Pericardial diseases Pericardial anatomy PERICARDIAL EFFUSION ETIOLOGY Viral





































- Slides: 93

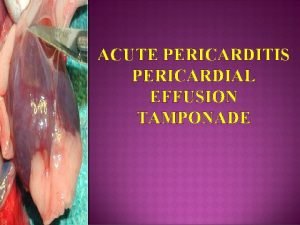
Pericardial diseases

Pericardial anatomy

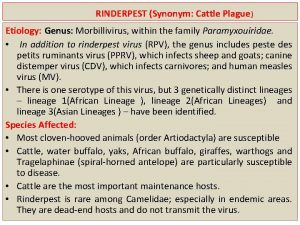
PERICARDIAL EFFUSION (ETIOLOGY) • • Viral (most common) Uremic (chronic renal failure) Metastatic (breast or lung CA) Post MI (Dresslers syndrome) Post cardiac surgery (regional) CHF, systemic diseases (lupus, AIDS) Trauma Infectious

HIV

PERICARDIAL DISEASES (CLINICAL PRESENTATION) • • • Chest pain with respiration, fever Shortness of breath Enlarged cardiac silhouette on chest X ray EKG changes with diffuse ST elevation Pulsus paradoxus, tachycardia, hypotension, neck vein distention, decreased heart sounds

PERICARDIAL FLUID • Serosanguinous (clear, pale yellow) - not echogenic • Bloody (consider metastatic, trauma) - may be echogenic • Infectious (brown, milky colored)

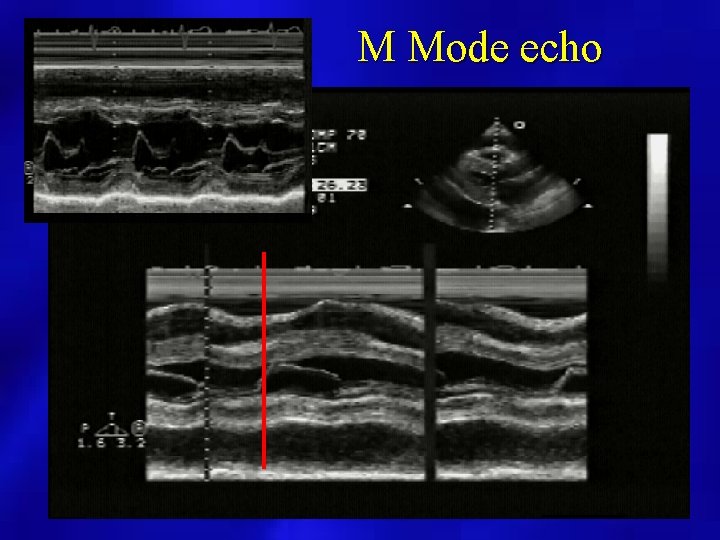
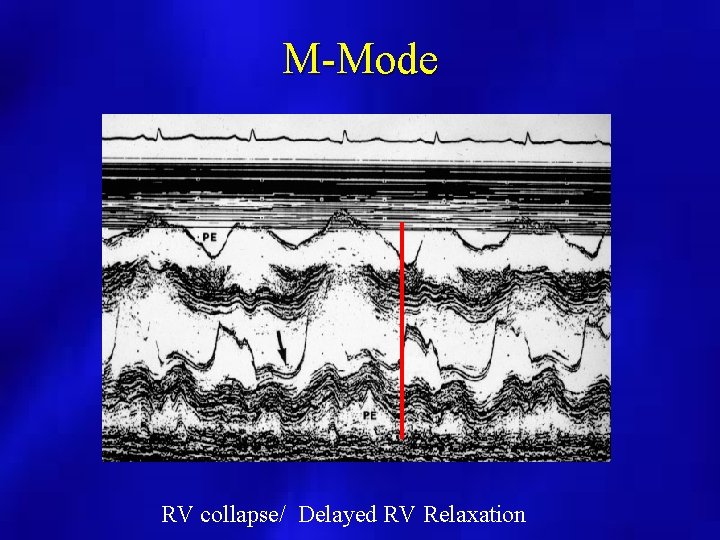
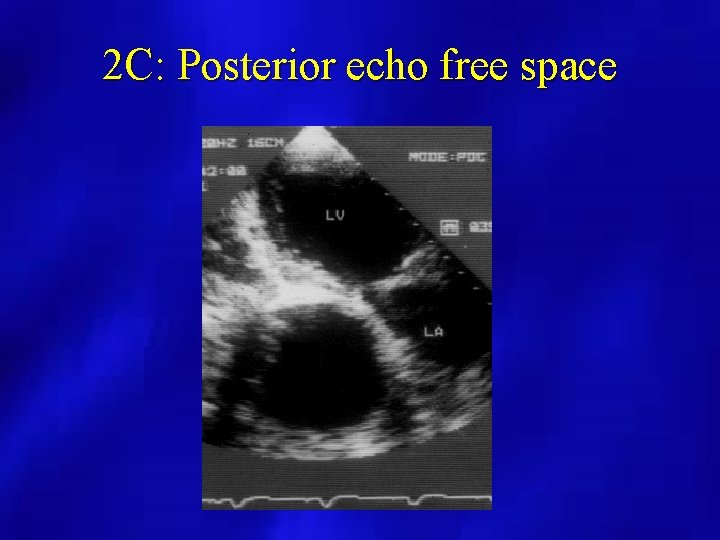
PERICARDIAL EFFUSION (M Mode Echocardiography) • may overestimate amount and not useful if loculated or localized • useful for timing of RV wall motion relative to mitral valve opening • Caution when only anterior echo free space present

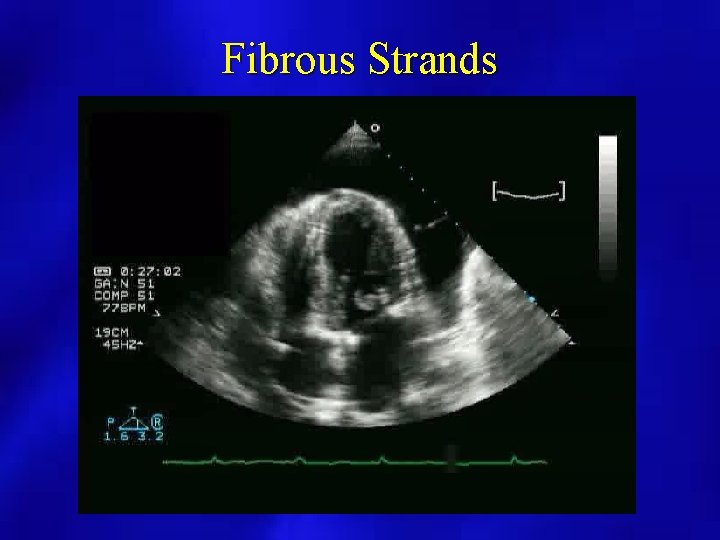
PERICARDIAL EFFUSION (2 D Echocardiography) • Superior to M Mode for extent and localization by use of multiple views • Assess for diastolic collapse of right heart chambers, IVC size and change with inspiration/expiration • Identify intrapericardial process (clot, tumor, fibrin strands) • Differentiate pericardial from pleural effusion by recognition of descending aorta • Non diagnostic for pericardial thickness

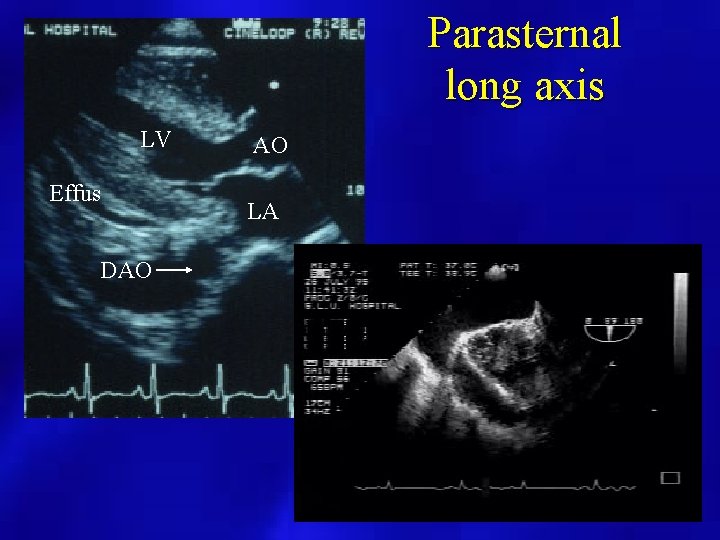
Parasternal long axis LV Effus DAO AO LA

