A Periodic Law z When elements are arranged






- Slides: 18

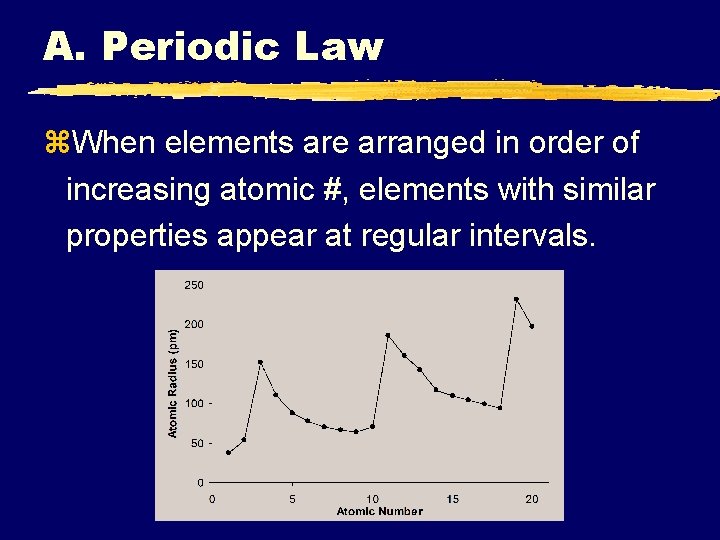
A. Periodic Law z. When elements are arranged in order of increasing atomic #, elements with similar properties appear at regular intervals.

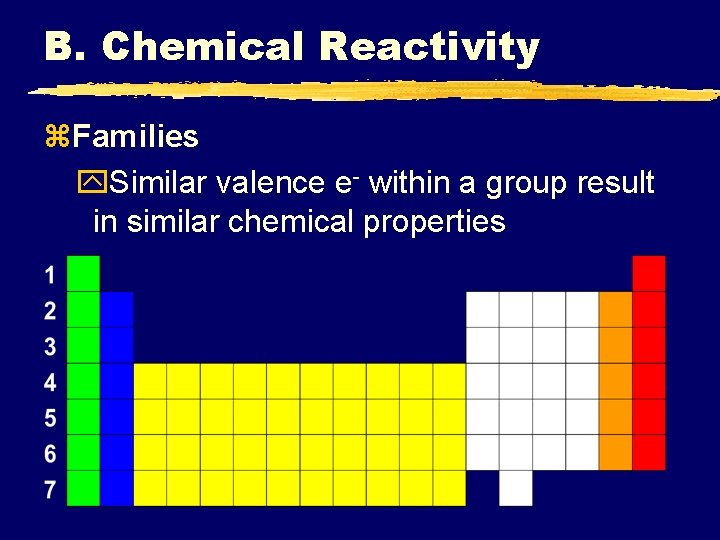
B. Chemical Reactivity z. Families y. Similar valence e- within a group result in similar chemical properties

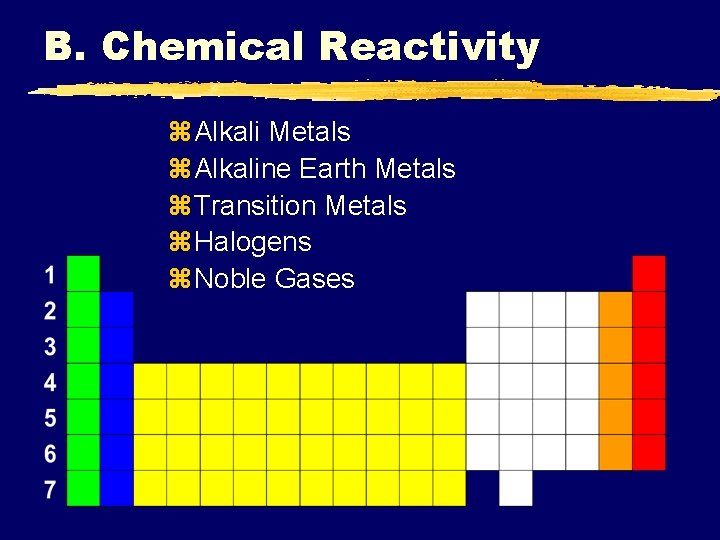
B. Chemical Reactivity z. Alkali Metals z. Alkaline Earth Metals z. Transition Metals z. Halogens z. Noble Gases

