Periodic trends Gradual changes or Large jumps Trends













- Slides: 37

Periodic trends Gradual changes -> or Large jumps

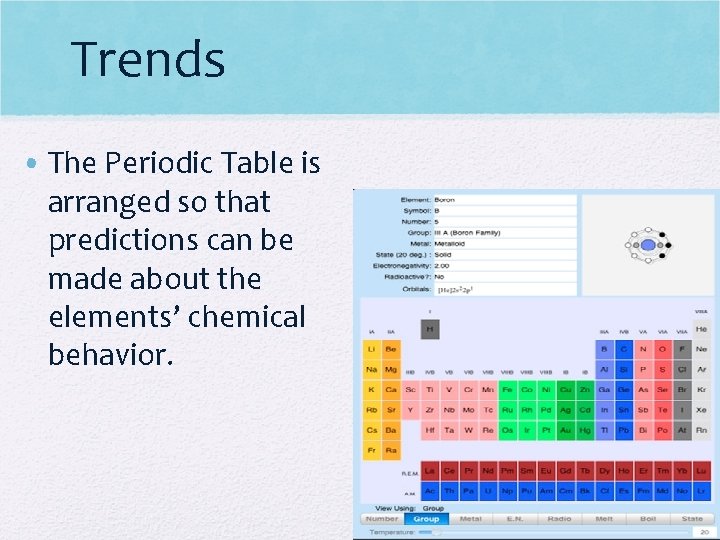
Trends • The Periodic Table is arranged so that predictions can be made about the elements’ chemical behavior.

Periods and Group • Period – horizontal row on P. T. • Group – vertical column P. T. • Each period represents an energy level (think back to models of the atom) • Each group represents a certain number of valence electrons • Atoms in period 1 have 1 energy level, atoms in period 5 have 5 energy levels • Also known as families

Valence Electrons • Electrons exist within orbitals. • The electrons in the outermost energy level are called valence electrons! • Valence electrons determine how an atom behaves (they perform all bonding)

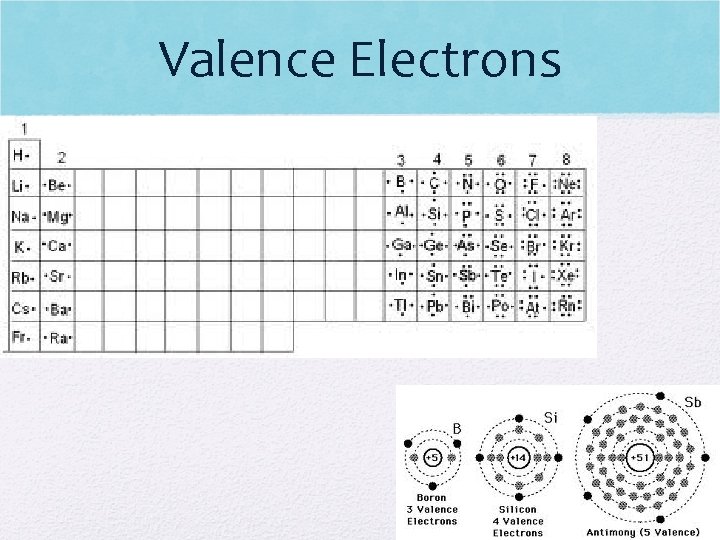
Valence Electrons Ctd • Each group number represents the number of valence electrons elements in that group have • Ex: group 1 has 1 valence electron • No atom can have more than 8 v. e. • For group numbers 13 - 18 subtract ten to figure the number of valence electrons • Ex: Group 18 = 8 valence electrons • Groups 3 -12, we assume to have 2 v. e. (not always the case)

Valence Electrons

8 is great (sometimes 2) • The noble gases (group 18) are stable atoms, meaning they do not react! (this is good) • The noble gases are stable because they have 8 valence electrons (or 2 as in helium) • All other atoms will gain or lose electrons to become like noble gases (remember cations and anions)