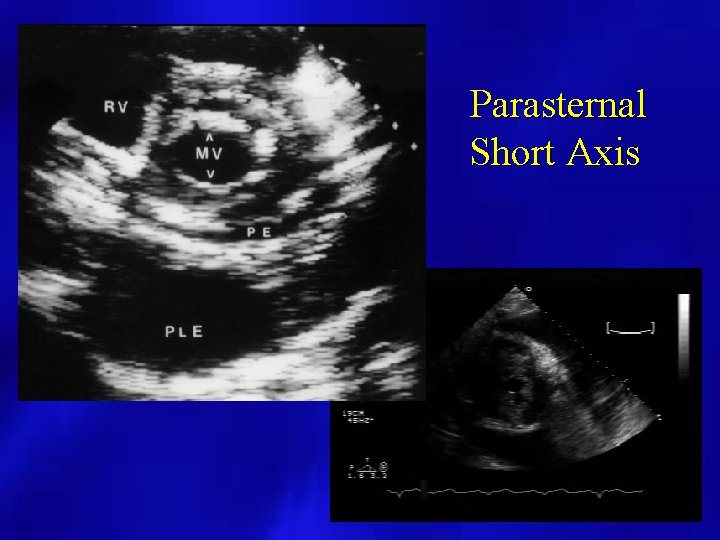
Parasternal Short Axis

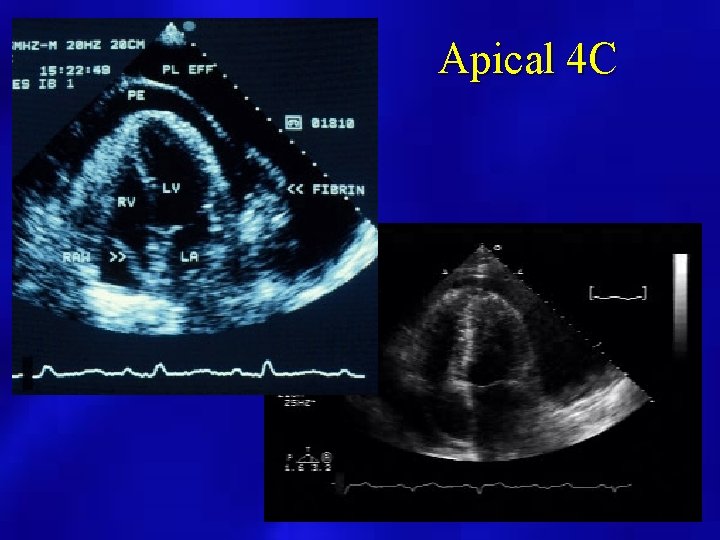
Apical 4 C

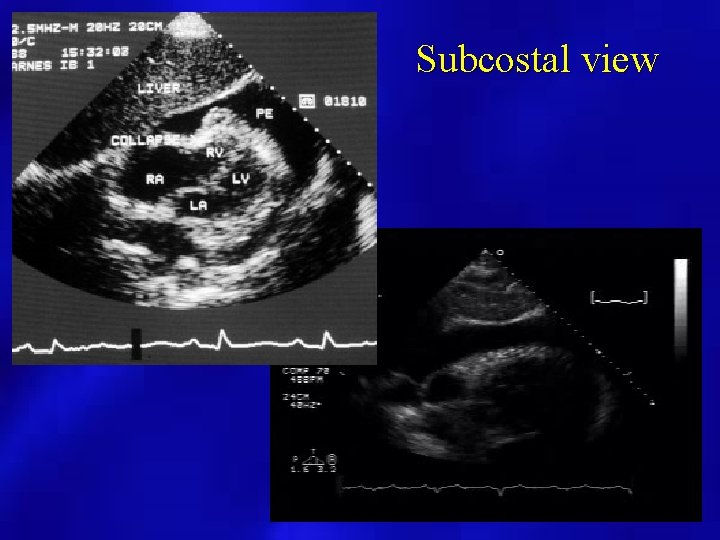
Subcostal view

Fibrous Strands

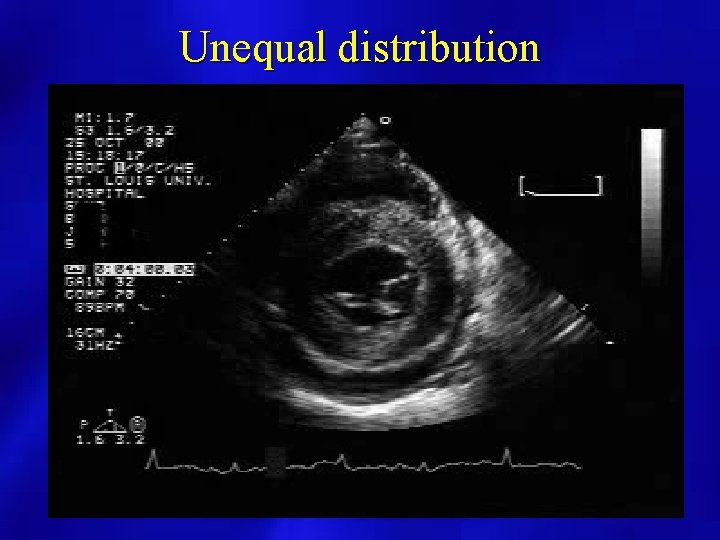
Unequal distribution

M Mode echo

M-Mode RV collapse/ Delayed RV Relaxation

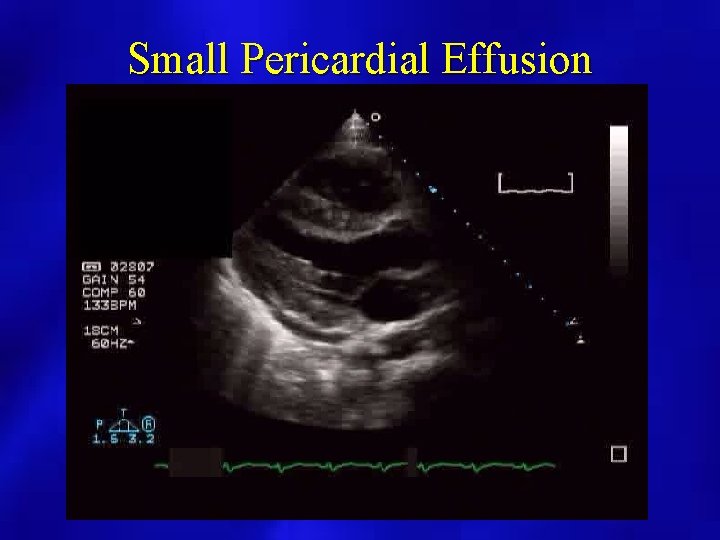
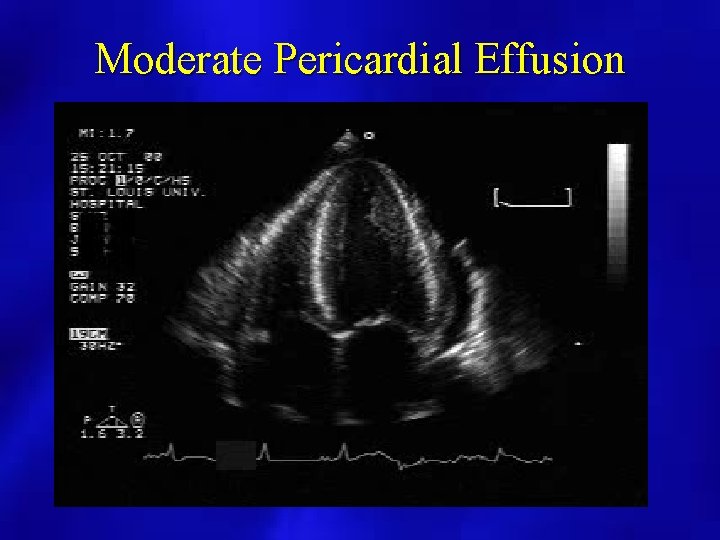
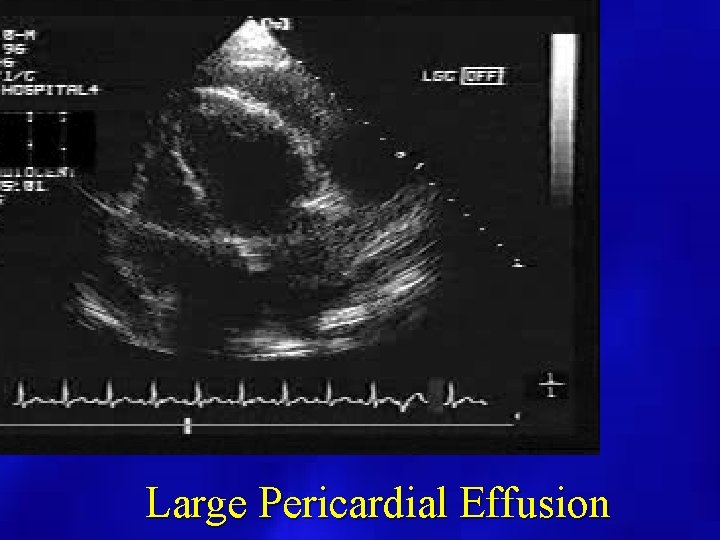
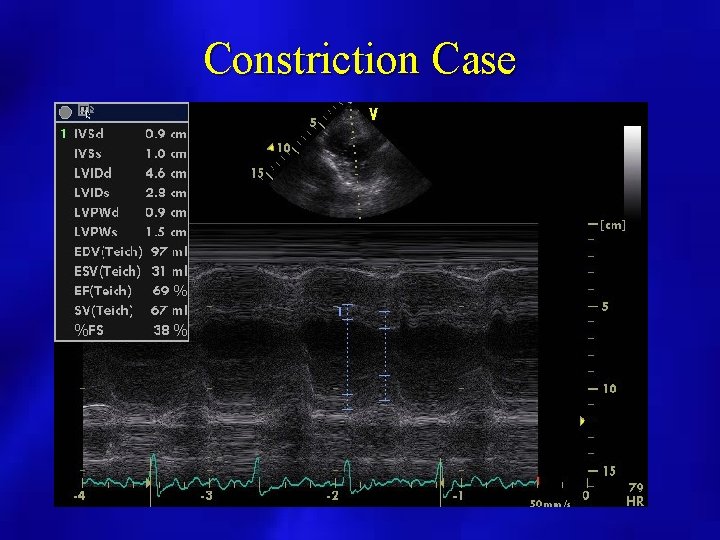
PERICARDIAL EFFUSION: SIZE • SMALL: echo free space present posterior and < 1 cm. • MODERATE: echo free space present anterior and posterior < 1 cm. • LARGE: echo free space anterior and posterior > 1 cm.