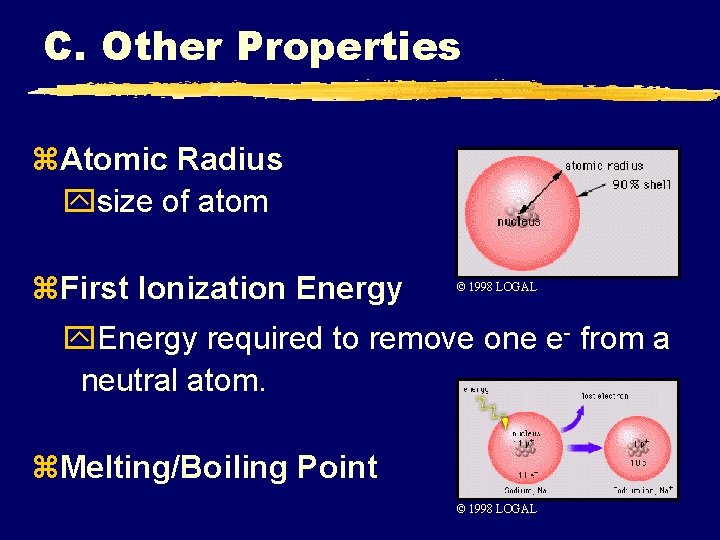
C. Other Properties z. Atomic Radius ysize of atom z. First Ionization Energy © 1998 LOGAL y. Energy required to remove one e- from a neutral atom. z. Melting/Boiling Point © 1998 LOGAL

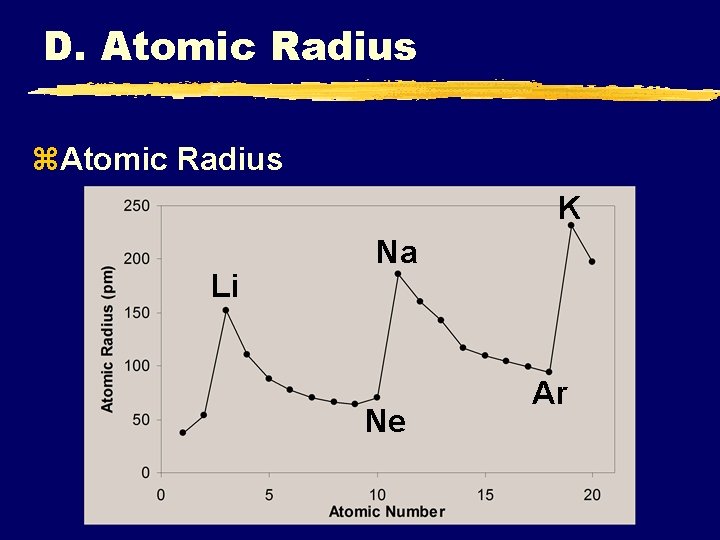
D. Atomic Radius z. Atomic Radius K Li Na Ne Ar

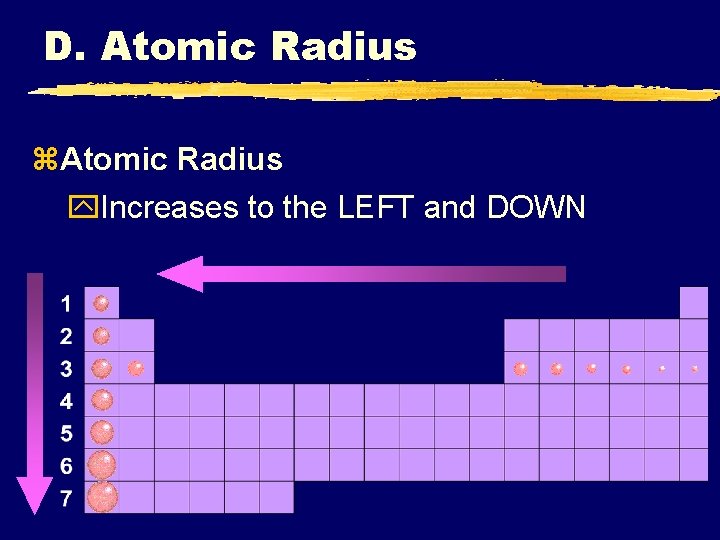
D. Atomic Radius z. Atomic Radius y. Increases to the LEFT and DOWN

D. Atomic Radius z. Why larger going down? y. Higher energy levels have larger orbitals y. Shielding - core e- block the attraction between the nucleus and the valence ez. Why smaller to the right? y. Increased nuclear charge without additional shielding pulls e- in tighter

