Ar]4 s 03 d 3 or [Ar]3 d 3 V+5: [Ar]4 s 03 d 0 or [Ar] Cu: [Ar]4 s 13 d 10 Cu+: [Ar]4 s 03 d 10 Cu+2: [Ar]4 s 03 d 9 Mn: Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Mn 7+: [Ar]4 s 03 d 0 or [Ar] As: [Ar]4 s 23 d 104 p 3 As+3 : [Ar]4 s 23 d 104 p 0 As+5 : [Ar]4 s 03 d 104 p 0 As-3 : [Ar]4 s 23 d 104 p 6 Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Pb: [Ar]6 s 24 f 145 d 106 p 2 Pb+2: [Ar]6 s 24 f 145 d 106 p 0 Pb+4: [Ar]6 s 04 f 145 d 106 p 0 [Ar]4 s 23 d 5

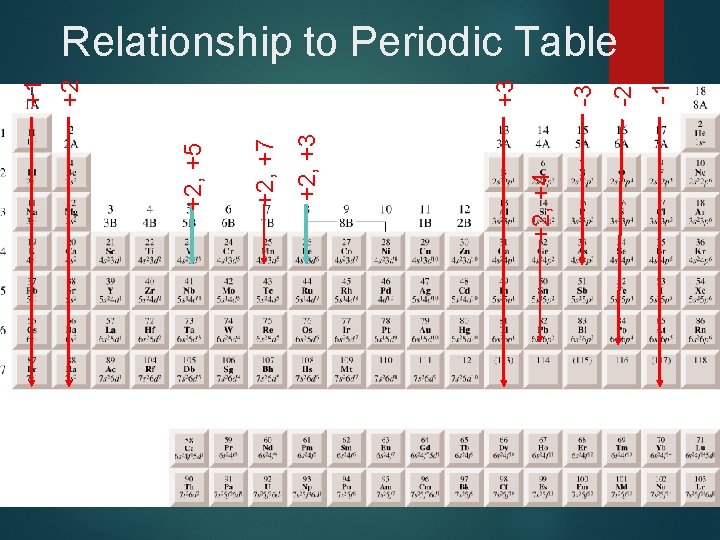
+2, +4 +2, +3 +2, +7 +2, +5 -1 -2 -3 +3 +2 +1 Relationship to Periodic Table

Metallic Character • • Metallic Character and Reactivity increases as you go from right to left across a period Metallic character and reactivity increase as you move down a group

Nonmetallic Character and Reactivity • • Nonmetallic character and reactivity increases as you go from left to right across a period. Nonmetallic character and reactivity increase as you move up a group.