Small Pericardial Effusion

Moderate Pericardial Effusion

Large Pericardial Effusion

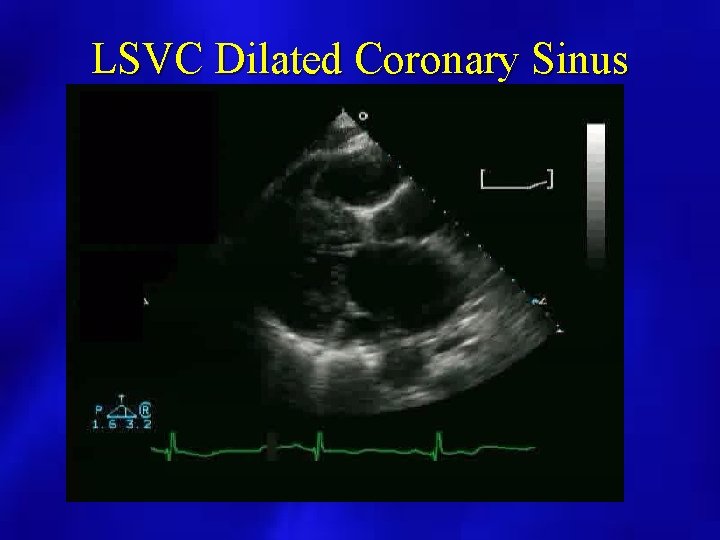
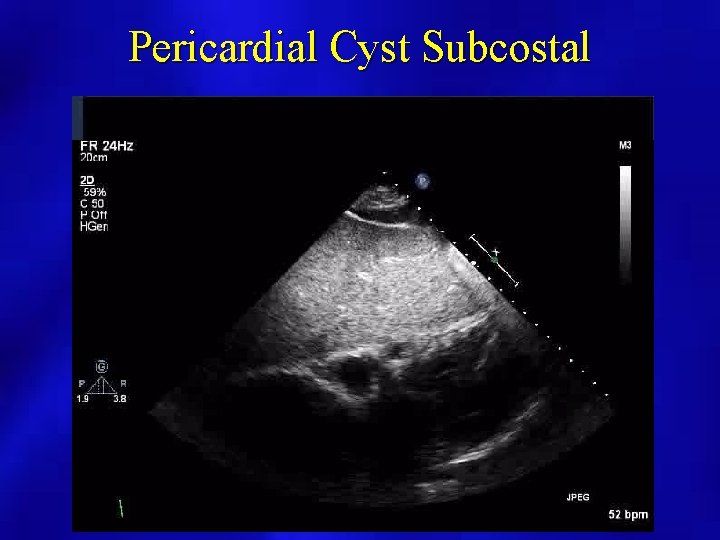
PERICARDIAL EFFUSION: POSSIBLE SOURCES OF FALSE POSITIVES • • • Pleural effusion Pericardial tumor or cyst Dilated coronary sinus LV pseudoaneurysm Large hiatal hernia

LSVC Dilated Coronary Sinus


Pericardial Cyst Subcostal

2 C: Posterior echo free space

DOPPLER • Assessment of flow velocities across mitral/tricuspid valves, LV outflow, and hepatic veins • Presence of respiratory variation > 20% in left heart flow velocities and more marked in right heart • Should be performed in all patients with suspicion or evidence of pericardial disease


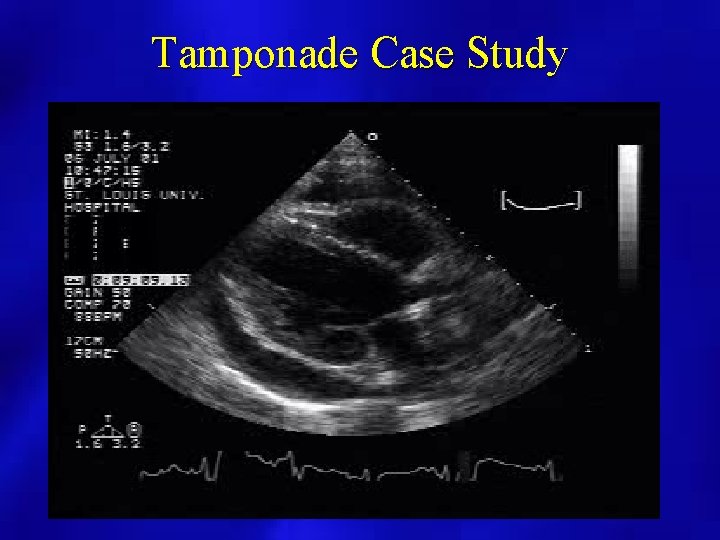
Tamponade Case Study





Pericardiocentesis • Needle aspiration of the pericardial effusion • Usually performed with needle entering subxiphoid • Echo guided – Evaluate fluid initially from subcostal – Imaging performed from the apical position

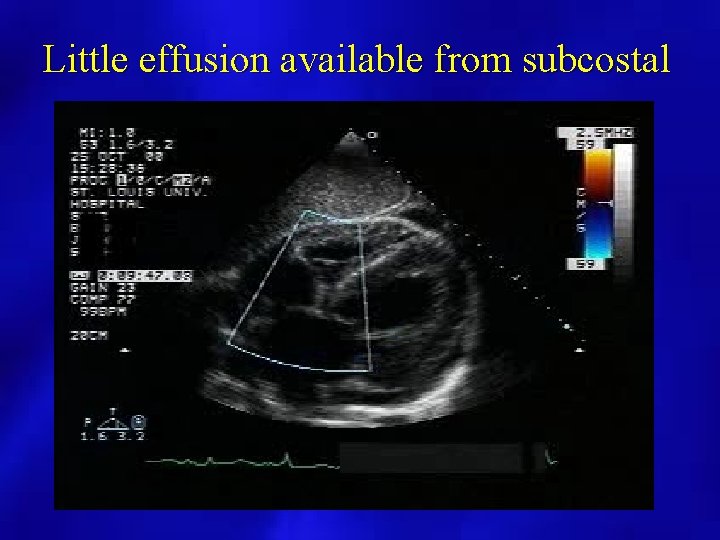
Little effusion available from subcostal

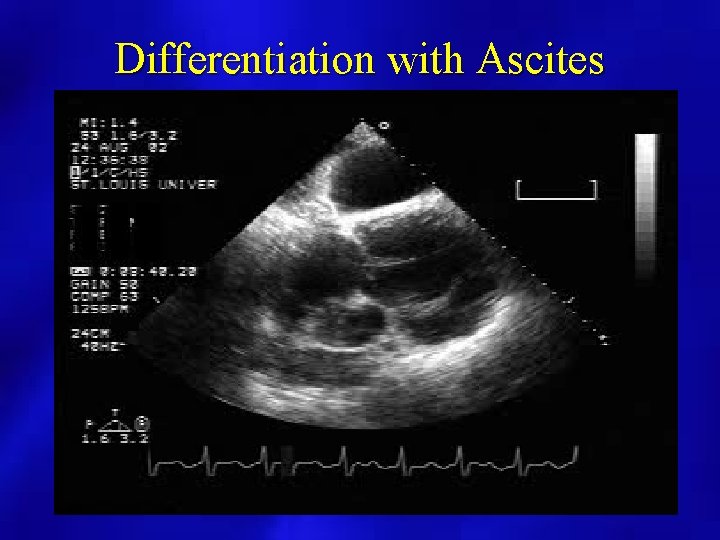
Differentiation with Ascites

Case 2 • • • 56 year old female transferred from outside hospital know breast cancer possible malignant pericardial effusion Pericardiocentesis