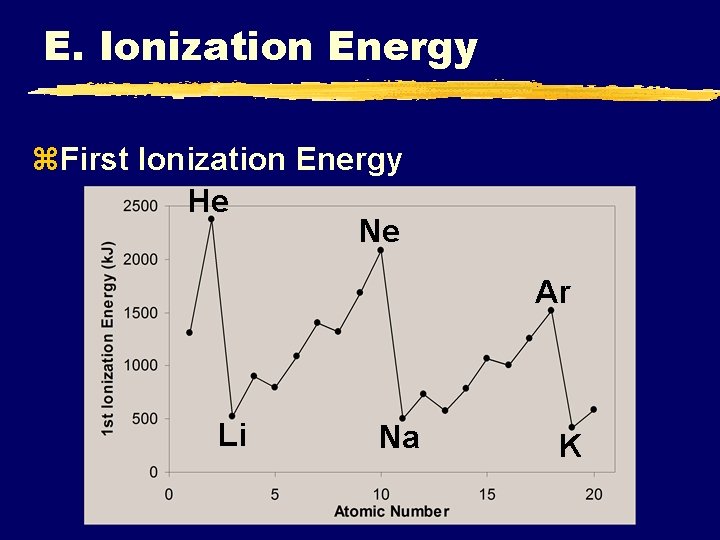
E. Ionization Energy z. First Ionization Energy He Ne Ar Li Na K

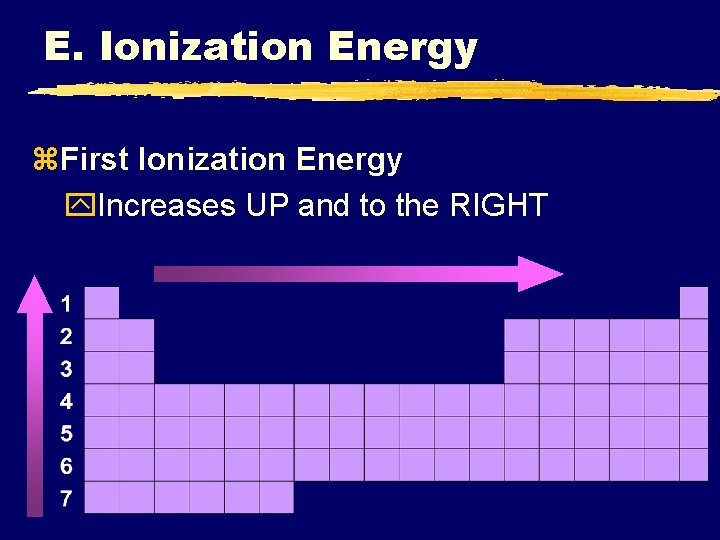
E. Ionization Energy z. First Ionization Energy y. Increases UP and to the RIGHT

E. Ionization Energy z. Why opposite of atomic radius? y. In small atoms, e- are close to the nucleus where the attraction is stronger z. Why small jumps within each group? y. Stable e- configurations don’t want to lose e-

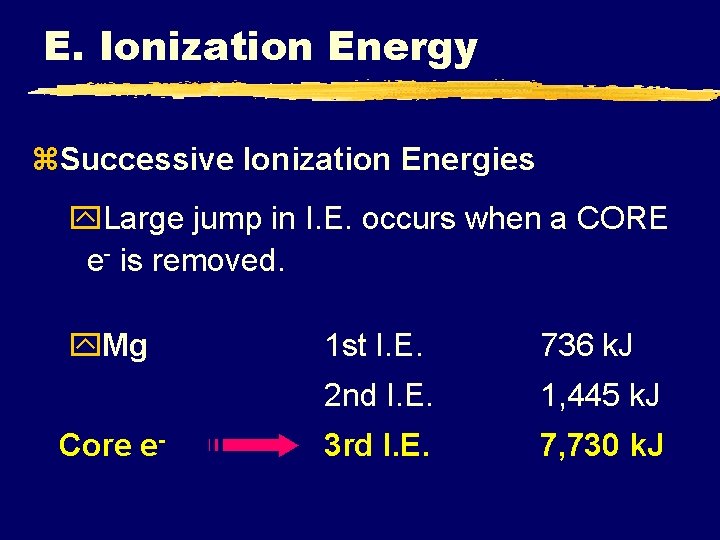
E. Ionization Energy z. Successive Ionization Energies y. Large jump in I. E. occurs when a CORE e- is removed. y. Mg Core e- 1 st I. E. 736 k. J 2 nd I. E. 1, 445 k. J 3 rd I. E. 7, 730 k. J