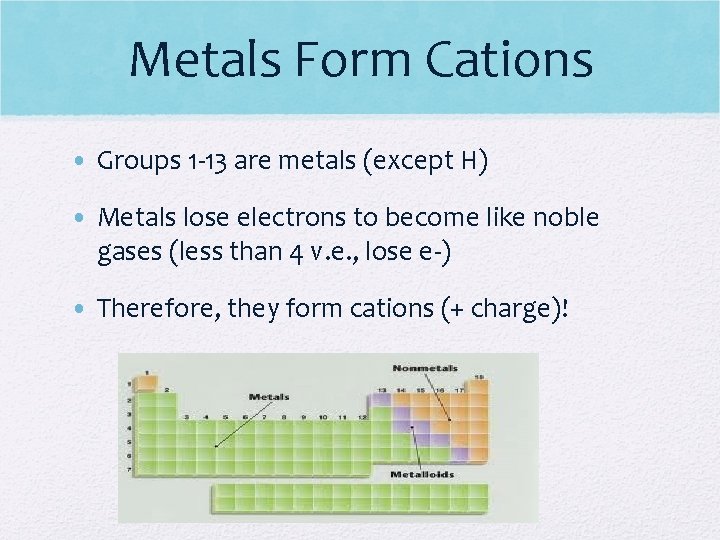
Metals Form Cations • Groups 1 -13 are metals (except H) • Metals lose electrons to become like noble gases (less than 4 v. e. , lose e-) • Therefore, they form cations (+ charge)!

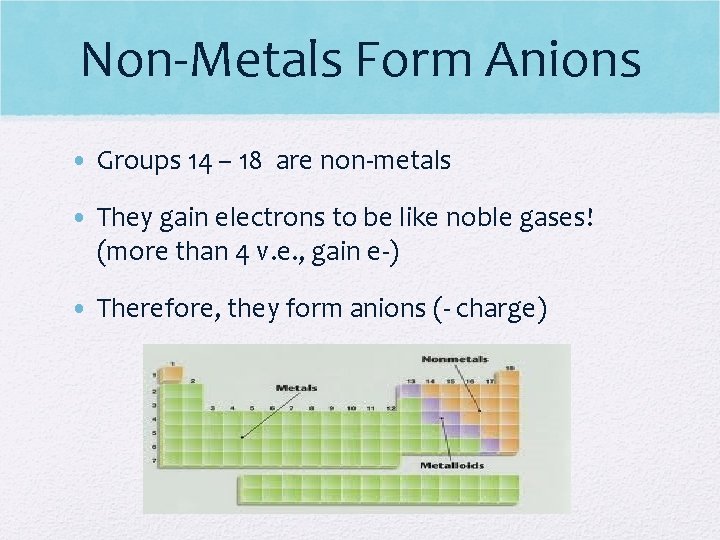
Non-Metals Form Anions • Groups 14 – 18 are non-metals • They gain electrons to be like noble gases! (more than 4 v. e. , gain e-) • Therefore, they form anions (- charge)

Practice • For each of the following elements, determine if they are a metal or non-metal and the charge they would form. Na Al S O Ba Cs F I P

Periodic Table Families • Group 1: Alkali Metals • Group 2: Alkaline Earth Metals • Groups 3 -12: Transition Metals • Group 17: Halogens • Group 18: Noble Gases

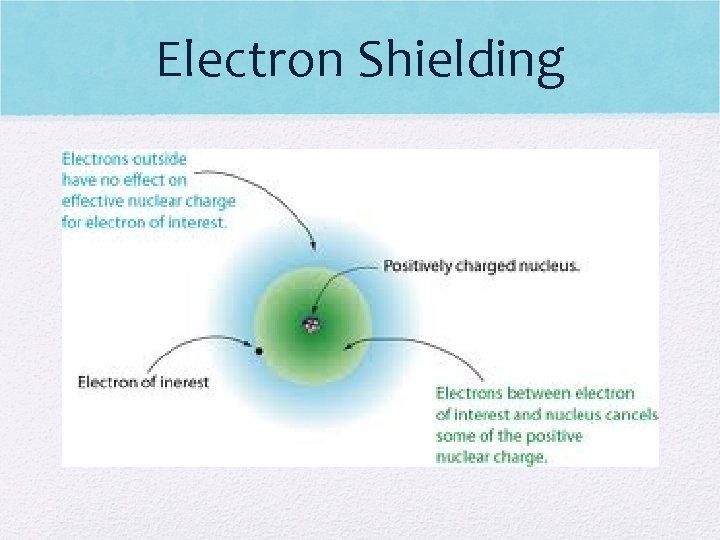
Shielding • As atom gets bigger, more E levels are added • Therefore, valence electrons get farther from pull of nucleus • Shielding occurs when the inner electrons block the positive pull from the nucleus from reaching the valence electrons • �Only impacts trends down a group!