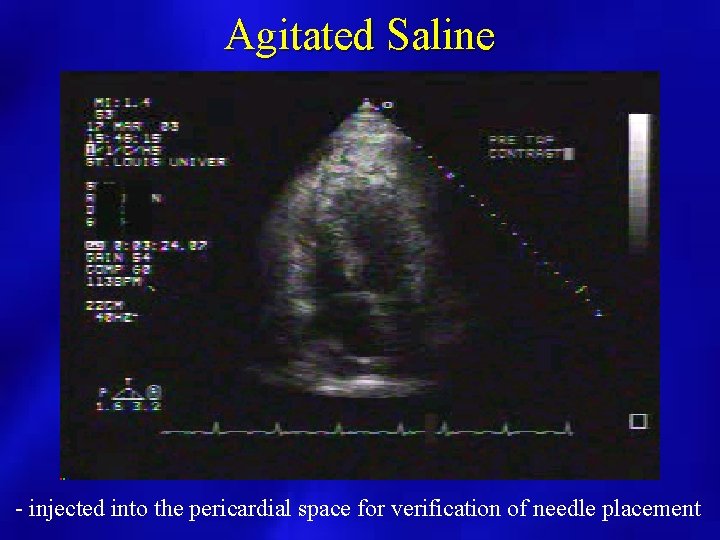
Agitated Saline - injected into the pericardial space for verification of needle placement

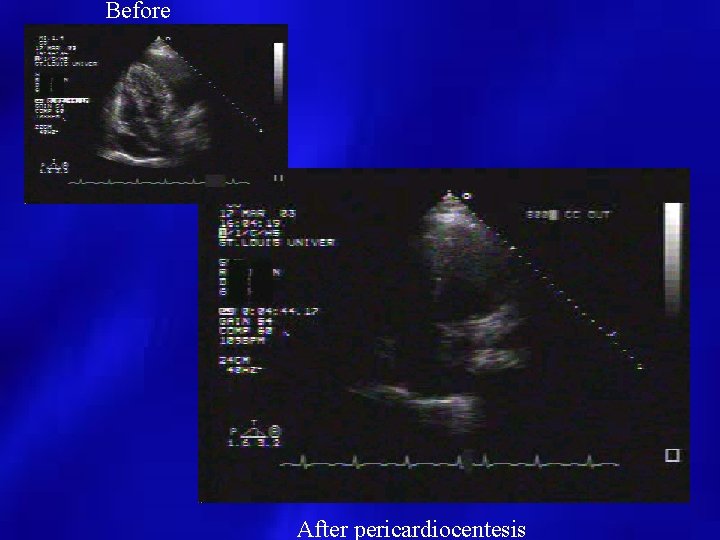
Before After pericardiocentesis

Case 4 • Patient presents post MI • New pericardial effusion • What is the differential?




EP application • 56 year old female comes into the hospital after being discharged from outside hospital after pacemaker insertion • Continued severe chest pain • When pacer activated, diaphragm stimulated





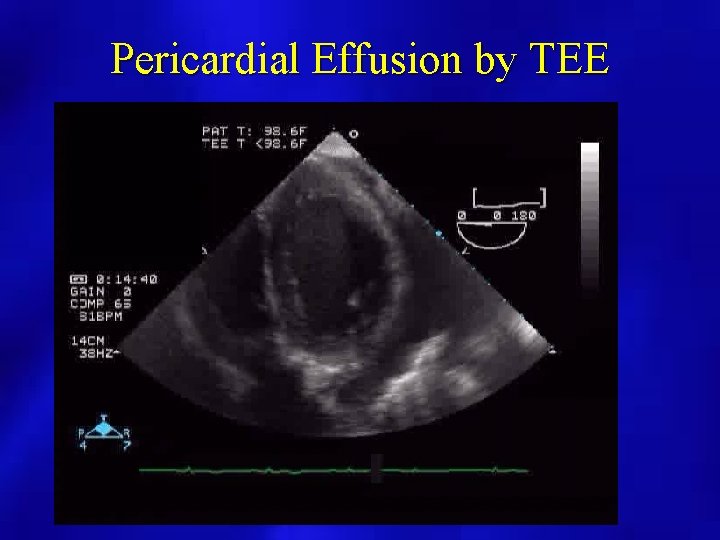
Pericardial Effusion by TEE

Pericardial Disease: Constriction versus Restriction

Constrictive Pericardial Diseases: Etiologies • • • Idiopathic/recurrent pericarditis Post cardiac surgery Prior chest radiation Infectious (Tuberculosis) Metastatic process Difficult diagnosis to establish

Less Common Etiologies • • Infectious (Fungal) Neoplasms Uremia Connective tissue disorders (SLE, Scleroderma) • Drug Induced (Procainamide, hydralazine) • Trauma • Post MI (Dressler’s)

Clinical Signs • • • Shortness of breath Peripheral edema Increased jugular venous pressure Normal heart size on chest X ray Similar in presentation to CHF Often confused with restrictive cardiomyopathy

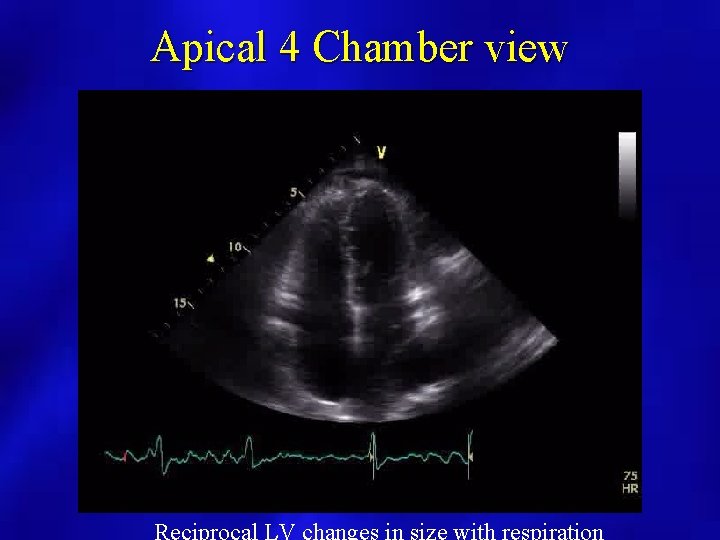
Physiology • Dissociation between intrathoracic and intracardiac pressures • Normally with inspiration, intrathoracic pressure falls and intrathoracic structures fall • In constriction, the pressure change is not transmitted to intrapericardial structures and cavities

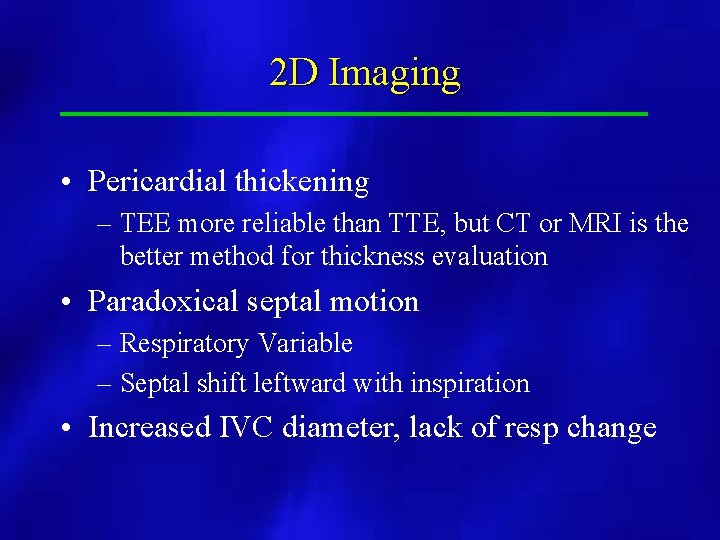
2 D Imaging • Pericardial thickening – TEE more reliable than TTE, but CT or MRI is the better method for thickness evaluation • Paradoxical septal motion – Respiratory Variable – Septal shift leftward with inspiration • Increased IVC diameter, lack of resp change

M-mode Evaluation • Parietal pericardial tracking with epicardial/endocardial motion • M Mode posterior LV wall motion is flat during mid and late diastole • Respiratory variation in ventricular chamber size