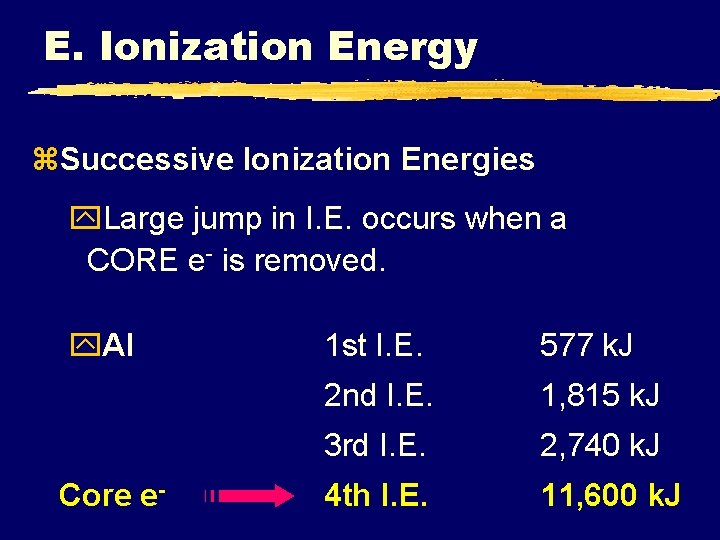
E. Ionization Energy z. Successive Ionization Energies y. Large jump in I. E. occurs when a CORE e- is removed. y. Al Core e- 1 st I. E. 577 k. J 2 nd I. E. 1, 815 k. J 3 rd I. E. 2, 740 k. J 4 th I. E. 11, 600 k. J

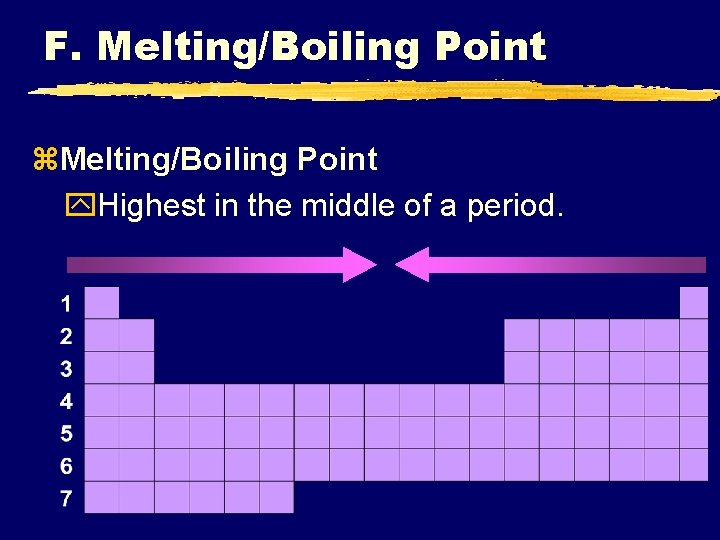
F. Melting/Boiling Point z. Melting/Boiling Point y. Highest in the middle of a period.

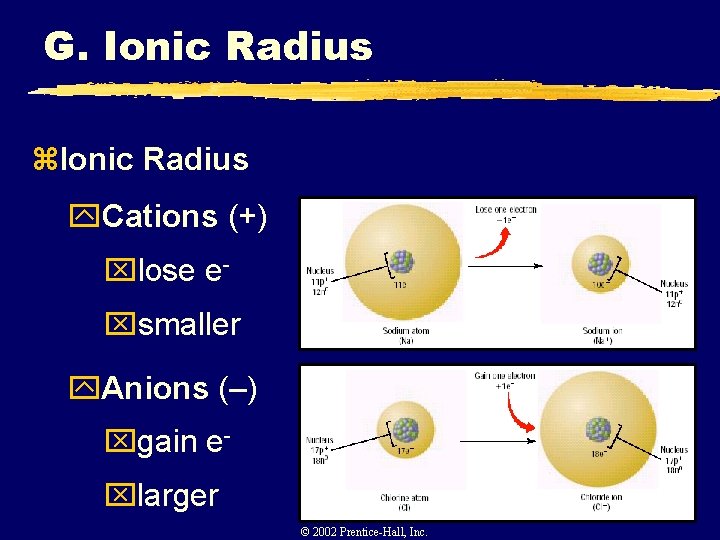
G. Ionic Radius z. Ionic Radius y. Cations (+) xlose exsmaller y. Anions (–) xgain exlarger © 2002 Prentice-Hall, Inc.

Examples z. Which atom has the larger radius? y. Beor Ba Ba y. Ca Ca or Br

Examples z. Which atom has the higher 1 st I. E. ? y. N or Bi N y. Baor Ne Ne

Examples z. Which atom has the higher melting/boiling point? y. Li or C C y. Cr or Kr Cr

Examples z. Which particle has the larger radius? y. S or S 2 - y. Al or Al 3+ Al