Electron Shielding

Things to Consider • P+ and e- within an atom are attracted to each other, but P+ pull e- because e- have less mass • As you go down a group, each atom adds an energy level! • As you go across a period each element adds a p+ and e-! (we consider all elements on P. T. neutral)

Atomic Radius – size of atom • What do you think? • Does atomic radius increase/decrease across a period? • Why? • Does atomic radius increase/decrease down a group? • Why?

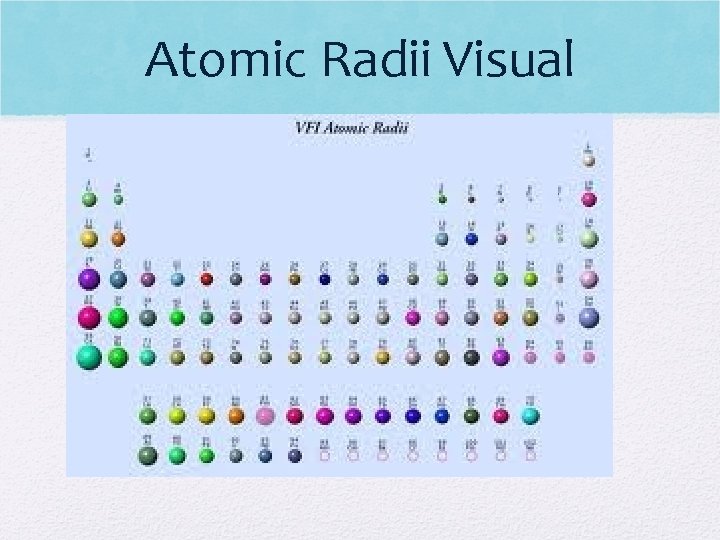
Atomic Radii Visual

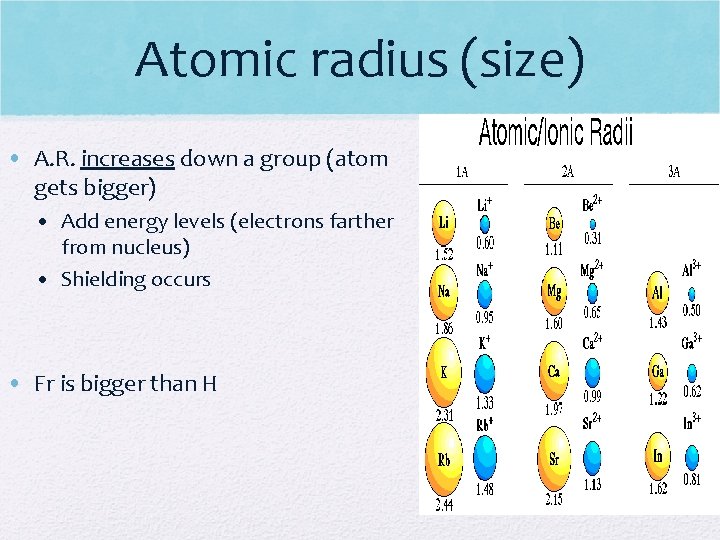
Atomic radius (size) • A. R. increases down a group (atom gets bigger) • Add energy levels (electrons farther from nucleus) • Shielding occurs • Fr is bigger than H

Atomic Radius (cont) • Decreases as you move across a period (atom gets smaller) • Adding more protons, so outermost electrons are pulled closer • Ex: K is bigger than Br

Determine which atom has a larger A. R. • Be vs. Mg Mg, more energy levels & more shielding • Co vs. Ir Ir, more energy levels & more shielding • Re vs. At Re, less protons • Mn vs. Zn Mn, less protons • He vs. Ar Ar, more energy levels & more shielding • B vs. Ga Ga, more energy levels & more shielding

Ionization Energy (I. E. ) • Energy required to remove an outermost electron (valence e-) • What do you think? • Does I. E. increase/decrease across a period? • Why? • Does I. E. increase/decrease down a group? • Why?