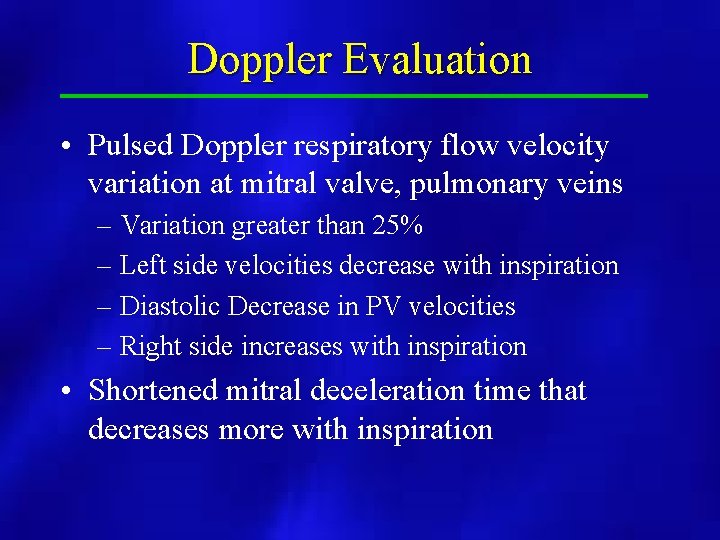
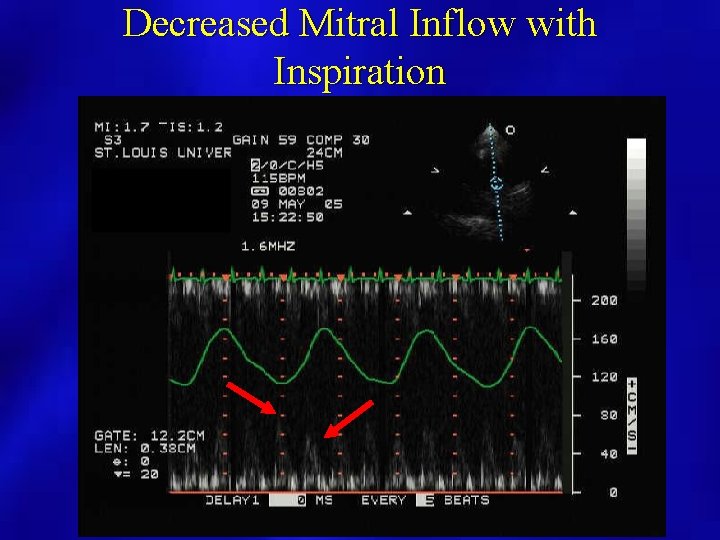
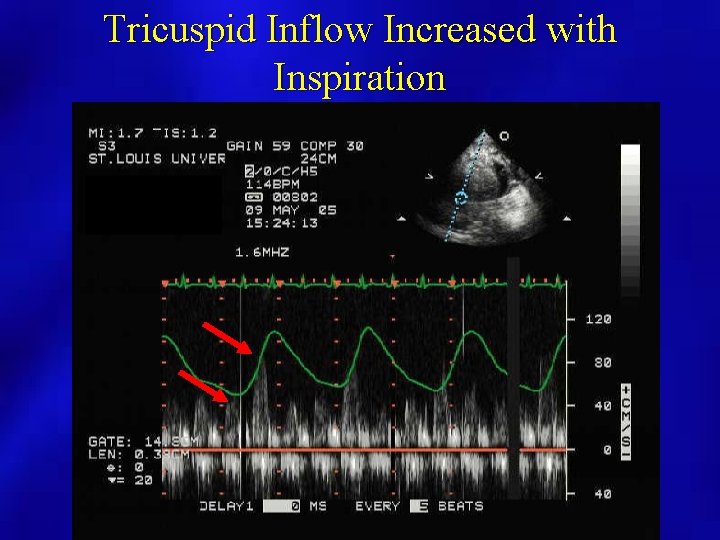
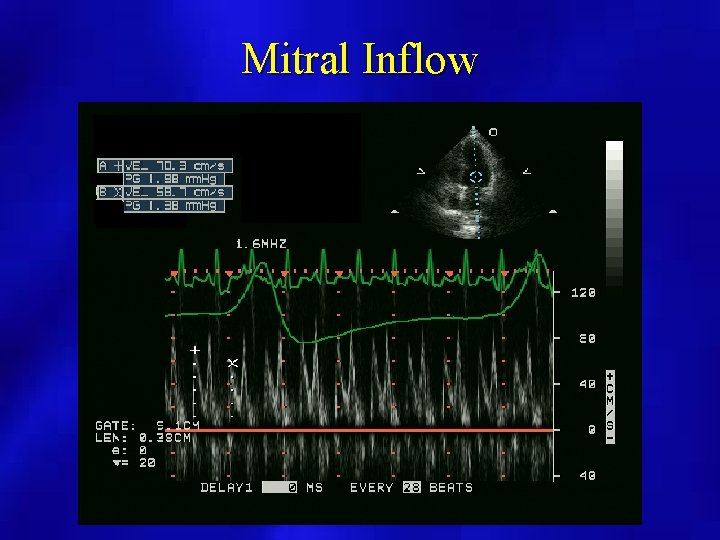
Doppler Evaluation • Pulsed Doppler respiratory flow velocity variation at mitral valve, pulmonary veins – Variation greater than 25% – Left side velocities decrease with inspiration – Diastolic Decrease in PV velocities – Right side increases with inspiration • Shortened mitral deceleration time that decreases more with inspiration

Decreased Mitral Inflow with Inspiration

Tricuspid Inflow Increased with Inspiration

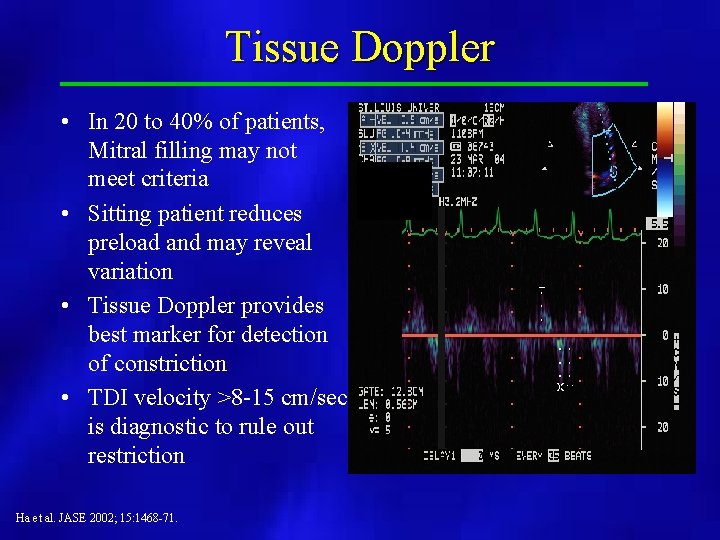
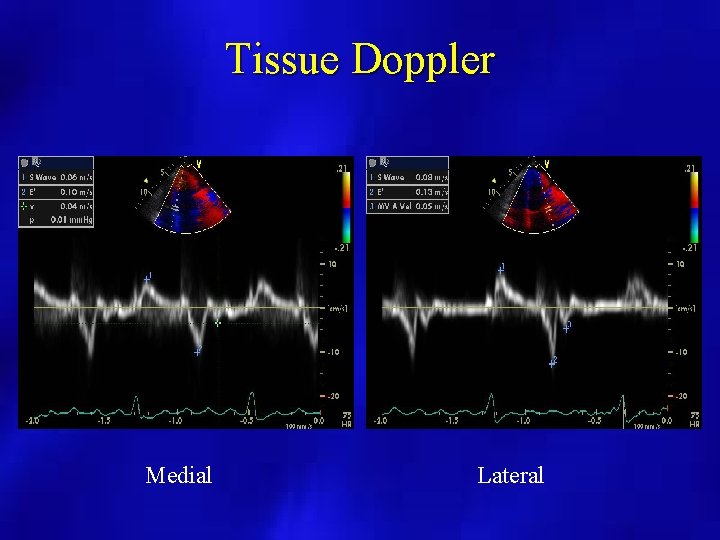
Tissue Doppler • In 20 to 40% of patients, Mitral filling may not meet criteria • Sitting patient reduces preload and may reveal variation • Tissue Doppler provides best marker for detection of constriction • TDI velocity >8 -15 cm/sec is diagnostic to rule out restriction Ha et al. JASE 2002; 15: 1468 -71.

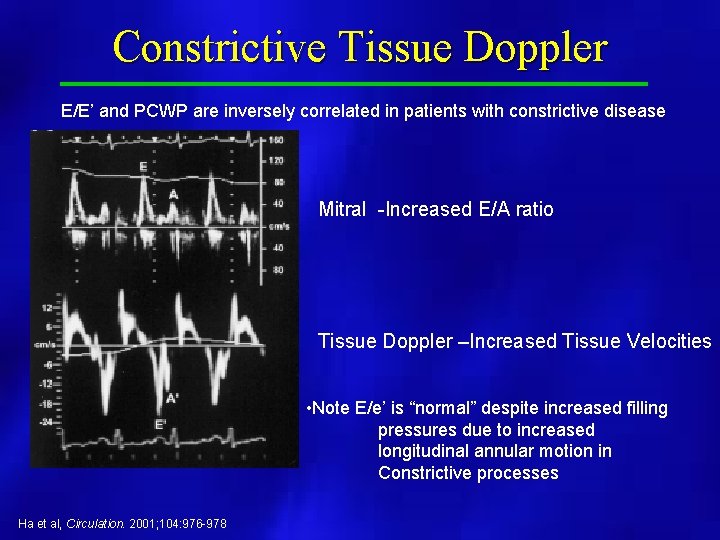
Constrictive Tissue Doppler E/E’ and PCWP are inversely correlated in patients with constrictive disease Mitral -Increased E/A ratio Tissue Doppler –Increased Tissue Velocities • Note E/e’ is “normal” despite increased filling pressures due to increased longitudinal annular motion in Constrictive processes Ha et al, Circulation. 2001; 104: 976 -978

Additional Doppler findings • Expiratory decrease in hepatic diastolic forward flow and increases in hepatic vein flow reversals

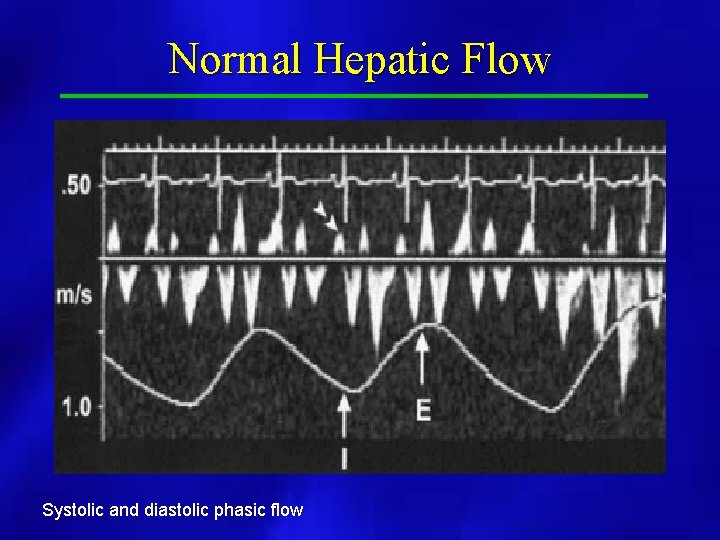
Normal Hepatic Flow Systolic and diastolic phasic flow

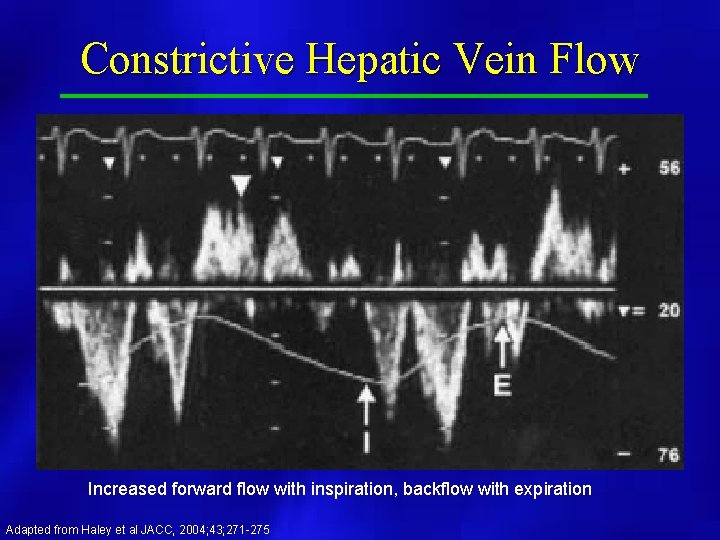
Constrictive Hepatic Vein Flow Increased forward flow with inspiration, backflow with expiration Adapted from Haley et al JACC, 2004; 43; 271 -275

Technical Concerns • COPD – May cause respiratory variability but not usually at the onset of inspiration/expiration – Mitral Inflow pattern is not necessarily increased E/A ratio as in constriction – SVC flow varies in COPD, not in constriction

Constriction Case

Mitral Inflow

Tissue Doppler Medial Lateral

Apical 4 Chamber view

Cardiac Catheterization Calcification LV function

Infiltrative/Restrictive Systemic Diseases

Etiology • Noninfiltrative – – – Idiopathic Familial HCM Scleroderma Diabetic • Infiltrative – Amyloidosis – Sarcoidosis • Storage – Hemochromatosis – Fabry’s • Hypereosinophilic Syndrome • Carcinoid

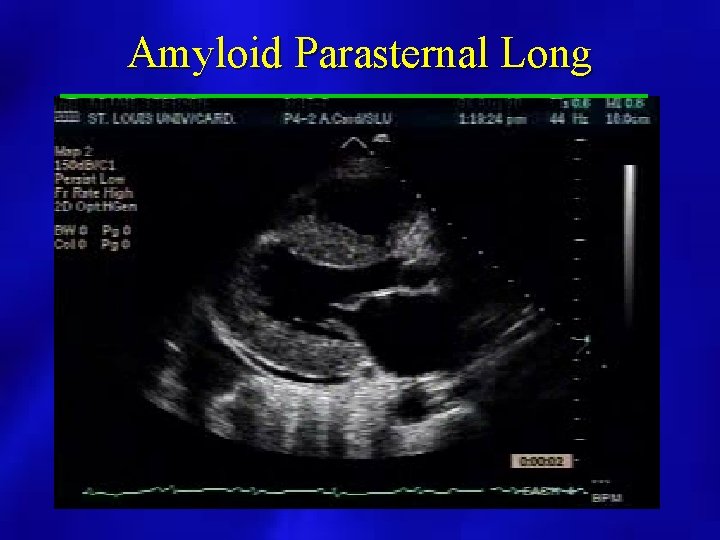
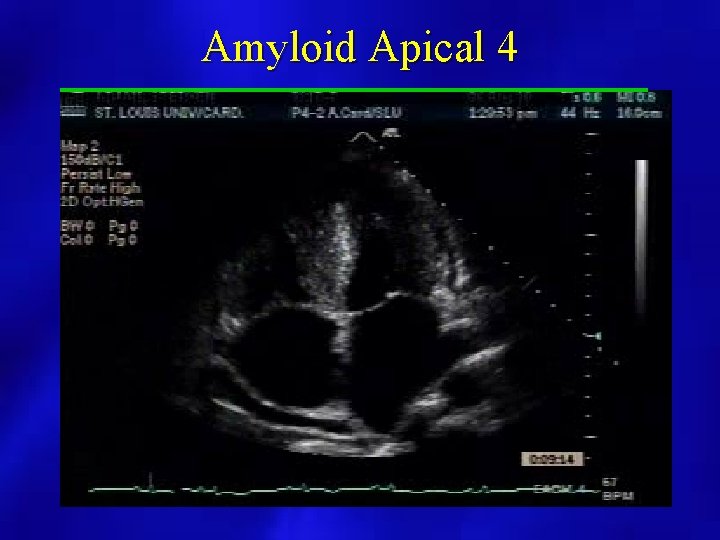
2 D Findings • • • Bilateral Atrial Enlargement Normal LV cavity size and function Hyperechoic Myocardium Possible Pericardial Effusion Dilated Hepatic Veins Granular appearance of the myocardium “Ground glass”

Amyloid Parasternal Long

Amyloid Apical 4

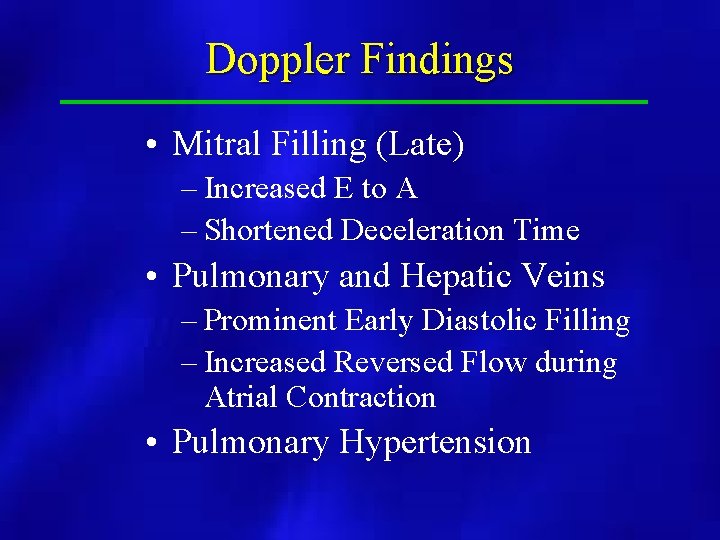
Doppler Findings • Mitral Filling (Late) – Increased E to A – Shortened Deceleration Time • Pulmonary and Hepatic Veins – Prominent Early Diastolic Filling – Increased Reversed Flow during Atrial Contraction • Pulmonary Hypertension