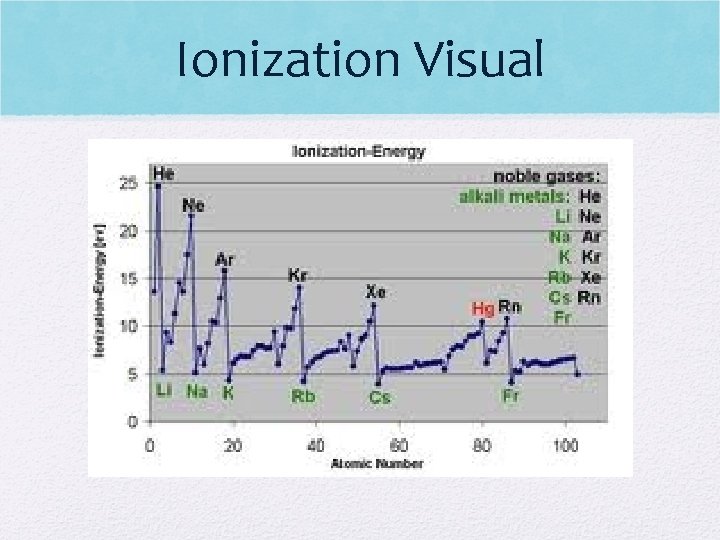
Ionization Visual

Ionization Energy (I. E. ) • I. E. decreases down a group (easier to take electron) • more energy levels, therefore valence e- far from the attractive force of the nucleus • Electron shielding occurs down a group • Ex: I. E. lower for Fr (francium) than H (hydrogen) • Stealing a basketball/Attacking Gazelle

Ionization Energy (cont) • I. E. increases across a period (harder to take electron) • More protons being added, pulling outer electrons in more! • Atoms are getting closer to being like a noble gas (do not want to give up e-!) • Ex: Kr (krypton) requires lower IE than K (potassium)

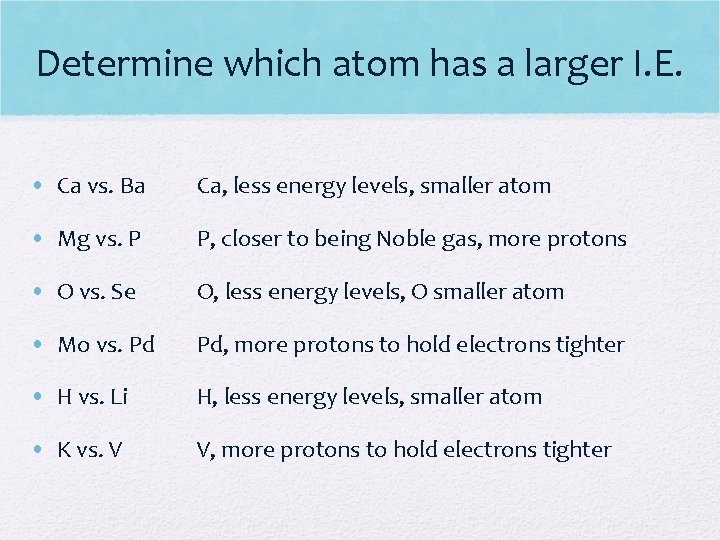
Determine which atom has a larger I. E. • Ca vs. Ba Ca, less energy levels, smaller atom • Mg vs. P P, closer to being Noble gas, more protons • O vs. Se O, less energy levels, O smaller atom • Mo vs. Pd Pd, more protons to hold electrons tighter • H vs. Li H, less energy levels, smaller atom • K vs. V V, more protons to hold electrons tighter