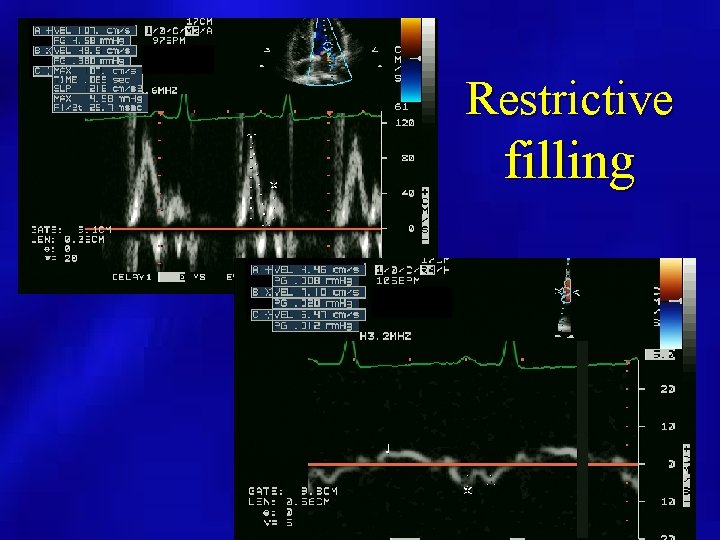
Restrictive filling

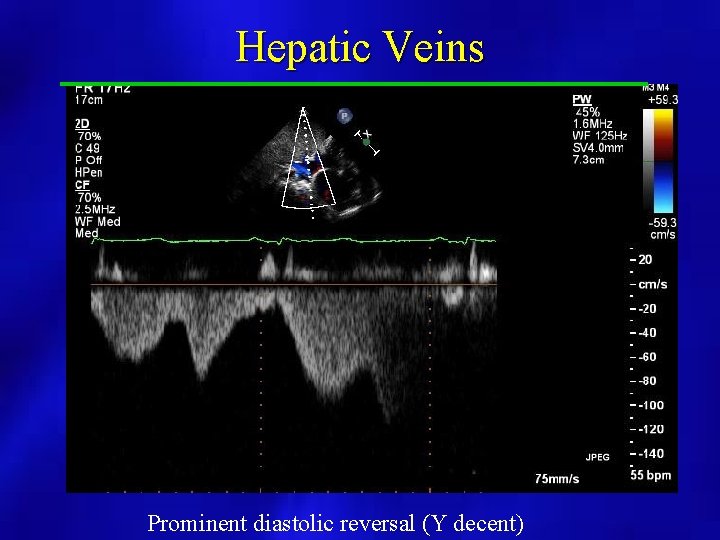
Hepatic Veins Prominent diastolic reversal (Y decent)

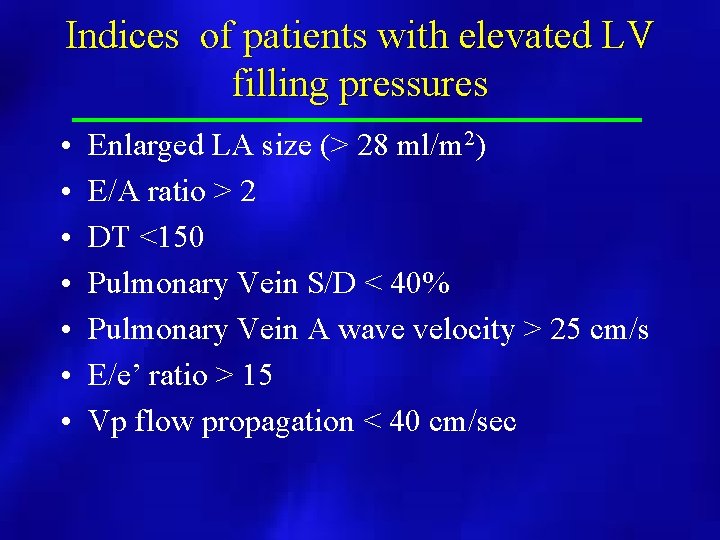
Indices of patients with elevated LV filling pressures • • Enlarged LA size (> 28 ml/m 2) E/A ratio > 2 DT <150 Pulmonary Vein S/D < 40% Pulmonary Vein A wave velocity > 25 cm/s E/e’ ratio > 15 Vp flow propagation < 40 cm/sec

Mitral / Tricuspid Inflow Constriction vs Restriction Normal I Mitral Tricuspid Constriction E I E Restriction I E

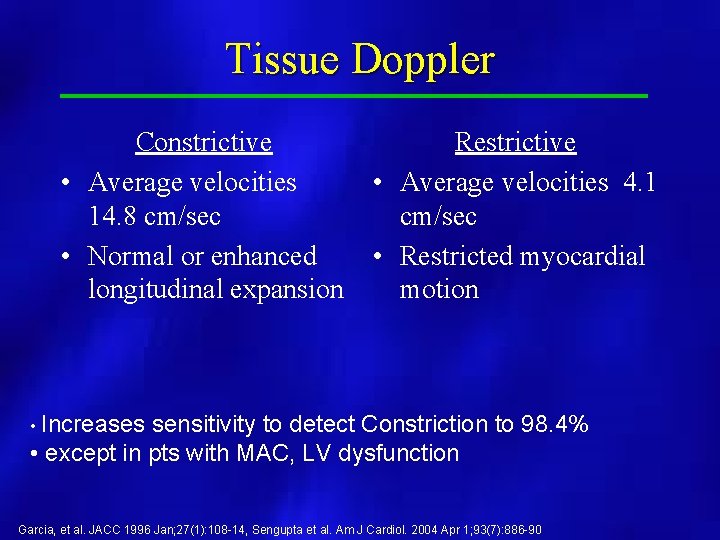
Tissue Doppler Constrictive • Average velocities 14. 8 cm/sec • Normal or enhanced longitudinal expansion Restrictive • Average velocities 4. 1 cm/sec • Restricted myocardial motion • Increases sensitivity to detect Constriction to 98. 4% • except in pts with MAC, LV dysfunction Garcia, et al. JACC 1996 Jan; 27(1): 108 -14, Sengupta et al. Am J Cardiol. 2004 Apr 1; 93(7): 886 -90

Mixed Constrictive/ Restrictive Physiology • Incidence varies, but around 20% of patients • May be found in Radiation Induced, CABG • Increased Mortality in Mixed physiology

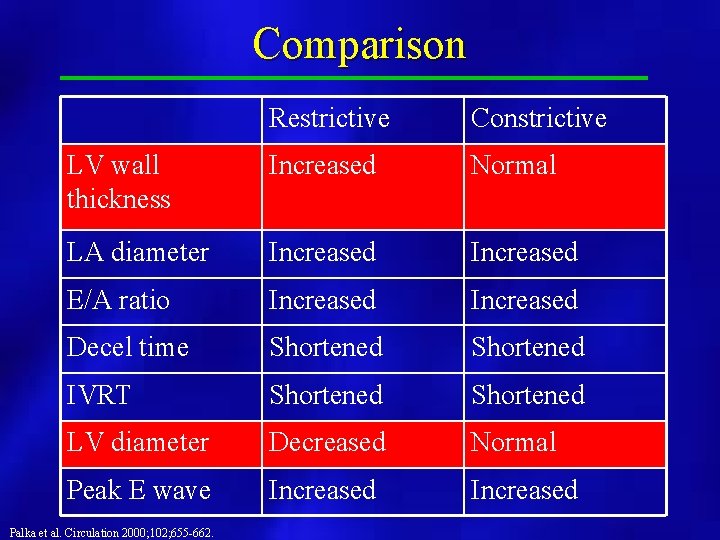
Comparison Restrictive Constrictive LV wall thickness Increased Normal LA diameter Increased E/A ratio Increased Decel time Shortened IVRT Shortened LV diameter Decreased Normal Peak E wave Increased Palka et al. Circulation 2000; 102; 655 -662.