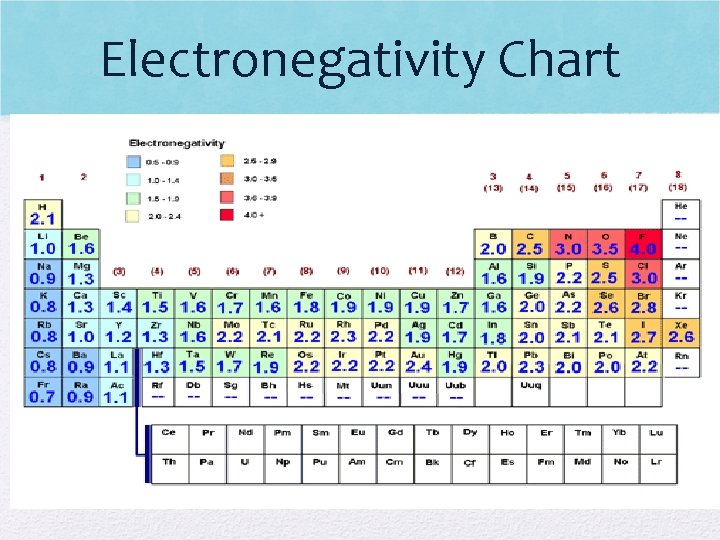
Electronegativity • Ability of an atom to attract an electron • Low electronegativity = hard to attract an e • High electronegativity = easy to attract an e- • What do you think? • Does E- increase/decrease across a period? • Why? • Does E- increase/decrease down a group? • Why?

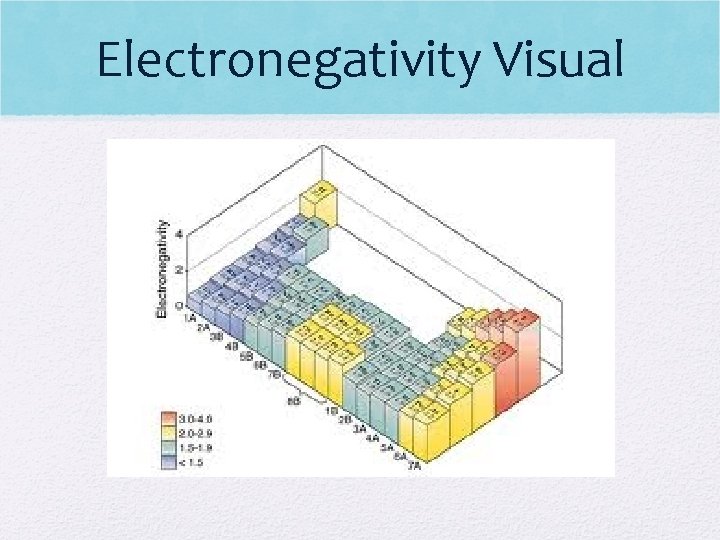
Electronegativity Visual

Electronegativity • E. N. decreases down a group (harder for atom to attract electrons) • E- shielding occurs • More energy levels so distance between nucleus and electrons increases • Ex: H has higher electronegativity than Fr (H has easier time attracting e- than Fr)

Electronegativity (cont. ) • E. N. increases as you move across a period • Nuclear charge increases (more p+) across a period causing e- to be attracted much more strongly • Metals do not want to gain e-, while nonmetals add e- to become like noble gas! • Ex: K has lower electronegativity than Br (K has a harder time attracting e-)

Electronegativity Chart

Determine which atom would have higher electronegativity • Be vs Ca Be, less energy levels, smaller atom, less shielding • N vs O O, closer to being Noble gas, more protons • F vs Cl F, less energy levels, smaller atom, less shielding • C vs. Pb C, less energy levels, smaller atom, less shielding • F vs. Li F, closer to being Noble gas, more protons • Sc vs. Co Co, more protons to attract e- more