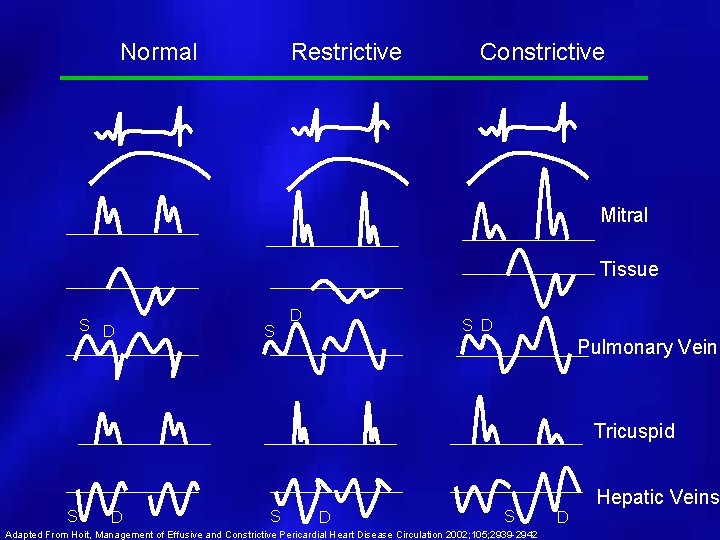
Normal Restrictive Constrictive Mitral Tissue S D S D Pulmonary Vein Tricuspid S D S Adapted From Hoit, Management of Effusive and Constrictive Pericardial Heart Disease Circulation 2002; 105; 2939 -2942 Hepatic Veins D

Case 1 Restrictive vs. Constrictive • 68 year old male • Admitted with shortness of breath • Known history of Amyloidosis





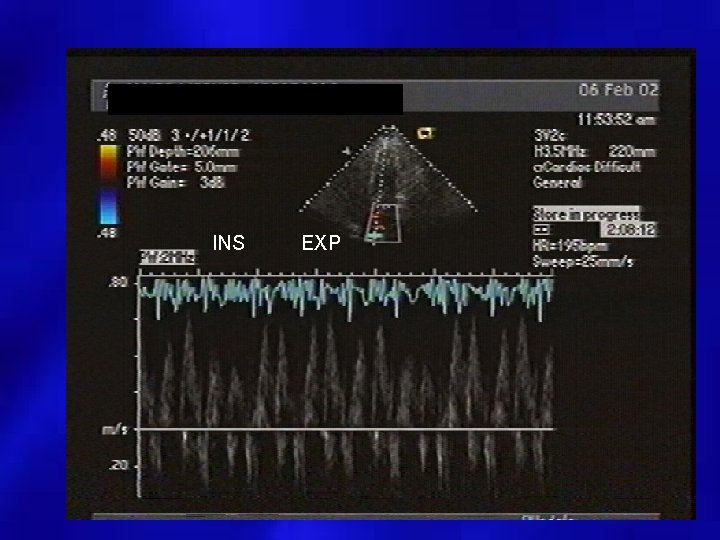
INS EXP

INS EXP

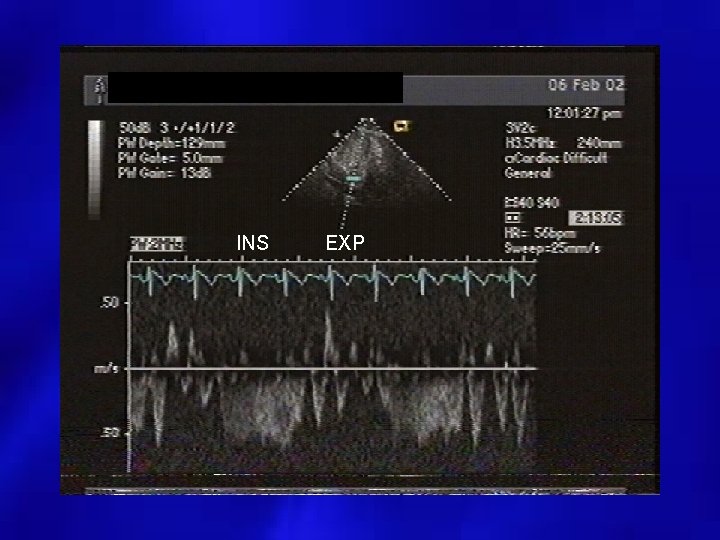
INS EXP

INS EXP
 Asthma grades
Asthma grades Etiology synonym
Etiology synonym Pes statement example
Pes statement example Cyst granuloma abscess
Cyst granuloma abscess Pathophysiology definition
Pathophysiology definition Lamacyl
Lamacyl Site:slidetodoc.com
Site:slidetodoc.com Etiology
Etiology Criminology etiology
Criminology etiology Potter face oligohydramnios
Potter face oligohydramnios Definition of acute appendicitis
Definition of acute appendicitis Etiology synonym
Etiology synonym Multicausal model of addiction
Multicausal model of addiction Etiology of cerebrovascular disease
Etiology of cerebrovascular disease Frank caries
Frank caries Etiology
Etiology Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Csf analysis interpretation
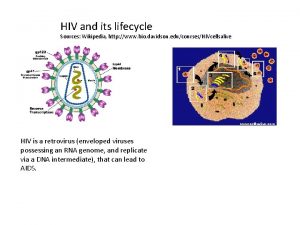
Csf analysis interpretation Viral life cycle
Viral life cycle Viral
Viral Morfologia viral
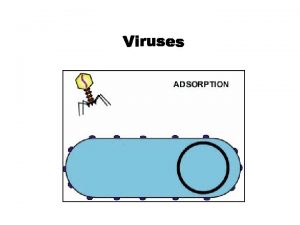
Morfologia viral Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Viral communications
Viral communications Viral entry
Viral entry Viral recombination
Viral recombination Ciclo viral
Ciclo viral Cultivation of viruses
Cultivation of viruses Antiperytique
Antiperytique Viral arthritis
Viral arthritis Equine rhinopneumonitis
Equine rhinopneumonitis Viral load sample collection
Viral load sample collection Inklüzyon cisimcikleri
Inklüzyon cisimcikleri Itil wikipedia fr
Itil wikipedia fr The dynamics of viral marketing
The dynamics of viral marketing Vaccins à vecteur viral
Vaccins à vecteur viral Spasmodic croup
Spasmodic croup Viral induced wheeze vs asthma
Viral induced wheeze vs asthma Viral shedding
Viral shedding An acute highly contagious viral disease
An acute highly contagious viral disease Viral dna
Viral dna Tratamiento meningitis viral
Tratamiento meningitis viral Viral infection
Viral infection Egg inoculation diagram
Egg inoculation diagram Vacinas subcutânea
Vacinas subcutânea Hgado
Hgado Viral receptors
Viral receptors Variola varicela
Variola varicela Vrus
Vrus Eline's viral
Eline's viral Rotarix live attenuated
Rotarix live attenuated Viral
Viral Causes of viral hemorrhagic fever
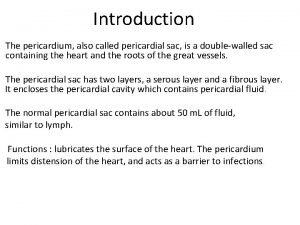
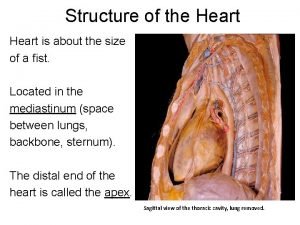
Causes of viral hemorrhagic fever Sinus of pericardium
Sinus of pericardium Pericardial membrane
Pericardial membrane Pericardial membranes
Pericardial membranes Septa of the heart
Septa of the heart Pericardial cavity
Pericardial cavity Middle mediastinum: contents mnemonic
Middle mediastinum: contents mnemonic Pericardial cavity
Pericardial cavity Pericardial friction
Pericardial friction Sinus venorum
Sinus venorum Oblique pericardial sinus
Oblique pericardial sinus Mediastinum contents
Mediastinum contents Pericardial friction
Pericardial friction Www.nisd.net
Www.nisd.net Effuse and diffuse
Effuse and diffuse Phase changes of matter
Phase changes of matter Hydrothorax vs pleural effusion
Hydrothorax vs pleural effusion Decreased tactile fremitus
Decreased tactile fremitus Hemarthrosis
Hemarthrosis Costophrenic angle
Costophrenic angle Concept map for mi
Concept map for mi Pleural effusion color chart
Pleural effusion color chart Normal pleural ldh
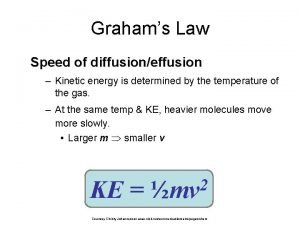
Normal pleural ldh How to calculate graham's law of diffusion
How to calculate graham's law of diffusion Transudate vs exudate
Transudate vs exudate Posterior lung fields
Posterior lung fields Pleura
Pleura Nccth
Nccth Ellis curve in pleural effusion
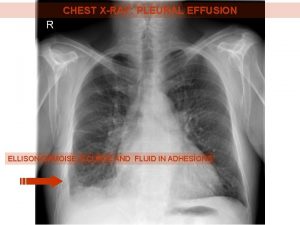
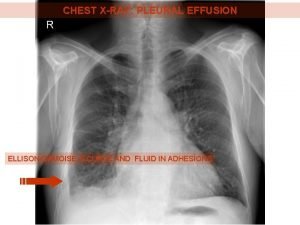
Ellis curve in pleural effusion Dr sasan beheshti
Dr sasan beheshti Meniscus sign pleural effusion
Meniscus sign pleural effusion Hip effusion
Hip effusion Graham's law of diffusion equation
Graham's law of diffusion equation Pleural effusion color chart
Pleural effusion color chart Acute inflammation
Acute inflammation Pleura parietal visceral
Pleura parietal visceral Percutaneous nephrostomy
Percutaneous nephrostomy Garland triangle pleural effusion
Garland triangle pleural effusion Pulmonary rub
Pulmonary rub Mid axillary line
Mid axillary line Graham's law real life example
Graham's law real life example Grahams law
Grahams law Effusion definition
Effusion definition