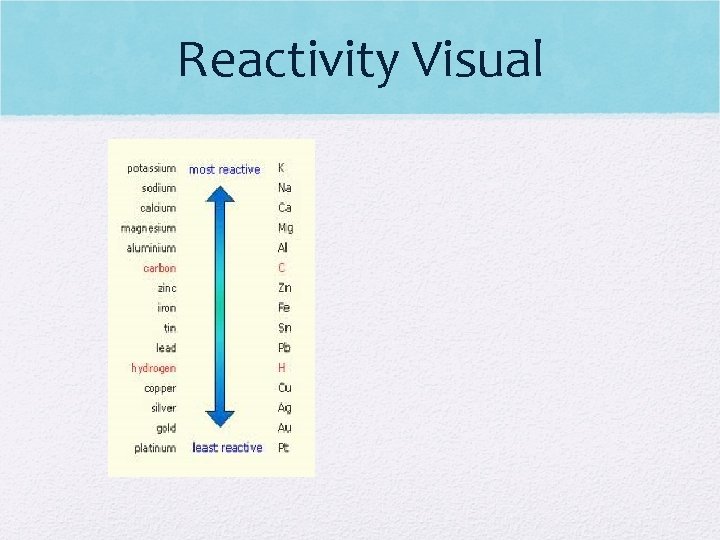
Reactivity • How easily an element will gain or lose electrons • Focuses on metal & non-metals in P. T.

Reactivity • What metals do you think are most reactive? Least reactive • Why? • What non-metals do you think are most reactive? Least reactive? • Why

Reactivity Visual

Reactivity – Down Group • Metals – increases (Li less reactive than Fr) • Metals want to lose electrons to gain stability • Easy to lose electrons as you add energy levels as electrons are further from pull of nucleus • Non-Metals – decreases (F more reactive than I) • Non-metals want to gain electrons to gain stability • Hard to gain electrons as you add energy levels as electrons are farther from pull of nucleus

Reactivity – Across Period • Metals – decreases (K more reactive than Ca) • Metals want to lose electrons to have a full octet • as you move across period, metals are adding electrons & protons • Non-metals – increases (N less reactive than F) • Non-metals want to gain electrons to have a full octet • As you move across period, non-metals are adding electrons

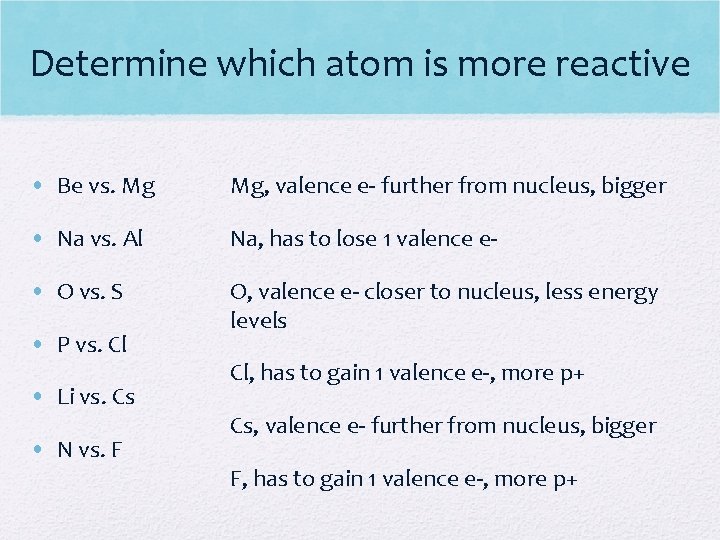
Determine which atom is more reactive • Be vs. Mg Mg, valence e- further from nucleus, bigger • Na vs. Al Na, has to lose 1 valence e- • O vs. S O, valence e- closer to nucleus, less energy levels • P vs. Cl • Li vs. Cs • N vs. F Cl, has to gain 1 valence e-, more p+ Cs, valence e- further from nucleus, bigger F, has to gain 1 valence e-, more p+

Tips for Trends • Focus on if element wants to gain or lose e- to become stable • Re-state trend in your own words • Know what shielding does! • Do not try to memorize!